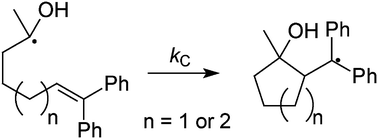
The 1-hydroxy-1-methyl-6,6-diphenyl-5-hexenyl radical (4a) and the 1-hydroxy-1-methyl-7,7-diphenyl-6-heptenyl radical (4b) were prepared from the corresponding PTOC esters (anhydrides of a carboxylic acid and N-hydroxypyridine-2-thione). The key step in the synthetic method for the precursors was a coupling reaction of the respective carboxylic acids with the thiohydroxamic acid, which was conducted for ca. 5 min and followed rapidly by chromatography. Rate constants for cyclizations of radicals 4a and 4b in acetonitrile and in THF were measured directly between –30 and 60 °C by laser flash photolysis methods. The Arrhenius functions in acetonitrile are log k = 9.9–2.6/2.303RT and log k = 8.9–4.4/2.303RT (kcal mol−1) for 4a and 4b, respectively. Rate constants for cyclizations at room temperature of 9 × 107s−1 and 4 × 105s−1 are somewhat larger than the rate constants for cyclizations of analogous alkyl radicals. Crude rate constants at room temperature for H-atom trapping of 4a by thiophenol and 4b by t-butylthiol were kT = 1.2 × 109 M−1s−1 and kT = 2 × 107 M−1s−1, respectively, which are modestly larger than rate constants for reactions of alkyl radicals with the same trapping agents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...