Cycloaddition of homochiral dihydroimidazoles: A 1,3-dipolar cycloaddition route to optically active pyrrolo[1,2-a]imidazoles†
Raymond C. F.
Jones
*a,
Kevin J.
Howard
b,
John S.
Snaith‡
b,
Alexander J.
Blake
b,
Wang-Shei
Li
b and
Peter J.
Steel
c
aDepartment of Chemistry, Loughborough University, Loughborough, Leics, UK LE11 3TU. E-mail: r.c.f.jones@lboro.ac.uk; Fax: +44 1509 223925; Tel: +44 1509 222557
bDepartment of Chemistry, University of Nottingham, University Park, Nottingham, UK NG7 2RD
cDepartment of Chemistry, University of Canterbury, Private Bag 4800, Christchurch, 8140, New Zealand
First published on 29th November 2010
Abstract
N-Alkylation of optically active 1-benzyl-4-phenyl-4,5-dihydroimidazole with active alkyl halides and treatment of the so-formed 4,5-dihydroimidazolium ions with DBU in the presence of a range of electron-deficient alkene dipolarophiles, constitutes a ‘one-pot’ cascade terminating in a 1,3-dipolar cycloaddition reaction that affords optically active pyrrolo[1,2-a]imidazoles. Three bonds of the so-formed pyrrolidine moiety are constructed in this cascade. The cycloaddition follows an endo approach of dipole and dipolarophile with anti geometry of the dipole and facial selectivity derived from the phenyl substituent. Inter- and intramolecular cycloaddition modes are observed.
Introduction
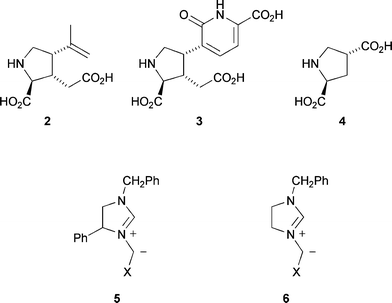
The five-membered saturated nitrogen heterocyclic pyrrolidine ring system is a common sub-unit in many natural products and synthetic molecules of biological significance.1 The use of azomethine ylides 1 as 1,3-dipoles in cycloaddition reactions with alkenes is a valuable method for the assembly of pyrrolidines (Scheme 1).2 These pericyclic reactions have well-defined transition states, ideally suited to the asymmetric synthesis of such molecules. Simple naturally occurring pyrrolidines with interesting biological properties include the marine metaboliteskainic acid 2 and acromelic acid 3,3 and pyrrolidine dicarboxylic acid 4, a potent inhibitor of glutamate transport.4 | ||
| Scheme 1 1,3-Dipolar cycloaddition of azomethine ylides to generate pyrrolidines (X, Y usually electron-withdrawing). | ||
Our approach to achieving stereocontrol in such dipolar cycloadditions is to attach a chiral auxiliary to nitrogen, but to restrict rotational freedom about the N-to-auxiliary bond by constraining the auxiliary in a ring, affording optically active 4,5-dihydroimidazolium (imidazolinium) ylides 5. The facial selectivity of the ylides in cycloadditions is thus fully predictable; the phenyl substituent facilitates ultimate removal of the templating atoms. The ylides are generated in situ from dihydroimidazoles and a suitable alkylating agent, and undergo enantioselective cycloaddition to afford optically active pyrrolo[1,2-a]imidazoles as potential precursors to the corresponding pyrrolidines.5,6 In these sequences three of the bonds in the newly-formed pyrrolidine ring are made during the alkylation-deprotonation-cycloaddition cascade. In a previous publication we have detailed the diasteroselective cycloadditions of achiral dihydroimidazolium ylides 6;7 we now report in full the results of our investigations in this area using the optically active ylides 5. Related studies of asymmetric induction in azomethine ylide cycloaddition by an auxiliary rotationally constrained at nitrogen have been disclosed by others.8
Results and Discussion
The precursors to the ylides 5 are the dihydroimidazoles 8a,b. These were prepared from the enantiomerically pure diamines 7a,b (themselves prepared from commercially available enantiomers of phenylglycine as we have reported earlier9) by heating at reflux in triethyl orthoformate with catalytic p-toluenesulfonic acid. Initial attempts at quaternisation proved very slow, being incomplete after several days at 20 °C, or 23 h at reflux in THF, even with active halides such as methyl bromoacetate; addition of KI had no effect. This led to the development of a revised ‘one-pot’ procedure whereby the salt is consumed by deprotonation and cycloaddition as it is formed.10 Thus, imidazolines 8a,b were separately mixed with an alkylating agent and a dipolarophile (3 mol equiv.) in dry THF and heated to reflux, when 1 mol equiv. of 1,8-diazabicyclo[5.4.0]undec-8-ene (DBU) was added dropwise over 4 h followed by a further 2 h at reflux. Isolation then provided the hexahydropyrrolo[1,2-a]imidazole cycloadducts, forming three of the five bonds of the new pyrrolidine ring in one pot (Scheme 2).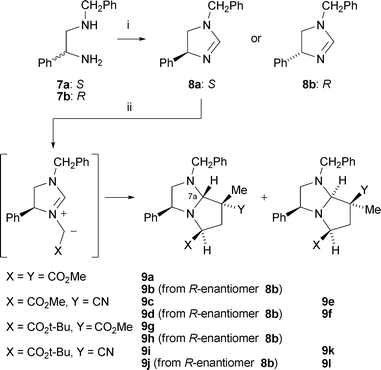 | ||
Scheme 2 Preparation of pyrroloimidazoles 9 by dipolar cycloaddition of azomethine ylides derived from optically active dihydroimidazoles 8 Reagents: i, (EtO)3CH, p-TsOH; ii, BrCH2X, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)Y, THF reflux, then DBU. C(Me)Y, THF reflux, then DBU. | ||
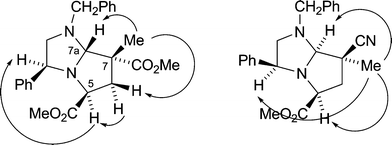
A variety of alkylating agents and dipolarophiles were employed in these reactions. Thus, with methyl bromoacetate as alkylating agent, smooth reactions occurred with methyl 2-methylpropenoate as dipolarophile, affording cycloadducts 9a (53%) and 9b (55%) from 8a and 8b, respectively (Scheme 2; only 3S-series cycloadducts shown). Employing 2-methylpropenonitrile as dipolarophile gave cycloadducts 9c (34%) and 9d (56%). The absolute stereochemistry of the cycloadducts is as illustrated and was secured by 1H NOE difference spectroscopy to determine relative stereochemistry, as exemplified in Fig. 1, combined with the fixed configuration of C-3 derived originally from phenylglycine. For example, for cycloadduct 9a, NOE enhancements were observed between protons on the following carbon atoms: C-5→C-3, C-6(pro-S)→C-5, C-7(Me)→C-6(pro-R), C-7(Me)→C-7a. This stereochemical outcome of the cycloaddition is consistent with an endo approach of the dipole and dipolarophile, with the dipole having the anti geometry and facial selectivity provided by the 4-phenyl substituent in the dipole, as summarised in the transition state model illustrated in Fig. 2.5 We did not generally observe any other cycloadduct diastereoisomers with ester-activated dipolarophiles, but with nitrile-based dipolarophiles a minor isomer corresponding to exo approach could sometimes be isolated. Thus, nitrile 9c was isolated along with small amounts of 9e (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo 8
exo 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and nitrile 9d with small amounts of 9f (endo
1), and nitrile 9d with small amounts of 9f (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo 11
exo 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
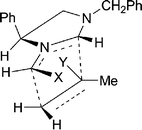
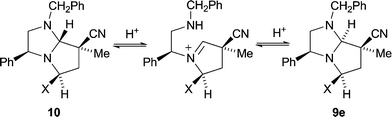
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Interestingly, however, 1H NOE difference spectroscopy (e.g.Fig. 1 for adduct 9e) showed that the stereochemistry at C-7a in the exo adducts was opposite to what would be predicted for exo addition by our transition state model. It is likely that exo addition occurs in the expected fashion and is followed by an epimerisation at C-7a, since the alternative antarafacial addition across the dipole is energetically disfavoured.11 A possible mechanism for this reversible epimerisation would involve protonation at N-1 of the predicted exo-adduct 10 followed by ring opening to produce an N-substituted pyrrolidinium species. Such species would be capable of ring closing from the opposite face to the ring opening and so provide the epimerised exo-adduct 9e (Scheme 3).
1). Interestingly, however, 1H NOE difference spectroscopy (e.g.Fig. 1 for adduct 9e) showed that the stereochemistry at C-7a in the exo adducts was opposite to what would be predicted for exo addition by our transition state model. It is likely that exo addition occurs in the expected fashion and is followed by an epimerisation at C-7a, since the alternative antarafacial addition across the dipole is energetically disfavoured.11 A possible mechanism for this reversible epimerisation would involve protonation at N-1 of the predicted exo-adduct 10 followed by ring opening to produce an N-substituted pyrrolidinium species. Such species would be capable of ring closing from the opposite face to the ring opening and so provide the epimerised exo-adduct 9e (Scheme 3).
 | ||
| Fig. 1 n.O.e correlations to determine relative stereochemistry of cycloadducts 9a and 9e. | ||
 | ||
| Fig. 2 Transition state model for cycloaddition to give 9a (X = Y = CO2Me). | ||
 | ||
| Scheme 3 Mechanism proposed for epimerisation at C-7a of pyrroloimidazoles. | ||
Using t-butyl bromoacetate as alkylating agent permitted smooth cycloaddition with a variety of dipolarophiles. With methyl 2-methylpropenoate adducts 9g (from 8a) and 9h (from 8b) were isolated as single stereoisomers. Once again the stereochemical outcome of the cycloaddition was in agreement with our transition state model. It proved possible to crystallise pyrroloimidazole 9g, and a single crystal X-ray analysis (Fig. 3) confirmed the structure to be as shown, in agreement with our 1H NOE studies. As in the previous examples, reaction with 2-methylpropenonitrile was slightly less stereoselective, affording adducts 9i (from 8a) and 9j (from 8b) along with a small amount of the product of exo addition: 9i with 9k (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo 8
exo 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 9j with 9l (endo
1); 9j with 9l (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo 8
exo 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
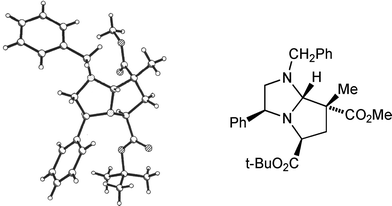 | ||
| Fig. 3 X-Ray crystal structure of cycloadduct 9g. | ||
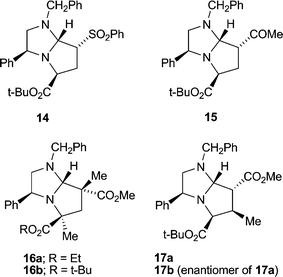
Seeking an approach to natural products that contain a 2,4-disubstituted pyrrolidine skeleton, we were interested in achieving cycloadditions with dipolarophiles lacking a substituent α to the activating group; in particular, we were concerned about the stereochemical integrity of the resulting C-7 substituent. Our work with the achiral imidazoline 11 had shown that cycloadducts such as 12 existed as an inseparable mixture of epimers at C-7, presumably via elimination (through ring-opening, cf.Scheme 3, and proton loss) and conjugate addition across C(7)–C(7a).5 Gratifyingly, methyl and t-butyl propenoates with the appropriate dihydroimidazoles 8a or 8b and t-butyl bromoacetate as alkylating agent, gave single stereoisomers of bicycles 13a (65%) and its enantiomer 13b (63%), and 13c (59%) and its enantiomer 13d (49%) (Scheme 4). Once again we were able to support our 1H NOE studies with a single crystal X-ray analysis of adduct 13a (Fig. 4), which was in agreement with our transition state model. In the case of t-butyl propenoate, some of the exo isomer was also produced. Hence 13c was isolated along with 13e (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo 20
exo 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 13d with 13f (endo
1) and 13d with 13f (endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo 25
exo 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Once again, 1H NOE studies showed that the exo adducts had the opposite stereochemistry at C-7a to that predicted by our transition state model.
1). Once again, 1H NOE studies showed that the exo adducts had the opposite stereochemistry at C-7a to that predicted by our transition state model.
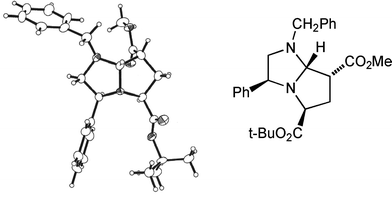 | ||
| Fig. 4 X-Ray crystal structure of cycloadduct 13a. | ||
 | ||
Scheme 4
Cycloaddition of azomethine ylides formed from dihydroimidazoles 8 using t-butyl bromoacetate as alkylating agent Reagents: i, BrCH2CO2t-Bu, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2R, THF reflux, then DBU. CHCO2R, THF reflux, then DBU. | ||
It was pleasing to find that these cycloadditions using t-butyl bromoacetate as alkylating agent were the most efficient to date. Using this alkylating agent it was possible to extend the range of dipolarophiles to those incorporating sulfone and ketone activating groups. Hence phenyl ethenyl sulfone gave 14 (33%)12 and but-3-en-2-one afforded 15 (81%), both from (S)-dihydroimidazole 8a. Employing secondary bromides as alkylating agents also afforded cycloadducts, although the yields were low. In cycloadditions with dihydroimidazole 8a and methyl 2-methylpropenoate, ethyl 2-bromopropanoate afforded the adduct 16a (29%) having quaternary centres at both C-5 and C-7, whilst t-butyl 2-bromopropanoate afforded adduct 16b (23%). Both of these cycloadducts were formed as single stereoisomers, with the more bulky ester function of the dipole adopting an anti disposition and the dipolarophile approaching endo as in previous examples.
