Molecular level studies on binding modes of labeling molecules with polyalanine peptides†
Xiaobo
Mao
,
Chenxuan
Wang
,
Xiaojing
Ma
,
Min
Zhang
,
Lei
Liu
,
Lan
Zhang
,
Lin
Niu
,
Qindao
Zeng
,
Yanlian
Yang
* and
Chen
Wang
*
National Center for Nanoscience and Technology, Beijing, 100190, China. Fax: +86-10-62656765; Tel: +86-10-82545559 (Yang YL) Tel: +86-10-82545561 (Wang C)E-mail: yangyl@nanoctr.cn; wangch@nanoctr.cn
First published on 31st January 2011
Abstract
In this work, the binding modes of typical labeling molecules (thioflavin T (ThT), Congo red (CR) and copper(II) phthalocyanine tetrasulfonic acid tetrasodium salt (PcCu(SO3Na)4)) on pentaalanine, which is a model peptide segment of amyloid peptides, have been resolved at the molecular level by using scanning tunneling microscopy (STM). In the STM images, ThT molecules are predominantly adsorbed parallel to the peptide strands and two binding modes could be identified. It was found that ThT molecules are preferentially binding on top of the peptide strand, and the mode of intercalated between neighboring peptides also exists. The parallel binding mode of CR molecules can be observed with pentaalanine peptides. Besides the binding modes of labeling molecules, the CR and PcCu(SO3Na)4 display different adsorption affinity with the pentaalanine peptides. The results could be beneficial for obtaining molecular level insight of the interactions between labeling molecules and peptides.
Introduction
The formation of β-sheet-rich amyloid has attracted extensive attention due to its association with a number of neurodegenerative processes.1,2 The accumulation of intracellular amyloid-like aggregates by mutant proteins is the hallmark of two groups of codon reiteration disorders, for which there are currently few treatment options. An example is the Huntington's disease that is caused by polyglutamine repeats resulting from CAG trinucleotide repeat mutations. Another example could be found in the oculopharyngeal muscular dystrophy (OPMD).3–5OPMD is caused by the abnormal expansion of a (GCG)n repeat coding the polyalanine (polyA) peptide. Polyalanine stretches may cause cytotoxicity through mitochondrial dysfunction4,6 and the exact mechanism responsible for its toxicity in OPMD is still unknown. By using X-ray diffraction (XRD) techniques, alanine-rich domains of prions are also reported to be the core domains of the amyloid formation due to the strong van der Waals interactions between polyalanines, such as SHa106-122 (KTNMKHMAGAAAAGAVV) and similar fragments.7–11Investigations on amyloid fibrillation kinetics are crucial in understanding the fibril formation mechanisms and for examination of drugs to inhibit amyloid formation.12 The molecular dynamics simulations (MD) on kinetics of fibril formation by polyalanine peptides suggested a conformational conversion process in which small amorphous aggregates → β-sheets → ordered nucleus → subsequent rapid growth of a small stable fibril or protofilament.13–15
The soluble model β-sheet complexes, polyalanine-based peptides, have also been designed for studying the β-sheet conformation in neurodegenerative disorders.11,16 Among the most commonly used and convenient methods for the measurement of amyloid fibrillation formation and its kinetics are fluorescent and birefringent detections by using two kinds of representative dye molecules, thioflavin T (ThT) and Congo red (CR).12,17–19 Many efforts have been made for studying the interactions between dye molecules and amyloid peptides, the underlying specific binding modes and fluorescence enhancement mechanisms.20,21 A length-dependent effect has been reported for analyzing β-sheet formed by polyalanines with fluorescent method by using ThT assay.22 The binding affinities of CR for amyloid-β and amylin fibrils vary considerably, and certain metal ions (Cu2+, Ni2+, Cd2+) could change the affinity significantly.23 The affinity of ThT to common β-sheet structures also gives rise to the interest for developing inhibiting species for treating amyloid diseases.24,25 As another widely used labeling agent, CR has also been proved to reduce amyloid aggregation and cell death in cell models of OPMD and amyloid diseases.24,26 Another kind of labeling molecules, phthalocyanine (Pc) and its derivatives, have also been suggested to bind to amyloid peptide, which could affect the amyloid formation and the related cytotoxicity.27,28 To unveil the interactions between dye molecules and amyloid peptides at the molecular level, it is essential to develop the detection methods for studying the structural characteristics of amyloids under various conditions.
