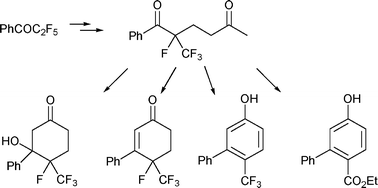
The diketone 2-fluoro-2-(trifluoromethyl)-1-phenylhexane-1,5-dione 3 was synthesized by a Mukaiyama Michael type reaction from the corresponding tetrafluoroenol silyl ether prepared from pentafluoropropiophenone. This diketone was treated under basic conditions and was converted, depending on the stoichiometry of the base, into the surprisingly stable ketol 4-fluoro-4-(trifluoromethyl)-3-hydroxy-3-phenylcyclohexanone 4 as a single diastereomer (catalytic KOH) or to the biphenylol 6-(trifluoromethyl)biphenyl-3-ol (excess KOH, THF) 5. Solvolysis of the trifluoromethyl group (anionic activation) occurred using excess KOH in alcohol. The corresponding cyclohexenone derivative 7, the usual product of Robinson annulation, might be prepared in good yield via mesylation of the ketol. Thus various unprecedented fluorinated cyclohexane and aromatic derivatives were achieved in a few steps from the commercially available pentafluoropropiophenone.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...