A number of key metabolites with interesting biological profiles, in particular neuroexcitatory properties, feature a 2,3,4-trisubstituted pyrrolidine ring.3 Clearly to approach such systems we needed to achieve cycloaddition with 1,2-disubstituted alkene dipolarophiles, and we selected methyl (E)-but-2-enoate for our initial experiments. Although this dipolarophile afforded encouraging yields of bicycles 17a (46%) and 17b (26%, not optimised) from 8a and 8b, respectively, we were unable to obtain cycloadducts with any of the other cyclic and acyclic 1,2-disubstituted dipolarophiles which were investigated. These included ethyl (E)-5-hydroxypent-2-enoate, ethyl (E)-5-(t-butyldimethylsilyloxy)pent-2-enoate, diethyl glutaconate, 2(5H)-furanone and 5,6-dihydro-2H-pyran-2-one.
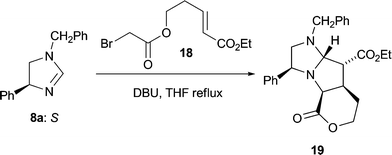
Success using 1,2-disubstituted alkene dipolarophiles finally came with an intramolecular variant of the cycloaddition reaction.13 Employing one equivalent of ethyl (E)-5-(bromoacetoxy)pent-2-enoate 18 whose convenient synthesis we have reported previously13 and which contains, tethered, both an alkylating agent and a dipolarophile, led to the crystalline tricyclic cycloadduct 19 from dihydroimidazole 8a (35%) as a single stereoisomer (Scheme 5); the enantiomer of 19 was also isolated (30% from 8b) but not fully characterised.14 The stereochemical outcome of this cycloaddition reaction was confirmed by an X-ray crystal structure analysis of 19 (Fig. 5). Once again the stereochemical result of the cycloaddition is fully consistent with our transition state model, furnishing a precursor to the desired 2,3,4-trisubstituted pyrrolidines with complete stereocontrol. Disappointingly the (Z)-bromoacetate failed to undergo the cycloaddition reaction, with a competing polymerisation processes appearing to dominate under the reaction conditions.
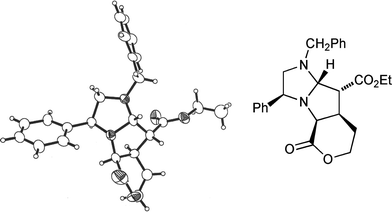 | ||
| Fig. 5 X-Ray crystal structure of cycloadduct 19. | ||
 | ||
| Scheme 5 Intramolecular cycloaddition of a dihydroimidazolium ylide. | ||
We have thus shown that optically active dihydroimidazoles, via dihydroimidazolium ylides, are useful precursors to optically active pyrrolo[1,2-a]imidazoles though an alkylation-deprotonation-cycloaddition cascade. The cycloaddition gives predictable stereochemistry and can operate in inter- and intramolecular modes. The cycloadducts are potential precursors of enantiopure pyrrolidines, as we have shown in preliminary form and will report in detail.15
Experimental
Melting points were obtained on a Gallenkamp capillary or a Reichert hot stage and are uncorrected. IR spectra were recorded using Pye Unicam SP1000, Pye Unicam SP3-100 or Perkin-Elmer 1820X FT spectrometers. Mass spectra were recorded using AEI MS902 or VG 8080E spectrometers. 1H NMR spectra were obtained using the following spectrometers: Perkin Elmer R32 or Jeol FX90Q at 90 MHz; Bruker WM200 at 200 MHz; Bruker WM250 at 250 MHz; Bruker AM400 or Jeol EX400 at 400 MHz. 13C NMR spectra were recorded using the following instruments: Jeol FX90Q spectrometer at 22.8 MHz; Bruker WM200 at 50.3 MHz; Bruker WM250 at 62.9 MHz; Jeol EX280 at 68 MHz; Bruker AM400 or Jeol EX400 at 100.6 or 100.4 MHz, respectively. 1H NMR spectra were determined in deuteriochloroform solution unless indicated, and chemical shifts are quoted in parts per million (ppm) from tetramethylsilane as internal standard; coupling constants are quoted in Hz. 13C NMR spectra were determined in deuteriochloroform solution and chemical shifts quoted in ppm from tetramethylsilane as internal standard or from tetramethylsilane using CDCl3 as internal standard. Column chromatography was carried out at medium pressure using Merck Kieselgel 60 (Art. 9385). Thin layer chromatography (TLC) was carried out on silica plates (Kieselgel 60, F254, Merck Art. 5554). Solvent extracts were dried over anhydrous magnesium sulfate or sodium sulfate for at least 10 min. Ether refers to diethyl ether and petroleum ether corresponds to the fraction with b.p. 40–60 °C. Tetrahydrofuran (THF) and ether were distilled from lithium aluminium hydride or potassium immediately prior to use. Other dry solvents were prepared as described in Perrinet al.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylamine (99
triethylamine (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and fractionally distilled under vacuum to yield the title compound as a colourless oil (5.40 g, 86%): b.p. 146–148 °C/0.08 mmHg; [α]23D 168.9 (c 1.01; EtOH); νmax(film)/cm−1 3061, 3028, 2924, 2844, 1670, 1600, 1581, 1493, 1453, 1168, 757; δH (250 MHz; CDCl3) 2.96 (1H, t, J = 9.2, PhCHCHH), 3.58 (1H, dd, J = 9.2, 10.7, PhCHCHH), 4.20, 4.36 (each 1H, d, J = 14.9, PhCH2), 5.14 (1H, m, PhCHCH2), 7.10 (1H, d, J = 1.8, NCHN), 7.28 (10H, m, Ar–H); δC (68 MHz; CDCl3) 51.3 (PhCH2), 55.55 (PhCHCH2), 69.7 (PhCHCH2) 126.3, 126.7, 127.4, 127.5, 128.2, 128.4 (6 × Ar-CH), 136.4, 143.5 (2 × Ar–C), 156.7 (NCHN); m/z 236 (M+, 39%), 120 (52), 91(100). HRMS: (EI) M+ 236.1294; C16H16N2 requires M+ 236.1313. Found: C, 81.3; H, 7.1; N, 11.9%; C16H16N2 requires C, 81.3; H, 6.8; N, 11.85%.
1 v/v) and fractionally distilled under vacuum to yield the title compound as a colourless oil (5.40 g, 86%): b.p. 146–148 °C/0.08 mmHg; [α]23D 168.9 (c 1.01; EtOH); νmax(film)/cm−1 3061, 3028, 2924, 2844, 1670, 1600, 1581, 1493, 1453, 1168, 757; δH (250 MHz; CDCl3) 2.96 (1H, t, J = 9.2, PhCHCHH), 3.58 (1H, dd, J = 9.2, 10.7, PhCHCHH), 4.20, 4.36 (each 1H, d, J = 14.9, PhCH2), 5.14 (1H, m, PhCHCH2), 7.10 (1H, d, J = 1.8, NCHN), 7.28 (10H, m, Ar–H); δC (68 MHz; CDCl3) 51.3 (PhCH2), 55.55 (PhCHCH2), 69.7 (PhCHCH2) 126.3, 126.7, 127.4, 127.5, 128.2, 128.4 (6 × Ar-CH), 136.4, 143.5 (2 × Ar–C), 156.7 (NCHN); m/z 236 (M+, 39%), 120 (52), 91(100). HRMS: (EI) M+ 236.1294; C16H16N2 requires M+ 236.1313. Found: C, 81.3; H, 7.1; N, 11.9%; C16H16N2 requires C, 81.3; H, 6.8; N, 11.85%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (4
ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to afford the title compound as a colourless oil (1.80 g, 52%): [α]22D −15.6 (c 1.04; EtOH); νmax(film)/cm−1 3062, 3028, 2949, 2804, 1731, 1454, 1265, 1205, 1132, 700; δH (250 MHz; CDCl3) 1.50 (3H, s, CH3), 2.20 (1H, dd, J = 9.9, 13.2, 6-CHH), 2.44 (1H, dd, J = 9.2, 10.0, PhCHCHH), 2.77 (1H, dd, J = 6.8, 13.2, 6-CHH), 3.21 (2H, m, PhCHH and PhCHCHH), 3.34, 3.81 (each 3H, s, OCH3), 4.08 (1H, dd, J = 6.8, 9.9, 5-CH), 4.13 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.25 (10H, m, Ar–H); δC (100 MHz; CDCl3) 23.0 (CH3), 43.3 (6-CH2), 51.5, 51.9 (2 × OCH3), 53.3 (7-C), 58.4 (PhCH2), 64.7 (PhCHCH2), 67.0 (5-CH), 69.4 (PhCHCH2), 96.2 (7a-CH), 127.0, 127.1, 127.2, 128.0, 128.2, 128.8 (6 × Ar-CH), 138.4, 141.3 (2 × Ar-C), 174.6, 175.3 (2 × CO); m/z 408 (M+, 4%), 309 (29), 308 (100), 249 (36), 217 (13), 130 (12), 104 (34), 91 (87). HRMS: (EI) M+ 408.2001; C24H28N2O4 requires M+ 408.2049. Found: C, 70.85; H, 7.2; N, 6.9%; C24H28N2O4 requires C, 70.6; H, 6.9; N, 6.9%.
1 v/v) to afford the title compound as a colourless oil (1.80 g, 52%): [α]22D −15.6 (c 1.04; EtOH); νmax(film)/cm−1 3062, 3028, 2949, 2804, 1731, 1454, 1265, 1205, 1132, 700; δH (250 MHz; CDCl3) 1.50 (3H, s, CH3), 2.20 (1H, dd, J = 9.9, 13.2, 6-CHH), 2.44 (1H, dd, J = 9.2, 10.0, PhCHCHH), 2.77 (1H, dd, J = 6.8, 13.2, 6-CHH), 3.21 (2H, m, PhCHH and PhCHCHH), 3.34, 3.81 (each 3H, s, OCH3), 4.08 (1H, dd, J = 6.8, 9.9, 5-CH), 4.13 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.25 (10H, m, Ar–H); δC (100 MHz; CDCl3) 23.0 (CH3), 43.3 (6-CH2), 51.5, 51.9 (2 × OCH3), 53.3 (7-C), 58.4 (PhCH2), 64.7 (PhCHCH2), 67.0 (5-CH), 69.4 (PhCHCH2), 96.2 (7a-CH), 127.0, 127.1, 127.2, 128.0, 128.2, 128.8 (6 × Ar-CH), 138.4, 141.3 (2 × Ar-C), 174.6, 175.3 (2 × CO); m/z 408 (M+, 4%), 309 (29), 308 (100), 249 (36), 217 (13), 130 (12), 104 (34), 91 (87). HRMS: (EI) M+ 408.2001; C24H28N2O4 requires M+ 408.2049. Found: C, 70.85; H, 7.2; N, 6.9%; C24H28N2O4 requires C, 70.6; H, 6.9; N, 6.9%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (4
ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless oil (1.90 g, 55%): [α]22D 16.8 (c 1.20; EtOH); νmax(film)/cm−1 3027, 2948, 2804, 1731, 1453, 1265, 1205, 1178, 701; δH (400 MHz; CDCl3) 1.50 (3H, s, CH3), 2.20 (1H, dd, J = 9.8, 13.3, 6-CHH), 2.44 (1H, t, J = 9.6, PhCHCHH), 2.77 (1H, dd, J = 7.0, 13.3, 6-CHH), 3.22 (2H, m, PhCHH and PhCHCHH), 3.34, 3.80 (each 3H, s, OCH3), 4.06 (1H, dd, J = 7.0, 9.8, 5-CH), 4.12 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.24 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.8 (CH3), 43.2 (6-CH2), 51.3, 51.75 (2 × OCH3), 53.1 (7-C), 58.2 (PhCH2), 64.5 (PhCHCH2), 66.8 (5-CH), 69.2 (PhCHCH2), 96.0 (7a-CH), 126.8, 126.9, 127.0, 127.9, 128.1, 128.7 (6 × Ar-CH), 138.2 141.1 (2 × Ar–C), 174.45, 175.1 (2 × CO); m/z 408 (M+, 2%), 309 (29), 308 (67), 249 (56), 217 (22), 130 (24), 113 (26), 104 (52), 91 (100). HRMS: (EI) M+ 408.2002; C24H28N2O4 requires M+ 408.2049. Found: C, 70.6; H, 7.2; N, 6.7%; C24H28N2O4 requires C, 70.6; H, 6.9; N, 6.9%.