A number of techniques have been applied to gain insights of the specificity of the interactions between dye molecules and amyloid peptides, such as XRD,12,29,30 fluorescence,31 circular dichroism,20isothermal titration calorimetry,12,20,32confocal microscopy,21 molecular dynamics simulations,33,34 computational modeling based on the crystal results.31,35 One of the proposed binding sites is in the central pore region of amyloid fibrils determined by small angle X-ray scattering and XRD.12,29 Several reports have suggested that the possible binding site for dye molecules is in the “channels” formed by side chains of residues along the long fibril axis.20,21,31,33,34 The binding sites in parallel configuration have also been proposed experimentally and theoretically.30,33–35 It should be noted that considerable challenges for the proposed binding modes still remain because of the lacking of direct evidence from structural analysis in both crystallization and assembly states.
In recent studies on molecular adsorption and assembling processes, scanning tunneling microscopy (STM) has provided a helpful venue for studying biological molecules due to its high structural resolution and adaptability to various environments, including liquid-solid interfaces36,37 and ultra-high vacuum conditions.38,39 Recently STM has been applied to study the core regions of amyloid assemblies with molecular level resolutions.40,41
In this work, using the organic-peptide co-assembly of pentaalanine (H2N-Ala-Ala-Ala-Ala-Ala-COOH) with a chaperon-like molecules, 4,4′-bipyridyl (4Bpy), we have studied the binding modes of labeling molecules, ThT, CR and phthalocyanine tetrasulfonates PcCu(SO3Na)4, by using STM. The labeling molecule ThT for amyloid stain are found to bind with pentaalanine peptide strands in parallel and two kinds of detailed binding modes for ThT could be identified. The parallel binding mode of CR molecules could also be identified by STM. Furthermore, the different adsorption affinity of CR and the Pc derivatives to the co-assembly of pentaalanine peptides and 4Bpy molecules could also be deduced.
Materials and methods
Synthetic peptides and labeling molecules
Pentaalanine, 4,4′-bipyridyl, Congo red, thioflavin T and copper(II) phthalocyanine tetrasulfonic acid tetrasodium salt (Scheme 1) were obtained from Sigma-Aldrich Co. Ltd and used without further purification. The physical sizes of the molecules are provided correspondingly.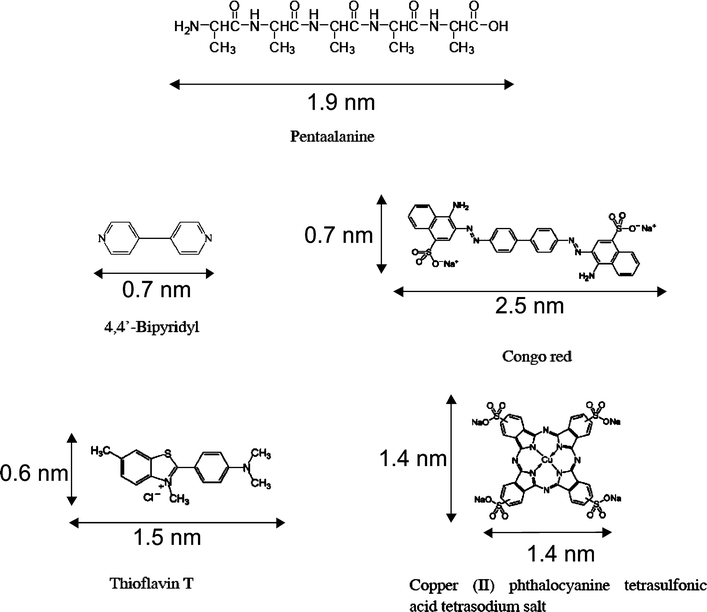 | ||
| Scheme 1 Molecular structures of pentaalanine, 4,4′-bipyridyl (4Bpy), Congo Red (CR), thioflavin T (ThT), and copper(II) phthalocyanine tetrasulfonic acid tetrasodium salt (PcCu(SO3Na)4). The dimensions of the molecules are provided correspondingly. | ||
Sample preparation for STM studies
The lyophilized powder of pentaalanine (1 mg) and the solid powder of 4Bpy (1 mg) were dissolved in chromatographic grade acetonitrile (ACN) (1 mL) (ESI†). 1 μL solution of the mixture with pentaalanine and 4Bpy were deposited on highly oriented pyrolytic graphite (HOPG) immediately after mixing. STM experiments were performed after 1 h the solvent ACN was completely evaporated from the HOPG surface. The labeling molecules were firstly mixed with the solutions of pentaalanine and 4Bpy. In the experiments, two concentrations (0.1 mg mL−1, 1 mg mL−1) of the ThT molecules have been used. The concentration of CR is 0.1 mg mL−1, and the concentration of PcCu(SO3Na)4 is 0.1 mg mL−1. 1 μL of mixed solution were deposited on HOPG surface, and followed by the similar procedure for the mixture of pentaalanine and 4Bpy.STM measurements
STM experiments were performed in constant-current mode under ambient conditions (Nanoscope IIIA scanning probe microscope (SPM) system, Veeco, USA). The tips were mechanically formed Pt/Ir wire (80/20). The STM tunneling conditions are described in the corresponding figure captions. The experiments were repeated more than 5 times independently using different tips for reproducibility for different labeling molecules.Statistical methods