1 v/v) yielded the title compound as a colourless oil (1.90 g, 55%): [α]22D 16.8 (c 1.20; EtOH); νmax(film)/cm−1 3027, 2948, 2804, 1731, 1453, 1265, 1205, 1178, 701; δH (400 MHz; CDCl3) 1.50 (3H, s, CH3), 2.20 (1H, dd, J = 9.8, 13.3, 6-CHH), 2.44 (1H, t, J = 9.6, PhCHCHH), 2.77 (1H, dd, J = 7.0, 13.3, 6-CHH), 3.22 (2H, m, PhCHH and PhCHCHH), 3.34, 3.80 (each 3H, s, OCH3), 4.06 (1H, dd, J = 7.0, 9.8, 5-CH), 4.12 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.24 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.8 (CH3), 43.2 (6-CH2), 51.3, 51.75 (2 × OCH3), 53.1 (7-C), 58.2 (PhCH2), 64.5 (PhCHCH2), 66.8 (5-CH), 69.2 (PhCHCH2), 96.0 (7a-CH), 126.8, 126.9, 127.0, 127.9, 128.1, 128.7 (6 × Ar-CH), 138.2 141.1 (2 × Ar–C), 174.45, 175.1 (2 × CO); m/z 408 (M+, 2%), 309 (29), 308 (67), 249 (56), 217 (22), 130 (24), 113 (26), 104 (52), 91 (100). HRMS: (EI) M+ 408.2002; C24H28N2O4 requires M+ 408.2049. Found: C, 70.6; H, 7.2; N, 6.7%; C24H28N2O4 requires C, 70.6; H, 6.9; N, 6.9%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the exo-adduct (9e) as a colourless solid (18 mg, 4%): m.p. 103–105 °C; νmax(film)/cm−1 3030, 2951, 2815, 2235, 1747, 1455, 1437, 1206, 1181, 701; δH (250 MHz; CDCl3) 1.51 (3H, s, CH3), 2.42 (1H, dd, J 6.0, 12.8, 6-CHH), 2.63 (1H, dd, J = 9.2, 10.1, PhCHCHH), 2.81 (1H, dd, J = 10.4, 12.8, 6-CHH), 3.35 (3H, s, OCH3), 3.38 (1H, m, PhCHCHH), 3.46 (1H, d, J = 13.1, PhCHH), 3.56 (1H, dd, J = 6.0, 10.4, 5-CH), 4.01 (1H, dd, J = 5.5, 10.1, PhCHCH2), 4.08 (1H, d, J = 13.1, PhCHH), 4.75 (1H, s, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.0 (CH3), 38.6 (7-C), 43.0 (6-CH2), 51.8 (OCH3), 57.7 (PhCH2), 64.2 (5-CH), 64.3 (PhCHCH2), 69.7 (PhCHCH2), 91.6 (7a-CH), 123.1 (CN), 126.9, 127.4, 127.6, 128.2, 128.5, 128.7 (6 × Ar-CH), 137.4, 140.0 (2 × Ar-C), 172.6 (CO); m/z 375 (M+, 3%), 309 (24), 308 (100), 249 (59), 217 (19), 130 (12), 104 (31), 91 (90). HRMS: (EI) M+ 375.1948; C23H25N3O2·0.33H2O requires M+ 375.1947. Found: C, 72.25; H, 6.7; N, 10.9%. C23H25N3O2·0.33H2O requires C, 72.4; H, 6.8; N, 11.0%; and the title compound (9c) as a colourless oil (0.16 g, 34%): [α]22D −43.5 (c 0.69; EtOH); νmax(film)/cm−1 3028, 2950, 2236, 1745, 1495, 1453, 1175; δH (250 MHz; CDCl3) 1.42 (3H, s, CH3), 2.36 (1H, dd, J = 10.1, 13.1, 6-CHH), 2.64 (1H, t, J = 9.6, PhCHCHH), 2.75 (1H, dd, J = 6.2, 13.1, 6-CHH), 3.36 (3H, s, OCH3), 3.50 (2H, m, PhCHH and PhCHCHH), 3.84 (1H, dd, J = 6.2, 10.1, 5-CH), 4.06 (1H, d, J = 12.8, PhCHH), 4.20 (1H, s, 7a-CH), 4.32 (1H, dd, J = 5.7, 9.9, PhCHCH2), 7.23 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.1 (CH3), 43.8 (6-CH2), 44.5 (7-C), 51.7 (OCH3), 58.5 (PhCH2), 64.5 (PhCHCH2), 65.1 (5-CH), 69.3 (PhCHCH2), 93.5 (7a-CH), 122.2 (CN), 126.8, 127.3, 127.4, 128.1, 128.4, 128.9 (6 × Ar-CH), 137.9, 140.3 (2 × Ar-C), 172.7 (CO); m/z 375 (M+, 1%), 309 (13), 308 (66), 249 (39), 217 (13), 146 (15), 130 (11), 104 (46), 91 (100). HRMS: (EI) M+ 375.1950; C23H25N3O2 requires M+ 375.1947. Found: C, 73.2; H, 6.9; N, 10.9%; C23H25N3O2 requires C, 73.6; H, 6.7; N, 11.1%.
1 v/v) yielded the exo-adduct (9e) as a colourless solid (18 mg, 4%): m.p. 103–105 °C; νmax(film)/cm−1 3030, 2951, 2815, 2235, 1747, 1455, 1437, 1206, 1181, 701; δH (250 MHz; CDCl3) 1.51 (3H, s, CH3), 2.42 (1H, dd, J 6.0, 12.8, 6-CHH), 2.63 (1H, dd, J = 9.2, 10.1, PhCHCHH), 2.81 (1H, dd, J = 10.4, 12.8, 6-CHH), 3.35 (3H, s, OCH3), 3.38 (1H, m, PhCHCHH), 3.46 (1H, d, J = 13.1, PhCHH), 3.56 (1H, dd, J = 6.0, 10.4, 5-CH), 4.01 (1H, dd, J = 5.5, 10.1, PhCHCH2), 4.08 (1H, d, J = 13.1, PhCHH), 4.75 (1H, s, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.0 (CH3), 38.6 (7-C), 43.0 (6-CH2), 51.8 (OCH3), 57.7 (PhCH2), 64.2 (5-CH), 64.3 (PhCHCH2), 69.7 (PhCHCH2), 91.6 (7a-CH), 123.1 (CN), 126.9, 127.4, 127.6, 128.2, 128.5, 128.7 (6 × Ar-CH), 137.4, 140.0 (2 × Ar-C), 172.6 (CO); m/z 375 (M+, 3%), 309 (24), 308 (100), 249 (59), 217 (19), 130 (12), 104 (31), 91 (90). HRMS: (EI) M+ 375.1948; C23H25N3O2·0.33H2O requires M+ 375.1947. Found: C, 72.25; H, 6.7; N, 10.9%. C23H25N3O2·0.33H2O requires C, 72.4; H, 6.8; N, 11.0%; and the title compound (9c) as a colourless oil (0.16 g, 34%): [α]22D −43.5 (c 0.69; EtOH); νmax(film)/cm−1 3028, 2950, 2236, 1745, 1495, 1453, 1175; δH (250 MHz; CDCl3) 1.42 (3H, s, CH3), 2.36 (1H, dd, J = 10.1, 13.1, 6-CHH), 2.64 (1H, t, J = 9.6, PhCHCHH), 2.75 (1H, dd, J = 6.2, 13.1, 6-CHH), 3.36 (3H, s, OCH3), 3.50 (2H, m, PhCHH and PhCHCHH), 3.84 (1H, dd, J = 6.2, 10.1, 5-CH), 4.06 (1H, d, J = 12.8, PhCHH), 4.20 (1H, s, 7a-CH), 4.32 (1H, dd, J = 5.7, 9.9, PhCHCH2), 7.23 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.1 (CH3), 43.8 (6-CH2), 44.5 (7-C), 51.7 (OCH3), 58.5 (PhCH2), 64.5 (PhCHCH2), 65.1 (5-CH), 69.3 (PhCHCH2), 93.5 (7a-CH), 122.2 (CN), 126.8, 127.3, 127.4, 128.1, 128.4, 128.9 (6 × Ar-CH), 137.9, 140.3 (2 × Ar-C), 172.7 (CO); m/z 375 (M+, 1%), 309 (13), 308 (66), 249 (39), 217 (13), 146 (15), 130 (11), 104 (46), 91 (100). HRMS: (EI) M+ 375.1950; C23H25N3O2 requires M+ 375.1947. Found: C, 73.2; H, 6.9; N, 10.9%; C23H25N3O2 requires C, 73.6; H, 6.7; N, 11.1%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (4
ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the exo-adduct (9f) as a colourless solid (38 mg, 5%), m.p. 100–102 °C: νmax KBr/cm−1 3062, 3029, 2950, 2815, 2234, 1745, 1454, 1204, 1180, 700; δH (250 MHz; CDCl3) 1.51 (3H, s, CH3), 2.42 (1H, dd, J = 6.0, 12.8, 6-CHH), 2.63 (1H, dd, J = 9.2, 10.0, PhCHCHH), 2.81 (1H, dd, J = 10.4, 12.8, 6-CHH), 3.34 (3H, s, OCH3) 3.36 (1H, m, PhCHCHH), 3.46 (1H, d, J = 13.1, PhCHH), 3.56 (1H, dd, J = 6.0, 10.4, 5-CH), 4.01 (1H, dd, J = 5.5, 10.0, PhCHCH2), 4.08 (1H, d, J = 13.0, PhCHH), 4.75 (1H, s, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.0 (CH3), 38.6 (7-C), 43.0 (6-CH2), 51.8 (OCH3), 57.7 (PhCH2), 64.2 (5-CH), 64.3 (PhCHCH2), 69.7 (PhCHCH2), 91.6 (7a-CH), 123.1 (CN), 126.9, 127.4, 127.55, 128.1, 128.45, 128.7 (6 × Ar-CH), 137.4, 140.0 (2 × Ar-C), 172.6 (CO); m/z 375 (M+, 1%), 308 (82), 249 (52), 217 (19), 130 (13), 104 (39) and 91 (100). HRMS: (EI) M+ 375.1951; C23H25N3O2 requires M+ 375.1947. Found: C, 73.7; H, 6.9; N, 11.5%; C23H25N3O2 requires C, 73.6; H, 6.7; N, 11.2%; and the title compound (9d) as a colourless solid (1.78 g, 56%), m.p. 67–69 °C: [α]22D 43.5 (c 0.63; EtOH); νmax KBr/cm−1 3028, 2948, 2811, 2236, 1744, 1454, 1204, 1176, 701; δH (250 MHz; CDCl3) 1.41 (3H, s, CH3), 2.35 (1H, dd, J = 10.1, 13.1, 6-CHH), 2.63 (1H, t, J = 9.7, PhCHCHH), 2.72 (1H, dd, J = 6.1, 13.1, 6-CHH), 3.35 (3H, s, OCH3), 3.50 (2H, m, PhCHH and PhCHCHH), 3.83 (1H, dd, J = 6.1, 10.1, 5-CH), 4.04 (1H, d, J = 12.9, PhCHH), 4.18 (1H, s, 7a-CH), 4.30 (1H, dd, J = 5.7, 9.7, PhCHCH2), 7.33 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.1 (CH3), 43.8 (6-CH2), 44.6 (7-C), 51.75 (OCH3), 58.5 (PhCH2), 64.5 (PhCHCH2), 65.1 (5-CH), 69.4 (PhCHCH2), 93.55 (7a-CH), 122.2 (CN), 126.85, 127.4, 127.4, 128.1, 128.4, 128.9 (6 × Ar-CH), 137.9, 140.3 (2 × Ar-C), 172.8 (CO); m/z 375 (M+, 2%), 309 (26), 308 (94), 249 (59), 217 (21), 104 (54), 91 (100). HRMS: (EI) M+ 375.1977; C23H25N3O2 requires M+ 375.1947. Found: C, 73.9; H, 6.6; N, 11.3%; C23H25N3O2 requires C, 73.6; H, 6.7; N, 11.2%.
1 v/v) yielded the exo-adduct (9f) as a colourless solid (38 mg, 5%), m.p. 100–102 °C: νmax KBr/cm−1 3062, 3029, 2950, 2815, 2234, 1745, 1454, 1204, 1180, 700; δH (250 MHz; CDCl3) 1.51 (3H, s, CH3), 2.42 (1H, dd, J = 6.0, 12.8, 6-CHH), 2.63 (1H, dd, J = 9.2, 10.0, PhCHCHH), 2.81 (1H, dd, J = 10.4, 12.8, 6-CHH), 3.34 (3H, s, OCH3) 3.36 (1H, m, PhCHCHH), 3.46 (1H, d, J = 13.1, PhCHH), 3.56 (1H, dd, J = 6.0, 10.4, 5-CH), 4.01 (1H, dd, J = 5.5, 10.0, PhCHCH2), 4.08 (1H, d, J = 13.0, PhCHH), 4.75 (1H, s, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.0 (CH3), 38.6 (7-C), 43.0 (6-CH2), 51.8 (OCH3), 57.7 (PhCH2), 64.2 (5-CH), 64.3 (PhCHCH2), 69.7 (PhCHCH2), 91.6 (7a-CH), 123.1 (CN), 126.9, 127.4, 127.55, 128.1, 128.45, 128.7 (6 × Ar-CH), 137.4, 140.0 (2 × Ar-C), 172.6 (CO); m/z 375 (M+, 1%), 308 (82), 249 (52), 217 (19), 130 (13), 104 (39) and 91 (100). HRMS: (EI) M+ 375.1951; C23H25N3O2 requires M+ 375.1947. Found: C, 73.7; H, 6.9; N, 11.5%; C23H25N3O2 requires C, 73.6; H, 6.7; N, 11.2%; and the title compound (9d) as a colourless solid (1.78 g, 56%), m.p. 67–69 °C: [α]22D 43.5 (c 0.63; EtOH); νmax KBr/cm−1 3028, 2948, 2811, 2236, 1744, 1454, 1204, 1176, 701; δH (250 MHz; CDCl3) 1.41 (3H, s, CH3), 2.35 (1H, dd, J = 10.1, 13.1, 6-CHH), 2.63 (1H, t, J = 9.7, PhCHCHH), 2.72 (1H, dd, J = 6.1, 13.1, 6-CHH), 3.35 (3H, s, OCH3), 3.50 (2H, m, PhCHH and PhCHCHH), 3.83 (1H, dd, J = 6.1, 10.1, 5-CH), 4.04 (1H, d, J = 12.9, PhCHH), 4.18 (1H, s, 7a-CH), 4.30 (1H, dd, J = 5.7, 9.7, PhCHCH2), 7.33 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.1 (CH3), 43.8 (6-CH2), 44.6 (7-C), 51.75 (OCH3), 58.5 (PhCH2), 64.5 (PhCHCH2), 65.1 (5-CH), 69.4 (PhCHCH2), 93.55 (7a-CH), 122.2 (CN), 126.85, 127.4, 127.4, 128.1, 128.4, 128.9 (6 × Ar-CH), 137.9, 140.3 (2 × Ar-C), 172.8 (CO); m/z 375 (M+, 2%), 309 (26), 308 (94), 249 (59), 217 (21), 104 (54), 91 (100). HRMS: (EI) M+ 375.1977; C23H25N3O2 requires M+ 375.1947. Found: C, 73.9; H, 6.6; N, 11.3%; C23H25N3O2 requires C, 73.6; H, 6.7; N, 11.2%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless oil (1.95 g, 51%): νmax(film)/cm−1 3061, 3027, 2976, 2803, 1731, 1453, 1366, 1263, 1151, 700; δH (250 MHz; CDCl3) 1.12 (9H, s, C(CH3)3), 1.49 (3H, s, 7-CH3), 2.18 (1H, dd, J = 9.8, 13.2, 6-CHH), 2.41 (1H, t, J = 9.7, PhCHCHH), 2.71 (1H, dd, J = 6.6, 13.2, 6-CHH), 3.20 (2H, m, PhCHH and PhCHCHH), 3.80 (3H, s, OCH3), 3.90 (1H, dd, J = 6.6, 9.8, 5-CH), 4.10 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.28 (10H, m, Ar–H); δC (68 MHz; CDCl3) 23.0 (7-CH3), 27.5 (C(CH3)3), 43.3 (6-CH2), 51.8 (OCH3), 53.2 (7-C), 58.4 (PhCH2), 64.9 (PhCHCH2), 68.2 (5-CH), 69.4 (PhCHCH2), 80.3 (C(CH3)3), 96.4 (7a-CH), 127.0, 127.1, 127.3, 128.0, 128.1, 128.8 (6 × Ar-CH), 138.4, 141.8 (2 × Ar-C), 173.5, 175.5 (2 × CO); m/z 450 (M+, 7%), 350 (57), 294 (45), 249 (39), 131 (42), 104 (40), 91 (100). HRMS: (EI) M+ 450.2491; C27H34N2O4 requires M+ 450.2519. Found: C, 72.1; H, 7.9; N, 6.1%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
1 v/v) yielded the title compound as a colourless oil (1.95 g, 51%): νmax(film)/cm−1 3061, 3027, 2976, 2803, 1731, 1453, 1366, 1263, 1151, 700; δH (250 MHz; CDCl3) 1.12 (9H, s, C(CH3)3), 1.49 (3H, s, 7-CH3), 2.18 (1H, dd, J = 9.8, 13.2, 6-CHH), 2.41 (1H, t, J = 9.7, PhCHCHH), 2.71 (1H, dd, J = 6.6, 13.2, 6-CHH), 3.20 (2H, m, PhCHH and PhCHCHH), 3.80 (3H, s, OCH3), 3.90 (1H, dd, J = 6.6, 9.8, 5-CH), 4.10 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.28 (10H, m, Ar–H); δC (68 MHz; CDCl3) 23.0 (7-CH3), 27.5 (C(CH3)3), 43.3 (6-CH2), 51.8 (OCH3), 53.2 (7-C), 58.4 (PhCH2), 64.9 (PhCHCH2), 68.2 (5-CH), 69.4 (PhCHCH2), 80.3 (C(CH3)3), 96.4 (7a-CH), 127.0, 127.1, 127.3, 128.0, 128.1, 128.8 (6 × Ar-CH), 138.4, 141.8 (2 × Ar-C), 173.5, 175.5 (2 × CO); m/z 450 (M+, 7%), 350 (57), 294 (45), 249 (39), 131 (42), 104 (40), 91 (100). HRMS: (EI) M+ 450.2491; C27H34N2O4 requires M+ 450.2519. Found: C, 72.1; H, 7.9; N, 6.1%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
Crystal data for 9g: C27H34N2O4, M = 450.6, colourless block, 0.70 × 0.59 × 0.30 mm; orthorhombic, P212121; a = 8.046(1), b = 14.280(2), c = 22.646(3) Å, U = 2602(1) Å3; T = 293 K, μ(Mo-Kα) = 0.08 mm−1, Dc = 1.15 g cm−3; Z = 4, F(000) = 968, 2θmax = 50°; 299 parameters, wR = 0.1034 for all 2620 data, R = 0.042 for 1393 data with Fo > 4σ(Fo).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless oil (1.95 g, 51%): νmax(film)/cm−1 2978, 1732, 1455, 1264, 1152, 701; δH (400 MHz; CDCl3) 1.12 (9H, s, C(CH3)3), 1.49 (3H, s, 7-CH3), 2.18 (1H, dd, J = 9.8, 13.2, 6-CHH), 2.41 (1H, t, J = 9.7, PhCHCHH), 2.71 (1H, dd, J = 6.6, 13.2, 6-CHH), 3.21 (2H, m, PhCHH and PhCHCHH), 3.80 (3H, s, OCH3), 3.90 (1H, dd, J = 6.6, 9.8, 5-CH), 4.11 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.29 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.9 (7-CH3), 27.3 (C(CH3)3), 43.2 (6-CH2), 51.6 (OCH3), 53.0 (7-C), 58.2 (PhCH2), 64.8 (PhCHCH2), 68.0 (5-CH), 69.3 (PhCHCH2), 80.1 (C(CH3)3), 96.3 (7a-CH), 126.8, 126.9, 127.1, 127.85, 128.0, 128.6 (6 × Ar-CH), 138.2, 141.65 (2 × Ar-C), 173.2, 175.2 (2 × CO); m/z 450 (M+, 1%), 350 (44), 294 (48), 249 (33), 104 (38), 91 (100). HRMS: (EI) M+ 450.2521; C27H34N2O4 requires M+ 450.2519. Found: C, 72.1; H, 7.9; N, 6.1%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
1 v/v) yielded the title compound as a colourless oil (1.95 g, 51%): νmax(film)/cm−1 2978, 1732, 1455, 1264, 1152, 701; δH (400 MHz; CDCl3) 1.12 (9H, s, C(CH3)3), 1.49 (3H, s, 7-CH3), 2.18 (1H, dd, J = 9.8, 13.2, 6-CHH), 2.41 (1H, t, J = 9.7, PhCHCHH), 2.71 (1H, dd, J = 6.6, 13.2, 6-CHH), 3.21 (2H, m, PhCHH and PhCHCHH), 3.80 (3H, s, OCH3), 3.90 (1H, dd, J = 6.6, 9.8, 5-CH), 4.11 (2H, m, PhCHH and PhCHCH2), 4.31 (1H, s, 7a-CH), 7.29 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.9 (7-CH3), 27.3 (C(CH3)3), 43.2 (6-CH2), 51.6 (OCH3), 53.0 (7-C), 58.2 (PhCH2), 64.8 (PhCHCH2), 68.0 (5-CH), 69.3 (PhCHCH2), 80.1 (C(CH3)3), 96.3 (7a-CH), 126.8, 126.9, 127.1, 127.85, 128.0, 128.6 (6 × Ar-CH), 138.2, 141.65 (2 × Ar-C), 173.2, 175.2 (2 × CO); m/z 450 (M+, 1%), 350 (44), 294 (48), 249 (33), 104 (38), 91 (100). HRMS: (EI) M+ 450.2521; C27H34N2O4 requires M+ 450.2519. Found: C, 72.1; H, 7.9; N, 6.1%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the exo-adduct (9k) as a colourless solid (62 mg, 4%), m.p. 100–102 °C [from petroleum ether (b.p. 40–60 °C)]: νmax (CHCl3)/cm−1 2822, 2236, 1732, 1369, 1153; δH (250 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.50 (3H, s, 7-CH3), 2.38 (1H, dd, J = 5.8, 12.7, 6-CHH), 2.62 (1H, apparent t, J = 9.5, PhCHCHH), 2.77 (1H, dd, J = 10.7, 12.7, 6-CHH), 3.35 (1H, dd, J = 5.5, 8.9, PhCHCHH), 3.45 (2H, m, PhCHH and 5-CH), 3.99 (1H, dd, J = 5.5, 9.9, PhCHCH2), 4.08 (1H, d, J = 13.0, PhCHH), 4.75 (1H, s, 7a-CH), 7.27 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.15 (7-CH3), 27.4 (C(CH3)3), 38.5 (7-C), 43.4 (6-CH2), 57.8 (PhCH2), 64.7 (PhCHCH2), 65.6 (5-CH), 69.8 (PhCHCH2), 81.3 (C(CH3)3), 92.0 (7a-CH), 123.4 (CN), 127.2, 127.4, 127.5, 128.2, 128.4, 128.7 (6 × Ar-CH), 137.6, 140.5 (2 × Ar-C), 171.4 (CO); m/z 350 (39%), 316 (12), 294 (48), 249 (32), 104 (40), 91 (100). Found: C, 74.85; H, 7.7; N, 10.0%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%; and the title compound (9i) as a colourless solid (0.47 g, 27%), m.p. 118–119 °C [from petroleum ether (b.p. 40–60 °C)]: [α]22D −15.8 (c 0.48; EtOH); νmax (KBr)/cm−1 3072, 3029, 2981, 2959, 2943, 2234, 1736, 1454, 1443, 1366, 1215, 1152, 1079, 702; δH (400 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.41 (3H, s, 7-CH3), 2.32 (1H, dd, J = 10.1, 13.1, 6-CHH), 2.59 (1H, apparent t, J = 9.5, PhCHCHH), 2.67 (1H, dd, J = 6.0, 13.1, 6-CHH), 3.47 (2H, m, PhCHH and PhCHCHH), 3.71 (1H, dd, J = 6.0, 10.1, 5-CH), 4.04 (1H, d, J = 12.7, PhCHH), 4.17 (1H, s, 7a-CH), 4.29 (1H, dd, J = 5.8, 9.8, PhCHCH2), 7.31 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.25 (7-CH3), 27.4 (C(CH3)3), 43.9 (6-CH2), 44.4 (7-C), 58.5 (PhCH2), 64.7 (PhCHCH2), 66.4 (5-CH), 69.4 (PhCHCH2), 81.1 (C(CH3)3), 93.8 (7a-CH), 122.4 (CN), 127.1, 127.3, 127.3, 128.1, 128.3, 128.9 (6 × Ar-CH), 138.0, 140.9 (2 × Ar-C), 171.45 (CO); m/z (FAB) 418 (MH+, 5%), 350 (48), 316 (17), 294 (57), 249 (32), 130 (16), 104 (44), 104 (100). HRMS: (EI) M+ 417.2424; C26H31N3O2 requires M+ 417.2416. Found: C, 74.75; H, 7.8; N, 10.1%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%.
1 v/v) yielded the exo-adduct (9k) as a colourless solid (62 mg, 4%), m.p. 100–102 °C [from petroleum ether (b.p. 40–60 °C)]: νmax (CHCl3)/cm−1 2822, 2236, 1732, 1369, 1153; δH (250 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.50 (3H, s, 7-CH3), 2.38 (1H, dd, J = 5.8, 12.7, 6-CHH), 2.62 (1H, apparent t, J = 9.5, PhCHCHH), 2.77 (1H, dd, J = 10.7, 12.7, 6-CHH), 3.35 (1H, dd, J = 5.5, 8.9, PhCHCHH), 3.45 (2H, m, PhCHH and 5-CH), 3.99 (1H, dd, J = 5.5, 9.9, PhCHCH2), 4.08 (1H, d, J = 13.0, PhCHH), 4.75 (1H, s, 7a-CH), 7.27 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.15 (7-CH3), 27.4 (C(CH3)3), 38.5 (7-C), 43.4 (6-CH2), 57.8 (PhCH2), 64.7 (PhCHCH2), 65.6 (5-CH), 69.8 (PhCHCH2), 81.3 (C(CH3)3), 92.0 (7a-CH), 123.4 (CN), 127.2, 127.4, 127.5, 128.2, 128.4, 128.7 (6 × Ar-CH), 137.6, 140.5 (2 × Ar-C), 171.4 (CO); m/z 350 (39%), 316 (12), 294 (48), 249 (32), 104 (40), 91 (100). Found: C, 74.85; H, 7.7; N, 10.0%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%; and the title compound (9i) as a colourless solid (0.47 g, 27%), m.p. 118–119 °C [from petroleum ether (b.p. 40–60 °C)]: [α]22D −15.8 (c 0.48; EtOH); νmax (KBr)/cm−1 3072, 3029, 2981, 2959, 2943, 2234, 1736, 1454, 1443, 1366, 1215, 1152, 1079, 702; δH (400 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.41 (3H, s, 7-CH3), 2.32 (1H, dd, J = 10.1, 13.1, 6-CHH), 2.59 (1H, apparent t, J = 9.5, PhCHCHH), 2.67 (1H, dd, J = 6.0, 13.1, 6-CHH), 3.47 (2H, m, PhCHH and PhCHCHH), 3.71 (1H, dd, J = 6.0, 10.1, 5-CH), 4.04 (1H, d, J = 12.7, PhCHH), 4.17 (1H, s, 7a-CH), 4.29 (1H, dd, J = 5.8, 9.8, PhCHCH2), 7.31 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.25 (7-CH3), 27.4 (C(CH3)3), 43.9 (6-CH2), 44.4 (7-C), 58.5 (PhCH2), 64.7 (PhCHCH2), 66.4 (5-CH), 69.4 (PhCHCH2), 81.1 (C(CH3)3), 93.8 (7a-CH), 122.4 (CN), 127.1, 127.3, 127.3, 128.1, 128.3, 128.9 (6 × Ar-CH), 138.0, 140.9 (2 × Ar-C), 171.45 (CO); m/z (FAB) 418 (MH+, 5%), 350 (48), 316 (17), 294 (57), 249 (32), 130 (16), 104 (44), 104 (100). HRMS: (EI) M+ 417.2424; C26H31N3O2 requires M+ 417.2416. Found: C, 74.75; H, 7.8; N, 10.1%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the exo-adduct (9l) as a colourless solid (24 mg, 3%), m.p. 99–101 °C: νmax (KBr)/cm−1 2981, 2233, 1724, 1636, 1366, 1162, 698; δH (400 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.49 (3H, s, 7-CH3), 2.38 (1H, dd, J = 5.9, 12.8, 6-CHH), 2.62 (1H, t, J = 9.2, PhCHCHH), 2.76 (1H, dd, J = 10.8, 12.8, 6-CHH), 3.35 (1H, dd, J = 5.5, 9.2, PhCHCHH), 3.44 (2H, m, PhCHH and 5-CH), 3.99 (1H, dd, J = 5.5, 10.0, PhCHCH2), 4.07 (1H, d, J = 13.1, PhCHH), 4.75 (1H, s, 7a-CH), 7.39 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.1 (7-CH3), 27.4 (C(CH3)3), 38.5 (7-C), 43.4 (6-CH2), 57.8 (PhCH2), 64.7 (PhCHCH2), 65.6 (5-CH), 69.8 (PhCHCH2), 81.3 (C(CH3)3), 91.95 (7a-CH), 123.4 (CN), 127.2, 127.4, 127.5, 128.2, 128.4, 128.7 (6 × Ar-CH), 137.6, 140.5 (2 × Ar-C), 171.4 (CO); m/z 350 (15%), 294 (20), 249 (17), 120 (35), 104 (15), 91 (100). Found: C, 75.0; H, 7.7; N, 10.3%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%; and the title compound (9j) as a colourless solid (194 mg, 22%), m.p. 119–120 °C: [α]22D 15.7 (c 1.07; EtOH); νmax (KBr)/cm−1 2978, 1732, 1455, 1264, 1152 and 701; δH (400 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.45 (3H, s, 7-CH3), 2.35 (1H, dd, J = 9.7, 13.2, 6-CHH), 2.65 (1H, t, J = 9.7, PhCHCHH), 2.78 (1H, dd, J = 6.4, 13.2, 6-CHH), 3.53 (2H, m, PhCHH and PhCHCHH), 3.78 (1H, dd, J = 6.4, 9.7, 5-CH), 4.12 (1H, d, J = 12.9, PhCHH), 4.23 (1H, s, 7a-CH), 4.35 (1H, dd, J = 5.9, 9.7, PhCHCH2), 7.29 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.1 (7-CH3), 27.2 (C(CH3)3), 43.7 (6-CH2), 44.2 (7-C), 58.3 (PhCH2), 64.55 (PhCHCH2), 66.25 (5-CH), 69.2 (PhCHCH2), 80.8 (C(CH3)3), 93.6 (7a-CH), 122.2 (CN), 126.9, 127.1, 127.15, 128.0, 128.2, 128.7 (6 × Ar-CH), 137.9, 140.75 (2 × Ar-C), 171.25 (CO); m/z (FAB) 418 (MH+, 63%), 350 (100), 316 (19), 294 (37), 249 (17), 208 (22). HRMS: (FAB) MH+ 418.2507; C26H31N3O2 requires MH+ 418.2494. Found: C, 74.7; H, 7.5; N, 9.9%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%.