The lengths of the peptide strands in STM images are measured by using the Nanoscope software. A step size of 0.325 nm for every residue are assumed in the statistical histogram of the length distribution of the peptide assemblies, which is fitted by Gaussian distribution. The measured lengths in the histograms represent the average value of the independent experiments. The number frequencies in the statistical results are all based on number of events.Results and discussion
Scheme 1 presents the schematic structures of pentaalanine and 4Bpy for the peptide assemblies, ThT and CR for amyloid stains, and Pc tetrasulfonates PcCu(SO3Na)4 as amyloid formation modulator. Pentaalanine is a peptide with methyl side groups only. ThT molecule consists of a pair of benzothiazole and benzaminic rings freely rotating around the inter-ring C–C bond. ThT shows enhanced fluorescence at 482 nm when bound to amyloid fibrils, which can be used as a sensitive diagnosis tool for amyloidosis. CR is a secondary diazo dye, which is water soluble, yielding a red colloidal solution. Apple-green birefringence of CR under polarized light is indicative for the presence of amyloid fibrils. PcCu(SO3Na)4 is a multifunctional molecule, which can be excited by visible or near-infrared light in photodynamic therapy (PDT).42Pentaalanine is a model peptide for amyloid studies due to its repeated sequential structure with only hydrophobic side groups and the high occurrence of alanine residues in the abnormal protein sequences.3,7–9 The assembly of pentaalanine contains nearly continuous lamellae structures in STM images (Fig. S1 in the ESI†). It has been reported that one of the characteristic features is 0.47 nm for the distance of two neighboring strands in the cross-β-sheet structure.52 In Fig. S1A, the marked distance covering 10 molecules is measured to be 4.7 ± 0.2 nm which is consistent with the previous results. Since high-resolution STM images could only be achieved for molecular species that are adsorbed stably under ambient conditions. The side groups of pentaalanine molecules can not be identified in our STM images due to its structural fluctuations.
In order to identify the terminal positions of the pentaalanine molecules in the assembly, directional noncovalent interactions derived from co-adsorbed chaperon-like molecules are introduced.28,43,44 In the current study 4Bpy is introduced as a chaperon-like molecule to modulate the pentaalanine assembly. The STM image of the 4Bpy assembly (Fig. S2 in the ESI†) shows the characteristic close-packing assembly structures. Fig. 1A is a typical large-scale STM image of pentaalanine/4Bpy co-assembly on HOPG surface. 4Bpy with the conjugated π-electron structure can be associated with the bright features in linear array arrangement due to the higher electron density of states. The lamella bands with reduced contrast could be attributed to the pentaalanine. In the high-resolution STM image (Fig. 1B), the molecular dimensions of 4Bpy and pentaalanine could be determined quantitatively. It could be proposed that the formation of the heterogeneous peptide-organic assembly structure is facilitated by strong N⋯H–O hydrogen bond between N atom of 4Bpy and the carboxyl group of pentaalanine C-terminus, in which two pentaalanine stripes between the neighboring 4Bpy arrays are connected with a head-to-head configuration with N-terminus in the middle.28,45 The stability of the hydrogen bond between 4Bpy and carboxyl group has been characterized by variable-temperature Fourier transform infrared spectroscopy (FTIR).46 Molecular packing arrangements with different angles between molecular axis and lamellae direction are influenced by the competitive and collaborative interactions between different functional groups, which have been studied extensively.47–49 The angle α between pentaalanine molecular axes and the stripe orientation is measured to be 32 ± 2°, which is associated with the hydrogen bond interaction between terminal functional groups.45
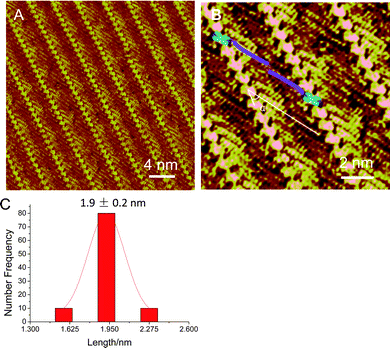 | ||