1 v/v) yielded the exo-adduct (9l) as a colourless solid (24 mg, 3%), m.p. 99–101 °C: νmax (KBr)/cm−1 2981, 2233, 1724, 1636, 1366, 1162, 698; δH (400 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.49 (3H, s, 7-CH3), 2.38 (1H, dd, J = 5.9, 12.8, 6-CHH), 2.62 (1H, t, J = 9.2, PhCHCHH), 2.76 (1H, dd, J = 10.8, 12.8, 6-CHH), 3.35 (1H, dd, J = 5.5, 9.2, PhCHCHH), 3.44 (2H, m, PhCHH and 5-CH), 3.99 (1H, dd, J = 5.5, 10.0, PhCHCH2), 4.07 (1H, d, J = 13.1, PhCHH), 4.75 (1H, s, 7a-CH), 7.39 (10H, m, Ar–H); δC (68 MHz; CDCl3) 20.1 (7-CH3), 27.4 (C(CH3)3), 38.5 (7-C), 43.4 (6-CH2), 57.8 (PhCH2), 64.7 (PhCHCH2), 65.6 (5-CH), 69.8 (PhCHCH2), 81.3 (C(CH3)3), 91.95 (7a-CH), 123.4 (CN), 127.2, 127.4, 127.5, 128.2, 128.4, 128.7 (6 × Ar-CH), 137.6, 140.5 (2 × Ar-C), 171.4 (CO); m/z 350 (15%), 294 (20), 249 (17), 120 (35), 104 (15), 91 (100). Found: C, 75.0; H, 7.7; N, 10.3%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%; and the title compound (9j) as a colourless solid (194 mg, 22%), m.p. 119–120 °C: [α]22D 15.7 (c 1.07; EtOH); νmax (KBr)/cm−1 2978, 1732, 1455, 1264, 1152 and 701; δH (400 MHz; CDCl3) 1.13 (9H, s, C(CH3)3), 1.45 (3H, s, 7-CH3), 2.35 (1H, dd, J = 9.7, 13.2, 6-CHH), 2.65 (1H, t, J = 9.7, PhCHCHH), 2.78 (1H, dd, J = 6.4, 13.2, 6-CHH), 3.53 (2H, m, PhCHH and PhCHCHH), 3.78 (1H, dd, J = 6.4, 9.7, 5-CH), 4.12 (1H, d, J = 12.9, PhCHH), 4.23 (1H, s, 7a-CH), 4.35 (1H, dd, J = 5.9, 9.7, PhCHCH2), 7.29 (10H, m, Ar–H); δC (68 MHz; CDCl3) 22.1 (7-CH3), 27.2 (C(CH3)3), 43.7 (6-CH2), 44.2 (7-C), 58.3 (PhCH2), 64.55 (PhCHCH2), 66.25 (5-CH), 69.2 (PhCHCH2), 80.8 (C(CH3)3), 93.6 (7a-CH), 122.2 (CN), 126.9, 127.1, 127.15, 128.0, 128.2, 128.7 (6 × Ar-CH), 137.9, 140.75 (2 × Ar-C), 171.25 (CO); m/z (FAB) 418 (MH+, 63%), 350 (100), 316 (19), 294 (37), 249 (17), 208 (22). HRMS: (FAB) MH+ 418.2507; C26H31N3O2 requires MH+ 418.2494. Found: C, 74.7; H, 7.5; N, 9.9%; C26H31N3O2 requires C, 74.8; H, 7.5; N, 10.1%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless solid (1.21 g, 65%), m.p. 98–99 °C [from petroleum ether (b.p. 40–60 °C)]: [α]22D −22.8 (c 1.08; EtOH); νmax (KBr)/cm−1 3032, 2980, 2946, 1728, 1367, 1166, 1152, 697; δH (400 MHz; CDCl3) 1.22 (9H, s, C(CH3)3), 2.26 (1H, m, 6-CHH), 2.40 (1H, t, J = 9.6, PhCHCHH), 2.58 (1H, m, 6-CHH), 3.27 (3H, m, PhCHH, 7-CH and PhCHCHH), 3.72 (3H, s, OCH3), 3.80 (1H, t, J = 6.8, 5-CH), 4.08 (1H, d, J = 12.9, PhCHH), 4.13 (1H, m, PhCHCH2), 4.68 (1H, d, J = 6.6, 7a-CH), 7.27 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.4 (C(CH3)3), 32.6 (6-CH2), 47.9 (7-CH), 51.3 (OCH3), 58.2 (PhCH2), 64.0 (PhCHCH2), 66.8 (5-CH), 68.6 (PhCHCH2), 80.1 (C(CH3)3), 88.4 (7a-CH), 126.7, 126.9, 127.8, 128.0, 128.3 (6 × Ar-CH), 138.2, 141.2 (2 × Ar-C), 172.7, 173.0 (2 × CO); m/z 436 (M+, 2%), 350 (36), 335 (15) 294 (41), 249 (33), 120 (24), 104 (52), 91 (100). HRMS: (EI) M+ 436.2377; C26H32N2O4 requires M+ 436.2362. Found: C, 71.6; H, 7.6; N, 6.4%; C26H32N2O4 requires C, 71.5; H, 7.4; N, 6.4%.
1 v/v) yielded the title compound as a colourless solid (1.21 g, 65%), m.p. 98–99 °C [from petroleum ether (b.p. 40–60 °C)]: [α]22D −22.8 (c 1.08; EtOH); νmax (KBr)/cm−1 3032, 2980, 2946, 1728, 1367, 1166, 1152, 697; δH (400 MHz; CDCl3) 1.22 (9H, s, C(CH3)3), 2.26 (1H, m, 6-CHH), 2.40 (1H, t, J = 9.6, PhCHCHH), 2.58 (1H, m, 6-CHH), 3.27 (3H, m, PhCHH, 7-CH and PhCHCHH), 3.72 (3H, s, OCH3), 3.80 (1H, t, J = 6.8, 5-CH), 4.08 (1H, d, J = 12.9, PhCHH), 4.13 (1H, m, PhCHCH2), 4.68 (1H, d, J = 6.6, 7a-CH), 7.27 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.4 (C(CH3)3), 32.6 (6-CH2), 47.9 (7-CH), 51.3 (OCH3), 58.2 (PhCH2), 64.0 (PhCHCH2), 66.8 (5-CH), 68.6 (PhCHCH2), 80.1 (C(CH3)3), 88.4 (7a-CH), 126.7, 126.9, 127.8, 128.0, 128.3 (6 × Ar-CH), 138.2, 141.2 (2 × Ar-C), 172.7, 173.0 (2 × CO); m/z 436 (M+, 2%), 350 (36), 335 (15) 294 (41), 249 (33), 120 (24), 104 (52), 91 (100). HRMS: (EI) M+ 436.2377; C26H32N2O4 requires M+ 436.2362. Found: C, 71.6; H, 7.6; N, 6.4%; C26H32N2O4 requires C, 71.5; H, 7.4; N, 6.4%.
A crystal was mounted on a thin glass fibre and transferred into the cold stream of the diffractometer low temperature device. Crystal data for 13a: C26H32N2O4, M = 436.54, monoclinic, P21; a = 10.276(2), b = 5.799(3), c = 20.259(5) Å, β = 103.07(2)°, U = 1175.9(7) Å3, T = 150(2) K, μ(Mo-Kα) = 0.083 mm−1, Dc = 1.233 g cm−3, Z = 2, 2284 unique data (Rint 0.072) were used in all calculations. Final R1 [1639 F > 4σ(F)] = 0.0734 and wR(all F2) was 0.146.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless solid (1.17 g, 63%), m.p. 92–94 °C [from petroleum ether (b.p. 40–60 °C)]: [α]22D 22.8 (c 1.08; EtOH); νmax (KBr)/cm−1 3032, 2980, 2946, 1728, 1367, 1166, 1152, 697; δH (270 MHz; CDCl3) 1.21 (9H, s, C(CH3)3), 2.27 (1H, m, 6-CHH), 2.40 (1H, t, J = 9.6, PhCHCHH), 2.58 (1H, m, 6-CHH), 3.28 (3H, m, PhCHH, 7-CH and PhCHCHH), 3.70 (3H, s, OCH3), 3.82 (1H, t, J = 6.9, 5-CH), 4.08 (1H, d, J = 13.2, PhCHH), 4.13 (1H, m, PhCHCH2), 4.68 (1H, d, J = 6.3, 7a-CH), 7.28 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.4 (C(CH3)3), 32.65 (6-CH2), 48.0 (7-CH), 51.4 (OCH3), 58.3 (PhCH2), 64.0 (PhCHCH2), 66.8 (5-CH), 68.7 (PhCHCH2), 80.2 (C(CH3)3), 88.45 (7a-CH), 126.8, 127.0, 127.9, 128.0, 128.3 (6 × Ar-CH), 138.3, 141.3 (2 × Ar-C), 172.8, 173.1 (2 × CO); m/z 436 (M+, 4%), 350 (43), 335 (16), 294 (48), 249 (37), 120 (32), 104 (58), 91 (100). HRMS: (EI) M+ 436.2345; C26H32N2O4 requires M+ 436.2362. Found: C, 71.3; H, 7.5; N, 6.3%; C26H32N2O4 requires C, 71.5; H, 7.4; N, 6.4%.