| Fig. 1 (A, B) STM images of co-assembly of pentaalanine and 4Bpy. Tunneling conditions: I = 323.5 pA, V = −846.6 mV. (B) The angle α is referred to the angle between the peptide stripes and the peptide backbones, and the schematic illustration of the pentaalanine/4Bpy co-assembly is superimposed on the image. (C) The statistical histogram of the length of pentaalanine molecules. The average length is measured to be 1.9 ± 0.2 nm. | ||
The distance between the neighboring pentaalanine molecules within one stripe is measured to be 4.7 ± 0.2 angstrom (Fig. S1, ESI†), indicative of a β-sheet-like structure of the pentaalanine assembly which has been suggested in the previous studies.13–15 The de novo designed polyalanine-based peptides may undergo conformational changes from random coil conformations into soluble β-pleated-sheet motifs driven by hydrophobic interactions between the methyl groups of the alanine side chains.16 In addition, fluorescence enhancement in ThT assay for the polyalanines also indicates the formation of β-sheet structures.22 In this work, the lengths of the pentaalanine peptides are measured to be about 1.9 nm, as shown in Fig. 1C, which is in agreement with the expected length of the extended pentaalanine peptides.40
It has been suggested that amyloid aggregation is caused by backbone hydrogen bonds,50 by maximizing the van der Waals interactions between side-chains,51 and possible shape complementarities between neighboring molecules.52,53 Then the introduction of 4Bpy molecules interacting with the C-terminus of pentaalanine molecules is unlikely to interfere with the related binding modes of labeling molecules. The presence of the 4Bpy arrays with higher contrast further provides an indicator for identifying the binding modes of the labeling molecules on the peptide stripes (Fig. 1B).
Upon the introduction of ThT into the pentaalanine/4Bpy co-assembly, Fig. 2A reveals the randomly distributed bright features, which can be as attributed to the individual ThT molecules marked by white circles. In the large-scale STM image, the directions of the long axes of ThT molecules are predominantly adsorbed parallel to the orientations of the pentaalanine strands. In the high-resolution STM image (Fig. 2B), the ThT molecule is revealed as a hedge-shaped rod with bright contrast (∼1.5 nm in length) with two parallel binding modes to the pentaalanine strands.33,35 In the preferential binding mode, ThT molecules tend to bind on top of the pentaalanine peptide strands rather than intercalated between two neighboring peptide strands, as shown in the histogram of the statistical distribution of Fig. 2B. The observations of the two parallel binding modes are in accordance with the previously reported experimental and theoretical results.30,33–35
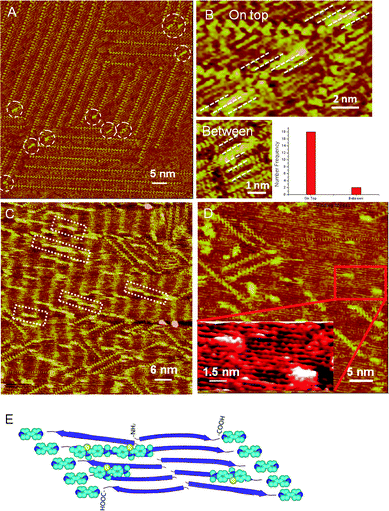 | ||
| Fig. 2 (A) Large-scale STM image of ThT-atop-pentaalanine/4Bpy with lower ThT/peptide ratio. Tunneling conditions: I = 402.8 pA, V = 422.1 mV. White dashed circles highlight the adsorbed ThT molecules. (B) High-resolution STM images of the two binding modes of ThT molecules marked by white dotted lines and the histogram of statistical distribution of two binding modes. Tunneling conditions: I = 402.8 pA, V = 422.1 mV. (C) Large-scale STM image of ThT-atop-pentaalanine/4Bpy with higher ThT/peptide ratio. The white dashed rectangles represent the self-assembly of ThT molecules on pentaalanine assembly. The molecular long axes of ThT molecules are parallel to the pentaalanine strands. Tunneling conditions: I = 314.3 pA, V = 821.8 mV. (D) High-resolution STM images of ThT-atop-pentaalanine self-assembly. (E) The schematic illustration of the adsorption mode of ThT with pentaalanine/4Bpy co-assembly. The backbones of the peptide strands are represented in blue arrows. The atoms of ThT and 4Bpy in the illustration are colored by element type, cyan, blue and yellow colors for C, N and S atoms respectively. | ||
At higher ThT concentration, the adsorption of ThT oligomers with parallel orientation to the peptide strands could also be observed, as shown in white dashed rectangles in Fig. 2C. ThT assemblies on the pentaalanine peptides could be stably observed during STM experiments, suggesting the strong affinity of ThT to pentaalanine peptides.
Based on the measured length of ThT monomer (∼1.5 nm) and the hedge-like features, the number of ThT molecules in the observed oligomers on pentaalanine assembly could be estimated as between 2 to 12, and the most probable value is 5 (Fig. S3 in the ESI†). Few ThT molecules could be observed binding on the 4Bpy site at the high concentration of ThT, which is also supportive to the consideration of high affinity of ThT molecules to pentaalanine strands. In addition, in the high-resolution STM images, as shown in Fig. 2D and the inset, the binding modes of ThT molecules to pentaalanine strands, can be clearly identified without 4Bpy molecules showing the predominant parallel orientations of ThT molecules to the pentaalanine strands. The binding modes of ThT molecules to pentaalanine/4Bpy co-assembly are schematically illustrated in Fig. 2E showing the preferential adsorption of ThT on peptide strands with parallel molecular orientations. The binding of ThT monomers and oligomers to pentaalanine assemblies could be ascribed to the nonspecific van der Waals interactions between the aromatic rings of ThT molecules and the hydrophobic peptide surface associated with the methyl side groups.
In a parallel study with the CR as the labeling agent, similar features could be observed in the STM images. In Fig. 3A, CR molecules marked by white circles can be clearly recognized as rod-like features with higher contrast and random distribution on the co-assembly of pentaalanine/4Bpy. The molecular long axes of CR molecules are predominantly aligned with the axes of pentaalanine peptides as revealed by the high-resolution STM image (Fig. 3B), in agreement with the previously proposed parallel binding mode.30,33–35 The location of CR molecules could be observed on top of the pentaalanine strands. In addition to the CR molecules bound to the peptide stripes, the CR molecules are also observed to self-assemble with distinctively different assembling characteristics, which are marked by the white dashed line in Fig. 3C. The affinity of CR to the pentaalanine surface could be related to the hydrophobic domain of amyloid peptides.54–57 It was suggested that such interaction could affect the amyloid aggregation relating to amyloid toxicity.24,26,58,59
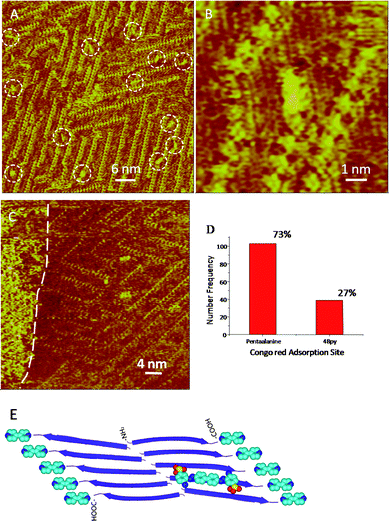 | ||
| Fig. 3 (A) STM image of CR-atop-pentaalanine/4Bpy. Tunneling conditions: I = 338.7 pA, V = 842.0 mV. White dashed circles highlight the adsorbed CR molecules. (B) High-resolution STM images of CR-atop-pentaalanine/4Bpy. Tunneling conditions: I = 338.7 pA, V = 842.0 mV. (C) Self-assembled CR molecules on the pentaalanine/4Bpy co-assembly can be observed on the left side of the image indicated by the white dashed line. Tunneling conditions: I = 305.2 pA, V = 609.1 mV. (D) The histogram of the statistical distribution for CR molecules on two adsorption sites: pentaalanine and 4Bpy. (E) The schematic illustration of the adsorption mode of CR with pentaalanine/4Bpy co-assembly. All the legends and the colors are same to those in Fig. 2E, and the atoms in red is O. | ||
It should be noted that in these STM images of the organic-peptide co-assemblies, both pentaalanine peptides and 4Bpy are candidate binding sites for CR. From the histogram distribution of the statistical results for CR molecules on two adsorption sites, as shown in Fig. 3D, the adsorption site atop pentaalanine peptides is the preferential one according to the ratio of the adsorbates on two adsorption sites (pentaalanineversus4Bpy) (103/39 or 73%: 27%). The preferential binding mode of CR atop pentaalanine peptide strands is schematically illustrated in Fig. 3E. In addition, a small portion of CR molecules could be immobilized in the trench regions between two stripes of pentaalanine peptides, and in the defects of the neighboring domains. The appearance of CR molecules seems to be discernible fluctuation both in shape and size, which could be a reflection of the difference in binding environment for the CR molecules.