1 v/v) yielded the title compound as a colourless solid (1.17 g, 63%), m.p. 92–94 °C [from petroleum ether (b.p. 40–60 °C)]: [α]22D 22.8 (c 1.08; EtOH); νmax (KBr)/cm−1 3032, 2980, 2946, 1728, 1367, 1166, 1152, 697; δH (270 MHz; CDCl3) 1.21 (9H, s, C(CH3)3), 2.27 (1H, m, 6-CHH), 2.40 (1H, t, J = 9.6, PhCHCHH), 2.58 (1H, m, 6-CHH), 3.28 (3H, m, PhCHH, 7-CH and PhCHCHH), 3.70 (3H, s, OCH3), 3.82 (1H, t, J = 6.9, 5-CH), 4.08 (1H, d, J = 13.2, PhCHH), 4.13 (1H, m, PhCHCH2), 4.68 (1H, d, J = 6.3, 7a-CH), 7.28 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.4 (C(CH3)3), 32.65 (6-CH2), 48.0 (7-CH), 51.4 (OCH3), 58.3 (PhCH2), 64.0 (PhCHCH2), 66.8 (5-CH), 68.7 (PhCHCH2), 80.2 (C(CH3)3), 88.45 (7a-CH), 126.8, 127.0, 127.9, 128.0, 128.3 (6 × Ar-CH), 138.3, 141.3 (2 × Ar-C), 172.8, 173.1 (2 × CO); m/z 436 (M+, 4%), 350 (43), 335 (16), 294 (48), 249 (37), 120 (32), 104 (58), 91 (100). HRMS: (EI) M+ 436.2345; C26H32N2O4 requires M+ 436.2362. Found: C, 71.3; H, 7.5; N, 6.3%; C26H32N2O4 requires C, 71.5; H, 7.4; N, 6.4%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless solid (1.20 g, 59%), m.p. 102–104 °C [from petroleum ether (b.p. 40–60 °C)], [α]22D −27.0 (c 0.97; EtOH): νmax (KBr)/cm−1 2927, 1734, 1628, 1384, 1369, 1145, 702; δH (400 MHz; CDCl3) 1.22, 1.49 (18H, 2 × s, 2 × C(CH3)3), 2.22 (1H, m, 6-CHH), 2.43 (1H, t, J = 9.5, PhCHCHH), 2.52 (1H, m, 6-CHH), 3.16 (1H, dd, J = 6.5, 11.8, 7-CH), 3.26 (2H, m, PhCHH and PhCHCHH), 3.71 (1H, t, J = 7.0, 5-CH), 4.13 (2H, m, PhCHH and PhCHCH2), 4.61 (1H, d, J = 6.5, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.5, 27.75 (2 × C(CH3)3), 33.1 (6-CH2), 49.2 (7-CH), 58.35 (PhCH2), 63.9 (PhCHCH2), 67.0 (5-CH), 68.9 (PhCHCH2), 80.1, 80.5 (2 × C(CH3)3), 88.4 (7a-CH), 126.8, 126.9, 127.0, 127.9, 129.0 (6 × Ar-CH), 137.9, 141.6 (2 × Ar-C), 171.6, 173.15 (2 × CO); m/z 478 (M+, 5%), 350 (53), 294 (62), 249 (35), 235 (25), 120 (17), 104 (42), 91 (100). HRMS: (EI) M+ 478.2853; C29H38N2O4 requires M+ 478.2832. Found: C, 72.6; H, 8.25; N, 6.0%; C29H38N2O4 requires C, 72.8; H, 8.0; N, 5.9%; and the exo-adduct (13e) as a colourless oil (70 mg, 3%): νmax (KBr)/cm−1 3062, 3028, 2977, 2931, 1732, 1628, 1367, 1150, 700; δH (400 MHz; CDCl3) 1.14, 1.47 (18H, 2 × s, 2 × C(CH3)3), 2.31 (1H, t, J = 9.5, PhCHCHH), 2.46, 2.56 (2H, 2 × m, 6-CH2), 2.99 (1H, m, 7-CH), 3.21 (1H, d, J = 12.9, PhCHH), 3.32 (1H, dd, J = 6.3, 9.3, PhCHCHH), 3.58 (1H, dd, J = 6.2, 9.4, 5-CH), 4.06 (2H, m, PhCHH and PhCHCH2), 4.39 (1H, d, J = 4.5, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.5, 28.05 (2 × C(CH3)3), 34.0 (6-CH2), 50.5 (7-CH), 56.6 (PhCH2), 64.0 (PhCHCH2), 68.75 (PhCHCH2), 69.0 (5-CH), 80.7, 81.0 (2 × C(CH3)3), 89.6 (7a-C), 126.9, 126.9, 127.1, 128.1, 128.1, 128.8 (6 × Ar-CH), 138.35, 142.6 (2 × Ar-C), 171.7, 171.9 (2 × CO); m/z 478 (M+, 21%), 350 (85), 294 (71), 249 (38), 235 (30), 104 (27), 91 (100). HRMS: (EI) M+ 478.2848; C29H38N2O4 requires M+ 478.2832. Found: C, 72.1; H, 8.1; N, 5.7%; C29H38N2O4·0.2H2O requires C, 72.1; H, 8.2; N, 5.7%.
1 v/v) yielded the title compound as a colourless solid (1.20 g, 59%), m.p. 102–104 °C [from petroleum ether (b.p. 40–60 °C)], [α]22D −27.0 (c 0.97; EtOH): νmax (KBr)/cm−1 2927, 1734, 1628, 1384, 1369, 1145, 702; δH (400 MHz; CDCl3) 1.22, 1.49 (18H, 2 × s, 2 × C(CH3)3), 2.22 (1H, m, 6-CHH), 2.43 (1H, t, J = 9.5, PhCHCHH), 2.52 (1H, m, 6-CHH), 3.16 (1H, dd, J = 6.5, 11.8, 7-CH), 3.26 (2H, m, PhCHH and PhCHCHH), 3.71 (1H, t, J = 7.0, 5-CH), 4.13 (2H, m, PhCHH and PhCHCH2), 4.61 (1H, d, J = 6.5, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.5, 27.75 (2 × C(CH3)3), 33.1 (6-CH2), 49.2 (7-CH), 58.35 (PhCH2), 63.9 (PhCHCH2), 67.0 (5-CH), 68.9 (PhCHCH2), 80.1, 80.5 (2 × C(CH3)3), 88.4 (7a-CH), 126.8, 126.9, 127.0, 127.9, 129.0 (6 × Ar-CH), 137.9, 141.6 (2 × Ar-C), 171.6, 173.15 (2 × CO); m/z 478 (M+, 5%), 350 (53), 294 (62), 249 (35), 235 (25), 120 (17), 104 (42), 91 (100). HRMS: (EI) M+ 478.2853; C29H38N2O4 requires M+ 478.2832. Found: C, 72.6; H, 8.25; N, 6.0%; C29H38N2O4 requires C, 72.8; H, 8.0; N, 5.9%; and the exo-adduct (13e) as a colourless oil (70 mg, 3%): νmax (KBr)/cm−1 3062, 3028, 2977, 2931, 1732, 1628, 1367, 1150, 700; δH (400 MHz; CDCl3) 1.14, 1.47 (18H, 2 × s, 2 × C(CH3)3), 2.31 (1H, t, J = 9.5, PhCHCHH), 2.46, 2.56 (2H, 2 × m, 6-CH2), 2.99 (1H, m, 7-CH), 3.21 (1H, d, J = 12.9, PhCHH), 3.32 (1H, dd, J = 6.3, 9.3, PhCHCHH), 3.58 (1H, dd, J = 6.2, 9.4, 5-CH), 4.06 (2H, m, PhCHH and PhCHCH2), 4.39 (1H, d, J = 4.5, 7a-CH), 7.26 (10H, m, Ar–H); δC (68 MHz; CDCl3) 27.5, 28.05 (2 × C(CH3)3), 34.0 (6-CH2), 50.5 (7-CH), 56.6 (PhCH2), 64.0 (PhCHCH2), 68.75 (PhCHCH2), 69.0 (5-CH), 80.7, 81.0 (2 × C(CH3)3), 89.6 (7a-C), 126.9, 126.9, 127.1, 128.1, 128.1, 128.8 (6 × Ar-CH), 138.35, 142.6 (2 × Ar-C), 171.7, 171.9 (2 × CO); m/z 478 (M+, 21%), 350 (85), 294 (71), 249 (38), 235 (30), 104 (27), 91 (100). HRMS: (EI) M+ 478.2848; C29H38N2O4 requires M+ 478.2832. Found: C, 72.1; H, 8.1; N, 5.7%; C29H38N2O4·0.2H2O requires C, 72.1; H, 8.2; N, 5.7%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless solid (0.49 g, 49%), m.p. 95–98 °C [from petroleum ether (b.p. 40–60 °C)], [α]22D 27.1 (c 0.98; EtOH): νmax (KBr)/cm−1 3031, 2975, 2935, 1733, 1369, 1146, 702; δH (400 MHz; CDCl3) 1.21, 1.48 (18H, 2 × s, 2 × C(CH3)3), 2.23 (1H, m, 6-CHH), 2.43 (1H, t, J = 9.6, PhCHCHH), 2.55 (1H, m, 6-CHH), 3.16 (1H, dd, J = 6.5, 11.9, 7-CH), 3.26 (2H, m, PhCHH and PhCHCHH), 3.73 (1H, t, J = 6.9, 5-CH), 4.13 (2H, m, PhCHH and PhCHCH2), 4.62 (1H, d, J = 6.5, 7a-CH), 7.27 (10H, m, ArH); δC (68 MHz; CDCl3) 27.5, 28.2 (2 × C(CH3)3), 33.1 (6-CH2), 49.2 (7-CH), 58.4 (PhCH2), 63.9 (PhCHCH2), 67.0 (5-CH), 69.0 (PhCHCH2), 80.2, 80.5 (2 × C(CH3)3), 88.5 (7a-CH), 126.8, 126.95, 127.0, 127.9, 129.0 (6 × Ar-CH), 138.0, 141.6 (2 × Ar-C), 171.6, 173.2 (2 × CO); m/z 478 (M+, 4%), 350 (73), 294 (76), 249 (45), 235 (34), 120 (17), 104 (38), 91 (100). HRMS: (EI) M+ 478.2827; C29H38N2O4 requires M+ 478.2832. Found: C, 72.5; H, 8.1; N, 5.8%; C29H38N2O4 requires C, 72.8; H, 8.0; N, 5.9%; and the exo-adduct (13f) as a colourless oil (22 mg, 2%): νmax(film)/cm−1 2977, 1729, 1367, 1150, 700; δH (400 MHz; CDCl3) 1.14, 1.47 (18H, 2 × s, 2 × C(CH3)3), 2.30 (1H, t, J = 9.5, PhCHCHH), 2.46, 2.57 (2H, 2 × m, 6-CHH), 2.97 (1H, m, 7-CH), 3.20 (1H, d, J = 12.9, PhCHH), 3.32 (1H, dd, J = 6.3, 9.2, PhCHCHH), 3.58 (1H, dd, J = 6.2, 9.4, 5-CH), 4.09 (2H, m, PhCHH and PhCHCH2), 4.38 (1H, d, J = 4.5, 7a-CH), 7.26 (10H, m, Ar–H); δC (100 MHz; CDCl3) 27.5, 28.1 (2 × C(CH3)3), 34.05 (6-CH2), 50.7 (7-CH), 56.7 (PhCH2), 64.1 (PhCHCH2), 68.9 (PhCHCH2), 69.1 (5-CH), 80.7, 81.0 (2 × C(CH3)3), 89.6 (7a-CH), 126.85, 126.9, 127.0, 128.1, 128.3, 128.8 (6 × Ar-CH), 138.6, 142.7 (2 × Ar-C), 171.8, 171.9 (2 × CO); m/z 478 (M+, 25%), 350 (96), 294 (83), 249 (42), 235 (33), 104 (29), 91 (100). HRMS: (EI) M+ 478.2837; C29H38N2O4 requires M+ 478.2832.
1 v/v) yielded the title compound as a colourless solid (0.49 g, 49%), m.p. 95–98 °C [from petroleum ether (b.p. 40–60 °C)], [α]22D 27.1 (c 0.98; EtOH): νmax (KBr)/cm−1 3031, 2975, 2935, 1733, 1369, 1146, 702; δH (400 MHz; CDCl3) 1.21, 1.48 (18H, 2 × s, 2 × C(CH3)3), 2.23 (1H, m, 6-CHH), 2.43 (1H, t, J = 9.6, PhCHCHH), 2.55 (1H, m, 6-CHH), 3.16 (1H, dd, J = 6.5, 11.9, 7-CH), 3.26 (2H, m, PhCHH and PhCHCHH), 3.73 (1H, t, J = 6.9, 5-CH), 4.13 (2H, m, PhCHH and PhCHCH2), 4.62 (1H, d, J = 6.5, 7a-CH), 7.27 (10H, m, ArH); δC (68 MHz; CDCl3) 27.5, 28.2 (2 × C(CH3)3), 33.1 (6-CH2), 49.2 (7-CH), 58.4 (PhCH2), 63.9 (PhCHCH2), 67.0 (5-CH), 69.0 (PhCHCH2), 80.2, 80.5 (2 × C(CH3)3), 88.5 (7a-CH), 126.8, 126.95, 127.0, 127.9, 129.0 (6 × Ar-CH), 138.0, 141.6 (2 × Ar-C), 171.6, 173.2 (2 × CO); m/z 478 (M+, 4%), 350 (73), 294 (76), 249 (45), 235 (34), 120 (17), 104 (38), 91 (100). HRMS: (EI) M+ 478.2827; C29H38N2O4 requires M+ 478.2832. Found: C, 72.5; H, 8.1; N, 5.8%; C29H38N2O4 requires C, 72.8; H, 8.0; N, 5.9%; and the exo-adduct (13f) as a colourless oil (22 mg, 2%): νmax(film)/cm−1 2977, 1729, 1367, 1150, 700; δH (400 MHz; CDCl3) 1.14, 1.47 (18H, 2 × s, 2 × C(CH3)3), 2.30 (1H, t, J = 9.5, PhCHCHH), 2.46, 2.57 (2H, 2 × m, 6-CHH), 2.97 (1H, m, 7-CH), 3.20 (1H, d, J = 12.9, PhCHH), 3.32 (1H, dd, J = 6.3, 9.2, PhCHCHH), 3.58 (1H, dd, J = 6.2, 9.4, 5-CH), 4.09 (2H, m, PhCHH and PhCHCH2), 4.38 (1H, d, J = 4.5, 7a-CH), 7.26 (10H, m, Ar–H); δC (100 MHz; CDCl3) 27.5, 28.1 (2 × C(CH3)3), 34.05 (6-CH2), 50.7 (7-CH), 56.7 (PhCH2), 64.1 (PhCHCH2), 68.9 (PhCHCH2), 69.1 (5-CH), 80.7, 81.0 (2 × C(CH3)3), 89.6 (7a-CH), 126.85, 126.9, 127.0, 128.1, 128.3, 128.8 (6 × Ar-CH), 138.6, 142.7 (2 × Ar-C), 171.8, 171.9 (2 × CO); m/z 478 (M+, 25%), 350 (96), 294 (83), 249 (42), 235 (33), 104 (29), 91 (100). HRMS: (EI) M+ 478.2837; C29H38N2O4 requires M+ 478.2832.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (5
ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to yield the title compound as a colourless solid (0.72 g, 33%), m.p. 142–145 °C (from ethyl acetate): νmax (KBr)/cm−1 2927, 1734, 1628, 1384, 1145; δH (250 MHz; CDCl3) 1.20 (9H, s, C(CH3)3), 2.18 (1H, m, 6-CHH), 2.46 (2H, m, PhCHCHH and 6-CHH), 3.32 (2H, m, PhCHH and PhCHCHH), 3.67 (1H, t, J = 6.5, PhCHCH2), 3.98 (1H, dd, J = 6.2, 13.7, 7-CH), 4.18 (1H, dd, J = 5.5, 10.1, 5-CH), 4.62 (1H, d, J = 11.9, PhCHH), 4.97 (1H, d, J = 6.2, 7a-CH), 7.59 (15H, m, Ar–H); δC (68 MHz; CDCl3) 27.55 (C(CH3)3), 32.0 (6-CH2), 59.1 (PhCH2), 63.45 (PhCHCH2), 64.6 (PhCHCH2), 66.45 (7-CH), 67.8 (5-CH), 81.0 (C(CH3)3), 87.7 (7a-CH), 126.9, 127.2, 128.1, 128.3, 129.1, 129.2 (9 × Ar-CH), 133.2, 138.3, 140.6 (3 × Ar-C), 171.9 (CO); m/z 461 (M+–C4H9, 6%), 417 (11), 350 (61), 294 (63), 249 (45), 235 (36), 120 (50), 104 (59), 91 (100). HRMS: (EI) M+–C4H9 461.1536; C30H34N2O4S requires M+–C4H9 461.1535. Found: C, 69.4; H, 6.9; N, 5.5%; C30H34N2O4S requires C, 69.5; H, 6.6; N, 5.4%.
1 v/v) to yield the title compound as a colourless solid (0.72 g, 33%), m.p. 142–145 °C (from ethyl acetate): νmax (KBr)/cm−1 2927, 1734, 1628, 1384, 1145; δH (250 MHz; CDCl3) 1.20 (9H, s, C(CH3)3), 2.18 (1H, m, 6-CHH), 2.46 (2H, m, PhCHCHH and 6-CHH), 3.32 (2H, m, PhCHH and PhCHCHH), 3.67 (1H, t, J = 6.5, PhCHCH2), 3.98 (1H, dd, J = 6.2, 13.7, 7-CH), 4.18 (1H, dd, J = 5.5, 10.1, 5-CH), 4.62 (1H, d, J = 11.9, PhCHH), 4.97 (1H, d, J = 6.2, 7a-CH), 7.59 (15H, m, Ar–H); δC (68 MHz; CDCl3) 27.55 (C(CH3)3), 32.0 (6-CH2), 59.1 (PhCH2), 63.45 (PhCHCH2), 64.6 (PhCHCH2), 66.45 (7-CH), 67.8 (5-CH), 81.0 (C(CH3)3), 87.7 (7a-CH), 126.9, 127.2, 128.1, 128.3, 129.1, 129.2 (9 × Ar-CH), 133.2, 138.3, 140.6 (3 × Ar-C), 171.9 (CO); m/z 461 (M+–C4H9, 6%), 417 (11), 350 (61), 294 (63), 249 (45), 235 (36), 120 (50), 104 (59), 91 (100). HRMS: (EI) M+–C4H9 461.1536; C30H34N2O4S requires M+–C4H9 461.1535. Found: C, 69.4; H, 6.9; N, 5.5%; C30H34N2O4S requires C, 69.5; H, 6.6; N, 5.4%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), yielded the title compound (530 mg, 29%) as a colourless oil, [α]22D 12.3 (c 1.00; CHCl3): νmax(film)/cm−1 2981, 2949, 1733, 1495, 1454, 1252, 1135, 702; δH (400 MHz; CDCl3) 1.25–1.28 (6H, m, CH3CH2 and 5-CH3), 1.37 (3H, s, 7-CH3), 2.24 (1H, d, J = 13.6, 6-CHH), 2.30-2.32 (1H, m, PhCHCHH), 2.88 (1H, d, J = 13.6, 6-CHH), 3.16 (1H, dd, J = 5.5, 9.0, PhCHCHH), 3.38 (1H, d, J = 12.7, PhCHH), 3.72 (3H, s, OCH3), 4.12-4.20 (3H, m, CH3CH2O and PhCHH), 4.33 (1H, s, 7a-CH), 4.54 (1H, dd, J = 5.5, 9.4, PhCHCH2), 7.14–7.28 (8H, m, Ar–H), 7.42 (2H, d, J = 8.0, Ar–H); δC (100.6 MHz; CDCl3) 14.2, 21.35, 23.95 (3 × CH3), 48.1 (6-CH2), 51.9 (OCH3), 54.3 (7-C), 59.7 (PhCH2), 60.8 (CH3CH2), 62.0 (PhCHCH2), 64.35 (PhCHCH2), 68.0 (5-C), 97.4 (7a-CH), 126.6, 126.9, 128.1, 128.6 (6 × Ar-CH, overlapping), 138.7, 143.1 (2 × Ar-C), 176.0, 176.4 (2 × CO); m/z 436 (M+, 1%), 405 (6), 363 (9), 337 (33), 336 (100), 263 (49), 91 (89). HRMS: (EI) M+ 436.2336; C26H32N2O4 requires M+ 436.2362.
1 v/v), yielded the title compound (530 mg, 29%) as a colourless oil, [α]22D 12.3 (c 1.00; CHCl3): νmax(film)/cm−1 2981, 2949, 1733, 1495, 1454, 1252, 1135, 702; δH (400 MHz; CDCl3) 1.25–1.28 (6H, m, CH3CH2 and 5-CH3), 1.37 (3H, s, 7-CH3), 2.24 (1H, d, J = 13.6, 6-CHH), 2.30-2.32 (1H, m, PhCHCHH), 2.88 (1H, d, J = 13.6, 6-CHH), 3.16 (1H, dd, J = 5.5, 9.0, PhCHCHH), 3.38 (1H, d, J = 12.7, PhCHH), 3.72 (3H, s, OCH3), 4.12-4.20 (3H, m, CH3CH2O and PhCHH), 4.33 (1H, s, 7a-CH), 4.54 (1H, dd, J = 5.5, 9.4, PhCHCH2), 7.14–7.28 (8H, m, Ar–H), 7.42 (2H, d, J = 8.0, Ar–H); δC (100.6 MHz; CDCl3) 14.2, 21.35, 23.95 (3 × CH3), 48.1 (6-CH2), 51.9 (OCH3), 54.3 (7-C), 59.7 (PhCH2), 60.8 (CH3CH2), 62.0 (PhCHCH2), 64.35 (PhCHCH2), 68.0 (5-C), 97.4 (7a-CH), 126.6, 126.9, 128.1, 128.6 (6 × Ar-CH, overlapping), 138.7, 143.1 (2 × Ar-C), 176.0, 176.4 (2 × CO); m/z 436 (M+, 1%), 405 (6), 363 (9), 337 (33), 336 (100), 263 (49), 91 (89). HRMS: (EI) M+ 436.2336; C26H32N2O4 requires M+ 436.2362.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), yielded the title compound (910 mg, 23%) as a colourless oil, [α]22D 2.35 (c 2.06; CH2Cl2): νmax (KBr)/cm−1 3028, 2977, 2876, 2813, 1728 (s, br), 1603, 1498, 1367, 1251, 1137, 1031, 702; δH (400 MHz; CDCl3) 1.15 (3H, s, 5-CH3), 1.40 (3H, s, 7-CH3), 1.47 (9H, s, C(CH3)3), 2.21 (1H, d, J = 13.6, 6-CHH), 2.30 (1H, t, J = 9.3, PhCHCHH), 2.83 (1H, d, J = 13.6, 6-CHH), 3.15 (1H, dd, J = 5.5, 9.3, PhCHCHH), 3.37 (1H, d, J = 12.7, PhCHH), 3.72 (3H, s, OCH3), 4.12 (1H, d, J = 12.7, PhCHH), 4.31 (1H, s, 7a-CH), 4.55 (1H, dd, J = 5.5, 9.3, PhCHCH2), 7.15–7.45 (10H, m, Ar–H); δC (100.6 MHz; CDCl3) 21.3, 23.9 (2 × CH3), 27.8 (C(CH3)3), 47.9 (6-CH2), 52.0 (OCH3), 54.2 (7-C), 60.2 (PhCH2), 61.9 (PhCHCH2), 64.3 (PhCHCH2), 68.3 (5-C), 80.25 (C(CH3)3), 97.4 (7a-CH), 126.7, 126.7, 126.8, 128.0, 128.1, 128.55 (6 × Ar-CH), 138.7, 143.2 (2 × Ar-C), 175.4, 176.1 (2 × CO); m/z (FAB) 465 (MH+, 13%), 463 (32), 407 (30), 365 (28), 364 (84), 363 (23), 308 (32), 263 (25), 237 (18), 210 (23), 208 (17), 104 (35), 91 (100). HRMS: (FAB) MH+ 465.2746; C28H36N2O4 requires MH+ 465.2753.
1 v/v), yielded the title compound (910 mg, 23%) as a colourless oil, [α]22D 2.35 (c 2.06; CH2Cl2): νmax (KBr)/cm−1 3028, 2977, 2876, 2813, 1728 (s, br), 1603, 1498, 1367, 1251, 1137, 1031, 702; δH (400 MHz; CDCl3) 1.15 (3H, s, 5-CH3), 1.40 (3H, s, 7-CH3), 1.47 (9H, s, C(CH3)3), 2.21 (1H, d, J = 13.6, 6-CHH), 2.30 (1H, t, J = 9.3, PhCHCHH), 2.83 (1H, d, J = 13.6, 6-CHH), 3.15 (1H, dd, J = 5.5, 9.3, PhCHCHH), 3.37 (1H, d, J = 12.7, PhCHH), 3.72 (3H, s, OCH3), 4.12 (1H, d, J = 12.7, PhCHH), 4.31 (1H, s, 7a-CH), 4.55 (1H, dd, J = 5.5, 9.3, PhCHCH2), 7.15–7.45 (10H, m, Ar–H); δC (100.6 MHz; CDCl3) 21.3, 23.9 (2 × CH3), 27.8 (C(CH3)3), 47.9 (6-CH2), 52.0 (OCH3), 54.2 (7-C), 60.2 (PhCH2), 61.9 (PhCHCH2), 64.3 (PhCHCH2), 68.3 (5-C), 80.25 (C(CH3)3), 97.4 (7a-CH), 126.7, 126.7, 126.8, 128.0, 128.1, 128.55 (6 × Ar-CH), 138.7, 143.2 (2 × Ar-C), 175.4, 176.1 (2 × CO); m/z (FAB) 465 (MH+, 13%), 463 (32), 407 (30), 365 (28), 364 (84), 363 (23), 308 (32), 263 (25), 237 (18), 210 (23), 208 (17), 104 (35), 91 (100). HRMS: (FAB) MH+ 465.2746; C28H36N2O4 requires MH+ 465.2753.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (10
ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless solid (0.87 g, 46%), m.p. 138–139 °C: νmax (KBr)/cm−1 2971, 1724, 1714, 1258, 1156, 1028, 698; δH (400 MHz; C6D6) 1.09 (3H, d, J = 6.8, 6-CH3), 1.29 (9H, s, C(CH3)3), 2.17 (1H, dd, J = 9.2, 9.9, PhCHCHH), 2.98 (1H, d, J = 13.2, PhCHH), 3.14 (2H, m, PhCHCHH and 6-CH), 3.38 (4H, m, OCH3 and 7-CH), 3.53 (1H, d, J = 7.5, 5-CH), 3.96 (1H, dd, J = 5.3, 9.9, PhCHCH2), 4.08 (1H, d, J = 13.2, PhCHH), 4.86 (1H, d, J = 6.9, 7a-CH), 7.17 (10H, m, Ar–H); δC (68 MHz; C6D6) 14.1 (6-CH3), 28.0 (C(CH3)3), 36.8 (6-CH), 51.1 (OCH3), 56.45 (7-CH), 59.3 (PhCH2), 62.7 (PhCHCH2), 68.4 (PhCHCH2), 70.75 (5-CH), 80.2 (C(CH3)3), 87.5 (7a-CH), 127.2, 127.2, 127.4, 128.4, 128.5, 128.85 (6 × Ar-CH), 139.5, 141.7 (2 × Ar-C), 171.6, 172.0 (2 × CO); m/z 450 (M+, 3%), 350 (29), 294 (36), 120 (23), 104 (32), 91 (100). HRMS: (EI) M+ 450.2520; C27H34N2O4 requires M+ 450.2519. Found: C, 71.9; H, 7.6; N, 6.1%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
1 v/v) yielded the title compound as a colourless solid (0.87 g, 46%), m.p. 138–139 °C: νmax (KBr)/cm−1 2971, 1724, 1714, 1258, 1156, 1028, 698; δH (400 MHz; C6D6) 1.09 (3H, d, J = 6.8, 6-CH3), 1.29 (9H, s, C(CH3)3), 2.17 (1H, dd, J = 9.2, 9.9, PhCHCHH), 2.98 (1H, d, J = 13.2, PhCHH), 3.14 (2H, m, PhCHCHH and 6-CH), 3.38 (4H, m, OCH3 and 7-CH), 3.53 (1H, d, J = 7.5, 5-CH), 3.96 (1H, dd, J = 5.3, 9.9, PhCHCH2), 4.08 (1H, d, J = 13.2, PhCHH), 4.86 (1H, d, J = 6.9, 7a-CH), 7.17 (10H, m, Ar–H); δC (68 MHz; C6D6) 14.1 (6-CH3), 28.0 (C(CH3)3), 36.8 (6-CH), 51.1 (OCH3), 56.45 (7-CH), 59.3 (PhCH2), 62.7 (PhCHCH2), 68.4 (PhCHCH2), 70.75 (5-CH), 80.2 (C(CH3)3), 87.5 (7a-CH), 127.2, 127.2, 127.4, 128.4, 128.5, 128.85 (6 × Ar-CH), 139.5, 141.7 (2 × Ar-C), 171.6, 172.0 (2 × CO); m/z 450 (M+, 3%), 350 (29), 294 (36), 120 (23), 104 (32), 91 (100). HRMS: (EI) M+ 450.2520; C27H34N2O4 requires M+ 450.2519. Found: C, 71.9; H, 7.6; N, 6.1%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (10
ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) yielded the title compound as a colourless solid (0.50 g, 26%), m.p. 139–140 °C: νmax (KBr)/cm−1 3027, 2974, 2960, 1725, 1714, 1258, 1156, 1028, 698; δH (270 MHz; C6D6) 1.09 (3H, d, J = 6.6, 6-CH3), 1.30 (9H, s, C(CH3)3), 2.17 (1H, dd, J = 8.9, 9.9, PhCHCHH), 2.99 (1H, d, J = 13.2, PhCHH), 3.18 (2H, m, PhCHCHH and 6-CH), 3.40 (4H, m, OCH3 and 7-CH), 3.52 (1H, d, J = 7.6, 5-CH), 3.96 (1H, dd, J = 5.3, 9.9, PhCHCH2), 4.08 (1H, d, J = 13.2, PhCHH), 4.85 (1H, d, J = 6.7, 7a-CH), 7.19 (10H, m, Ar–H); δC (68 MHz; C6D6) 14.1 (6-CH3), 28.0 (C(CH3)3), 36.8 (6-CH), 51.1 (OCH3), 56.5 (7-CH), 59.3 (PhCH2), 62.7 (PhCHCH2), 68.4 (PhCHCH2), 70.8 (5-CH), 80.2 (C(CH3)3), 87.5 (7a-CH), 127.2, 127.2, 127.4, 128.5, 128.8, 128.85 (6 × Ar-CH), 139.5, 141.75 (2 × Ar-C), 171.6, 172.0 (2 × CO); m/z (FAB) 451 (MH+, 22%), 350 (15), 210 (14), 154 (37), 136 (35), 120 (23), 107 (27), 91 (78). HRMS: (FAB) MH+ 451.2540; C27H34N2O4 requires MH+ 451.2597. Found: C, 72.1; H, 7.8; N, 6.2%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