Phthalocyanine (Pc) molecules have been used in PDT60–63 experiments due to its opto-electrical properties,64–66 and it has been suggested that Pc tetrasulfonates could suppress the amyloid formation and cytotoxicity. With the molecularly resolved assembled structure of pentaalanine peptide by using STM, it is possible to study the interaction between the peptides and Pcs. In the STM image (Fig. 4A), the Pc derivative, PcCu(SO3Na)4 molecules, represented as bright square-like features, are distributed randomly in the co-assembly of pentaalanine/4Bpy. Two adsorption sites could also be observed, namely atop pentaalanine peptides and atop 4Bpy molecules. In the labeled white rectangle of Fig. 4A, one molecular chain formed by five PcCu(SO3Na)4 molecules can be clearly observed to adsorb at the domain boundary of pentaalanine assemblies. In the high-resolution STM image (Fig. 4B), it can be clearly identified that the PcCu(SO3Na)4 is adsorbed preferentially atop pentaalanine peptides. The preferential binding of PcCu(SO3Na)4 on peptide strands are illustrated in the histogram distribution (Fig. 4C) showing higher ratio of the adsorbate populations on two adsorption sites (pentaalanineversus4Bpy) (77/7 or 92%: 8%). PcCu(SO3Na)4 can be considered as an aromatic moiety with planar structure and could interact with the pentaalanine assembly through van der Waals interactions. In addition, the introduction of the sulfonate groups to the Pc moiety is also critical for the interaction between the amyloid protein and Pc tetrasulfonates.27 The electrostatic interaction between –SO3− of PcCu(SO3Na)4 and –NH2 of pentaalanine should also be taken into account in the interactions between PcCu(SO3Na)4 and pentaalanine, which gives rise to the more preferential site on pentaalanine surfaces (Fig. 4D). As to the 4Bpy molecules, it can be suggested that PcCu(SO3Na)4 and 4Bpy interact mainly via π–π interaction.
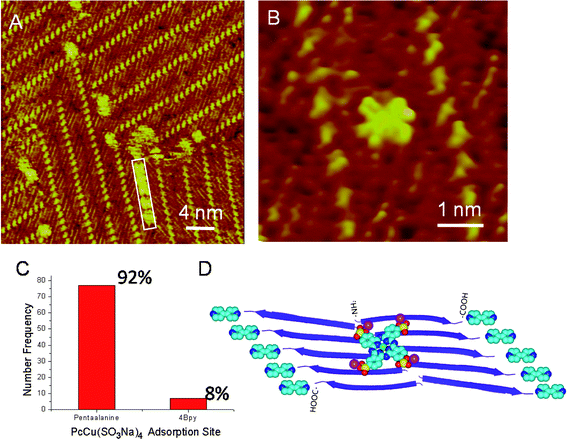 | ||
| Fig. 4 (A and B) STM images of PcCu(SO3Na)4-atop-pentaalanine/4Bpy co-assembly. Tunneling conditions: I = 363.2 pA, V = 660.1 mV. The molecular chain of PcCu(SO3Na)4 at the domain boundary is highlighted with a white rectangle. (C) The histogram of the statistical distribution of PcCu(SO3Na)4 molecules on two adsorption sites, pentaalanine and 4Bpy. (D) The schematic illustration of the adsorption model of PcCu(SO3Na)4 with pentaalanine/4Bpy co-assembly. All the legends and colors are same to those in Fig. 2E, and the atoms in magenta and green are Na and Cu elements of PcCu(SO3Na)4. | ||
Due to the insolubility and non-crystalline nature of amyloid peptides, it is still a challenging task to determine the molecular-level structures of the binding configurations of labeling molecules. The study on the binding sites of labeling molecules by using STM is still at the preliminary stage. The current STM experiments are performed at liquid/solid interfaces, therefore, could be relevant to biological conditions of interests. The differences between the aggregation behavior of surface-bound peptides and that in solutions should also be explored.67 It was suggested that dynamic exchanges of the adsorbed peptides with those in solutions could enable reversible conformational changes of the peptides.68 Common to the peptide assemblies in solution and at various interfaces is that the molecule-molecule interactions are central to the assembling characteristics, which could cause amyloidal diseases.
The relevance between STM techniques and other important spectroscopic and microscopic methods on the binding behaviors of labeling molecules to various amyloid peptides is an interesting topic to pursue. It has been reported that the fluorescence efficiency are strongly dependent on the conformation of ThT and the deviation from planar conformation may lead to dramatic reduction of the fluorescence of ThT molecules.69 Rigorous experimental and theoretical studies on the connection between the binding modes and enhanced fluorescence mechanism are keenly needed in the future efforts.
Conclusion
In summary, the binding modes of the labeling molecules to amyloid peptide model, pentaalanine, have been observed at the molecular level by using STM. In the co-assembly with the chaperon-like molecules, the C-termini of the pentaalanine peptides are recognized, providing a direct venue to identify various binding modes of labeling molecules. It has been identified that thioflavin T and Congo red could be bound parallel to the pentaalanine strands, and the CR and PcCu(SO3Na)4 molecules also tend to stay on pentaalanine peptide comparing to the lower affinity to 4Bpy binding sites. The identification of the binding behaviors could benefit the studies on the interactions between amyloid peptides and labeling molecular species.Acknowledgements
This work was supported by the National Basic Research Program of China (2009CB930100, 2011CB932800) and Chinese Academy of Sciences (KJCX2-YW-M15). Financial support from National Natural Science Foundation of China (20911130229) and CAS Key Laboratory for Biological Effects of Nanomaterials & Nanosafety are also gratefully acknowledged. The authors would like to thank for the supplier of the programs, PyMOL70 and NanoEngineer-171 for generation of the molecular models in Figures 2E, 3E and 3D.References
- D. J. Selkoe, Alzheimer's disease: Genes, proteins, and therapy, Physiol. Rev., 2001, 81, 741–766 CAS
.
- S. B. Prusiner, Prions, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 13363–13383 CrossRef CAS
.
- U. Kuhn and E. Wahle, Structure and function of poly(A) binding proteins, Biochim. Biophys. Acta, Gene Struct. Expr., 2004, 1678, 67–84 Search PubMed
.
- K. Toriumi, Y. Oma, Y. Kino, E. Futai, N. Sasagawa and S. J. Ishiura, Expression of polyalanine stretches induces mitochondrial dysfunction, J. Neurosci. Res., 2008, 86, 1529–1537 CrossRef CAS
.
- B. Brais, Oculopharyngeal muscular dystrophy: A polyalanine myopathy, Curr. Neurol. Neurosci. Rep., 2008, 9, 76–82 Search PubMed
.
- I. M. Nasrallah, J. C. Minarcik and J. A. Golden, A polyalanine tract expansion in Arx forms intranuclear inclusions and results in increased cell death, J. Cell Biol., 2004, 167, 411–416 CrossRef CAS
.
- H. Inouye and D. A. Kirschner, X-ray diffraction analysis of scrapie prion: Intermediate and folded structures in a peptide containing two putative alpha-helices, J. Mol. Biol., 1997, 268, 375–389 CrossRef CAS
.
- H. Inouye and D. A. Kirschner, Polypeptide chain folding in the hydrophobic core of hamster scrapie prion: Analysis by X-ray diffraction, J. Neurochem., 1998, 70, S7
.
- H. Inouye, J. Bond, M. A. Baldwin, H. L. Ball, S. B. Prusiner and D. A. Kirschner, Structural changes in a hydrophobic domain of the prion protein induced by hydration and by Ala->Val and Pro->Leu substitutions, J. Mol. Biol., 2000, 300, 1283–1296 CrossRef CAS
.
- J. T. Nguyen, H. Inouye, M. A. Baldwin, R. J. Fletterick, F. E. Cohen, S. B. Prusiner and D. A. Kirschner, X-Ray-Diffraction of scrapie prion rods and Prp peptides, J. Mol. Biol., 1995, 252, 412–422 CrossRef CAS
.
- L. M. Shinchuk, D. Sharma, S. E. Blondelle, N. Reixach, H. Inouye and D. A. Kirschner, Poly-(L-alanine) expansions form core beta-sheets that nucleate amyloid assembly, Proteins: Struct., Funct., Bioinf., 2005, 61, 579–589 Search PubMed
.
- M. Groenning, M. Norrman, J. M. Flink, M. van de Weert, J. T. Bukrinsky, G. Schluckebier and S. Frokjaer, Binding mode of Thioflavin T in insulin amyloid fibrils, J. Struct. Biol., 2007, 159, 483–497 CrossRef CAS
.
- H. D. Nguyen and C. K. Hall, Kinetics of fibril formation by polyalanine peptides, J. Biol. Chem., 2004, 280, 9074–9082 CrossRef
.
- H. D. Nguyen, A. J. Marchut and C. K. Hall, Solvent effects on the conformational transition of a model polyalanine peptide, Protein Sci., 2008, 13, 2909–2924 CrossRef
.
- Y. Levy, J. Jortner and O. M. Becker, Solvent effects on the energy landscapes and folding kinetics of polyalanine, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2188–2193 CrossRef CAS
.