1 v/v) yielded the title compound as a colourless solid (0.50 g, 26%), m.p. 139–140 °C: νmax (KBr)/cm−1 3027, 2974, 2960, 1725, 1714, 1258, 1156, 1028, 698; δH (270 MHz; C6D6) 1.09 (3H, d, J = 6.6, 6-CH3), 1.30 (9H, s, C(CH3)3), 2.17 (1H, dd, J = 8.9, 9.9, PhCHCHH), 2.99 (1H, d, J = 13.2, PhCHH), 3.18 (2H, m, PhCHCHH and 6-CH), 3.40 (4H, m, OCH3 and 7-CH), 3.52 (1H, d, J = 7.6, 5-CH), 3.96 (1H, dd, J = 5.3, 9.9, PhCHCH2), 4.08 (1H, d, J = 13.2, PhCHH), 4.85 (1H, d, J = 6.7, 7a-CH), 7.19 (10H, m, Ar–H); δC (68 MHz; C6D6) 14.1 (6-CH3), 28.0 (C(CH3)3), 36.8 (6-CH), 51.1 (OCH3), 56.5 (7-CH), 59.3 (PhCH2), 62.7 (PhCHCH2), 68.4 (PhCHCH2), 70.8 (5-CH), 80.2 (C(CH3)3), 87.5 (7a-CH), 127.2, 127.2, 127.4, 128.5, 128.8, 128.85 (6 × Ar-CH), 139.5, 141.75 (2 × Ar-C), 171.6, 172.0 (2 × CO); m/z (FAB) 451 (MH+, 22%), 350 (15), 210 (14), 154 (37), 136 (35), 120 (23), 107 (27), 91 (78). HRMS: (FAB) MH+ 451.2540; C27H34N2O4 requires MH+ 451.2597. Found: C, 72.1; H, 7.8; N, 6.2%; C27H34N2O4 requires C, 72.0; H, 7.6; N, 6.2%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (3
hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) afforded the title compound (1.06 g, 35%) as colourless plates, m.p. 109–111 °C (from diethyl ether), [α]22D 34.7 (c 1.23; CHCl3): νmax (KBr)/cm−1 3026, 2963, 2806, 1754(s), 1724(s), 1496, 1455, 1378, 1304, 1256, 1219, 1175, 1138, 1053, 1038, 758, 703; δH (400 MHz; CDCl3) 1.21 (3H, t, J = 7.2, CH3), 1.63–1.72, 2.18–2.25 (each 1H, m, CH2CH2O), 2.39 (1H, t, J = 9.0, PhCHCHH), 2.98 (1H, t, J = 7.0, CHCO2Et), 3.24 (1H, d, J = 13.2, NCHHPh), 3.29 (1H, dd, J = 5.9, 9.0, PhCHCHH), 3.39–3.47 (1H, m, CHCH2CH2), 3.86 (1H, d, J = 7.8, CHCO2), 4.06 (1H, d, J = 13.2, NCHHPh), 4.10–4.30 (4H, m, PhCHCH2, CH2CHHO, OCH2CH3), 4.40–4.45 (1H, m, CH2CHHO), 4.65 (1H, d, J = 7.0, NCHN), 7.20–7.30 (8H, m, Ar–H), 7.40 (2H, d, J = 8.1, Ar–H); δC (100.6 MHz; CDCl3) 13.9 (CH3), 27.6 (CH2CH2O), 38.2 (CHCH2CH2), 56.7 (CHCO2Et), 58.2 (PhCH2N), 60.8 (CO2CH2), 62.8 (PhCHCH2), 64.9 (CHCO2), 66.45 (CH2CH2O), 68.3 (PhCHCH2), 86.8 (NCHN), 126.4, 126.9, 127.1, 128.1, 128.15, 128.2 (6 × Ar-CH), 138.1, 140.5 (2 × Ar-C), 170.8, 171.3 (2 × CO); m/z 420 (M+, 28%), 374 (37), 245 (30), 235 (59), 120 (44), 104 (71), 91 (100), 77 (60). Found: C, 71.75; H, 7.0; N, 6.9; C25H28N2O4 requires C, 71.4; H, 6.7; N, 6.7).
1 v/v) afforded the title compound (1.06 g, 35%) as colourless plates, m.p. 109–111 °C (from diethyl ether), [α]22D 34.7 (c 1.23; CHCl3): νmax (KBr)/cm−1 3026, 2963, 2806, 1754(s), 1724(s), 1496, 1455, 1378, 1304, 1256, 1219, 1175, 1138, 1053, 1038, 758, 703; δH (400 MHz; CDCl3) 1.21 (3H, t, J = 7.2, CH3), 1.63–1.72, 2.18–2.25 (each 1H, m, CH2CH2O), 2.39 (1H, t, J = 9.0, PhCHCHH), 2.98 (1H, t, J = 7.0, CHCO2Et), 3.24 (1H, d, J = 13.2, NCHHPh), 3.29 (1H, dd, J = 5.9, 9.0, PhCHCHH), 3.39–3.47 (1H, m, CHCH2CH2), 3.86 (1H, d, J = 7.8, CHCO2), 4.06 (1H, d, J = 13.2, NCHHPh), 4.10–4.30 (4H, m, PhCHCH2, CH2CHHO, OCH2CH3), 4.40–4.45 (1H, m, CH2CHHO), 4.65 (1H, d, J = 7.0, NCHN), 7.20–7.30 (8H, m, Ar–H), 7.40 (2H, d, J = 8.1, Ar–H); δC (100.6 MHz; CDCl3) 13.9 (CH3), 27.6 (CH2CH2O), 38.2 (CHCH2CH2), 56.7 (CHCO2Et), 58.2 (PhCH2N), 60.8 (CO2CH2), 62.8 (PhCHCH2), 64.9 (CHCO2), 66.45 (CH2CH2O), 68.3 (PhCHCH2), 86.8 (NCHN), 126.4, 126.9, 127.1, 128.1, 128.15, 128.2 (6 × Ar-CH), 138.1, 140.5 (2 × Ar-C), 170.8, 171.3 (2 × CO); m/z 420 (M+, 28%), 374 (37), 245 (30), 235 (59), 120 (44), 104 (71), 91 (100), 77 (60). Found: C, 71.75; H, 7.0; N, 6.9; C25H28N2O4 requires C, 71.4; H, 6.7; N, 6.7).
A crystal was mounted on a glass fibre and transferred to the diffractometer. Crystal data for 19: C25H28N2O4, M = 420.49, monoclinic, a = 11.351(2), b = 9.7612(10), c = 19.942(3) Å, β = 91.07(2), U = 2209.1(4) Å3, T = 298(2) K, space groupP21 (No. 4), Z = 4, Dc = 1.265 g cm−3,μ(Mo-Kα) = 0.050 mm−1, 3081 unique reflections measured, of which 3072 were used in all calculations. Final R1 [2091 F > 4σ(F)] = 0.0494 and wR(all F2) was 0.141.
Acknowledgements
We thank EPSRC for postdoctoral funding (J. S. S., K. J. H.), University of Nottingham for a studentship (K. J. H.), Rhone-Poulenc Agriculture for additional financial support (K. J. H.), Dr D. Hawkins for helpful discussions, and the EPSRC National Mass Spectrometry Service Centre (Swansea) for some MS data.References
- See, for example: F. Bellina and R. Rossi, Tetrahedron, 2006, 62, 7213 Search PubMed; I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765 CrossRef; D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435 CrossRef CAS and previous articles in this series.
- See, for example: G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 Search PubMed; L. M. Harwood, and R. J. Vickers, in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, ed. A. Padwa, and W. H. Pearson, Wiley-Interscience: Hoboken, 2003, p. 169 CrossRef CAS.
- E. G. McGeer, J. W. Olney, and P. L. McGeer, Kainic Acid as a Tool in Neurobiology, Raven Press: New York, 1978 Search PubMed. For reviews, see: Q. Wang, S. Yu, A. Simonyi, G. Sun and A. Sun, Mol. Neurobiol., 2005, 31, 3 Search PubMed; M. G. Moloney, Nat. Prod. Rep., 2002, 19, 597 Search PubMed; A. F. Parsons, Tetrahedron, 1996, 52, 4149 Search PubMed.
- R. J. Bridges, M. S. Stanley, M. W. Anderson, C. W. Cotman and A. R. Chamberlin, J. Med. Chem., 1991, 34, 717 CrossRef CAS.
- R. C. F. Jones, K. J. Howard and J. S. Snaith, Tetrahedron Lett., 1996, 37, 1707 CrossRef CAS.
- R. C. F. Jones, K. J. Howard and J. S. Snaith, Tetrahedron Lett., 1996, 37, 1711 CrossRef CAS.
- R. C. F. Jones, K. J. Howard, J. R. Nichols and J. S. Snaith, J. Chem. Soc., Perkin Trans. 1, 1998, 2061 RSC.
- See, for example: W. N. Draffin and L. M. Harwood, Synlett, 2006, 857 Search PubMed; D. J. Aldous, M. G. B. Drew, W. N. Draffin, E. M. N. Hamelin, L. M. Harwood and S. Thurairatnam, Synthesis, 2005, 3271 CrossRef CAS; K. A. Ahrendt and R. M. Williams, Org. Lett., 2004, 6, 4539 CAS; T. Onishi, P. R. Sebahar and R. M. Williams, Tetrahedron, 2004, 60, 9503 CrossRef CAS; F. X. Lery, N. Kunesch, P. George and H.-P. Husson, Heterocycles, 2002, 57, 1599 CrossRef CAS.
- R. C. F. Jones, I. Turner and K. J. Howard, Tetrahedron Lett., 1993, 34, 6329 CrossRef CAS.
- R. C. F. Jones and K. J. Howard, J. Chem. Soc., Perkin Trans. 1, 1993, 2391 RSC.
- Preliminary molecular mechanics calculations (Macromodel 4.0, MM2) surprisingly show the isolated product of exo addition, 9e, as higher in energy than the expected exo-adduct 10, by over 60 kJ mol−1; cf. R. C. F. Jones, and K. J. Howard, Electronic Conference on Trends in Organic Chemistry (ECTOC-1) ISBN 0 85404 899 5, ed. H. S. Rzepa, and J. M. Goodman, (CD-ROM), Royal Society of Chemistry Publications, 1995, poster 30. See also http://www.ch.ic.ac.uk/ectoc/ Search PubMed.
- For a full discussion of the use of sulfur-activated dipolarophiles, see: R. C. F. Jones, S. Rafiq, M. R. J. Elsegood, V. McKee and M. J. Slater, Chem.–Asian J., 2010, 5, 461 Search PubMed.
- R. C. F. Jones, K. J. Howard, J. R. Nichols and J. S. Snaith, Tetrahedron Lett., 1997, 38, 1647 CrossRef CAS.
- For related intramolecular cycloadditions using a ketone tether, see: P. M. J. Lory, R. C. F. Jones, J. N. Iley, S. J. Coles and M. B. Hursthouse, Org. Biomol. Chem., 2006, 4, 3155 Search PubMed.
- R. C. F. Jones, K. J. Howard, J. S. Snaith, A. J. Blake, W.-S. Li, and P. J. Steel, unpublished results.
- D. R. Perrin, and W. L. F. Armarego, Purification of Laboratory Chemicals, Pergamon Press: Oxford, 3rd edition, 1988 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: X-Ray crystallographic data tables. CCDC reference numbers 787234–787236. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ob00529k |
| ‡ Present address: School of Chemistry, University of Birmingham, Birmingham B15 2TT |
| This journal is © The Royal Society of Chemistry 2011 |