- S. E. Blondelle, B. Forood, R. A. Houghten and E. PerezPaya, Polyalanine-based peptides as models for self-associated beta-pleated-sheet complexes, Biochemistry, 1997, 36, 8393–8400 CrossRef CAS
.
- G. Kelenyi, Thioflavin S fluorescent and Congo Red anisotropic stainings in histologic demonstration of amyloid, Acta Neuropathol., 1967, 7, 336–348 CrossRef CAS
.
-
E. Gurr, 1965, Congo Red, in Encyclopedia of Microscopic Stains, pp. 140–141, Leonard Hill, London Search PubMed
.
- H. I. LeVine, Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: Detection of amyloid aggregation in solution, Protein Sci., 2008, 2, 404–410 CrossRef
.
- R. Sabate, I. Lascu and S. J. Saupe, On the binding of Thioflavin-T to HET-s amyloid fibrils assembled at pH 2, J. Struct. Biol., 2008, 162, 387–396 CrossRef CAS
.
- M. R. H. Krebs, E. H. C. Bromley and A. M. Donald, The binding of thioflavin-T to amyloid fibrils: localisation and implications, J. Struct. Biol., 2005, 149, 30–37 CrossRef CAS
.
- K. Giri, N. P. Bhattacharyya and S. Basak, pH-dependent self-assembly of polyalanine peptides, Biophys. J., 2007, 92, 293–302 CrossRef CAS
.
- T. T. Ashburn, H. Han, B. F. Mc Guinness and P. T. Lansbury, Amyloid probes based on Congo Red distinguish between fibrils comprising different peptides, Chem. Biol., 1996, 3, 351–358 CrossRef CAS
.
- V. Heiser, E. Scherzinger, A. Boeddrich, E. Nordhoff, R. Lurz, N. Schugardt, H. Lehrach and E. E. Wanker, Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: Implications for Huntington's disease therapy, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 6739–6744 CrossRef CAS
.
- C. I. Stains, K. Mondal and I. Ghosh, Molecules that target beta-amyloid, ChemMedChem, 2007, 2, 1675–1692
.
- A. Lorenzo and B. A. Yankner, Beta-Amyloid neurotoxicity requires fibril formation and is inhibited by Congo Red, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 12243–12247 CAS
.
- E. N. Lee, H. J. Cho, C. H. Lee, D. Lee, K. C. Chung and S. R. Paik, Phthalocyanine tetrasulfonates affect the amyloid formation and cytotoxicity of alpha-synuclein, Biochemistry, 2004, 43, 3704–3715 CrossRef CAS
.
- L. Liu, L. Zhang, X. Mao, L. Niu, Y. Yang and C. Wang, Chaperon-Mediated single molecular approach toward modulating Aβ peptide aggregation, Nano Lett., 2009, 9, 4066–4072 CrossRef CAS
.
- M. Harel, L. K. Sonoda, I. Silman, J. L. Sussman and T. L. Rosenberry, Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site, J. Am. Chem. Soc., 2008, 130, 7856–7861 CrossRef CAS
.
- W. G. Turnell and J. T. Finch, Binding of the dye Congo Red to the amyloid protein pig insulin reveals a novel homology amongst amyloid-forming peptide sequences, J. Mol. Biol., 1992, 227, 1205–1223 CrossRef CAS
.
- M. Biancalana, K. Makabe, A. Koide and S. Koide, Molecular mechanism of Thioflavin-T binding to the surface of beta-Rich peptide self-assemblies, J. Mol. Biol., 2009, 385, 1052–1063 CrossRef CAS
.
- M. Groenning, L. Olsen, M. van de Weert, J. M. Flink, S. Frokjaer and F. S. Jorgensen, Study on the binding of Thioflavin T to beta-sheet-rich and non-beta-sheet cavities, J. Struct. Biol., 2007, 158, 358–369 CrossRef CAS
.
- C. Wu, Z. X. Wang, H. X. Lei, W. Zhang and Y. Duan, Dual binding modes of Congo red to amyloid protofibril surface observed in molecular dynamics simulations, J. Am. Chem. Soc., 2007, 129, 1225–1232 CrossRef CAS
.
- C. Wu, Z. X. Wang, H. X. Lei, Y. Duan, M. T. Bowers and J. E. Shea, The binding of Thioflavin T and its neutral analog BTA-1 to protofibrils of the Alzheimer's disease A beta(16–22) peptide probed by molecular dynamics simulations, J. Mol. Biol., 2008, 384, 718–729 CrossRef CAS
.
- D. B. Carter and K. C. Chou, A model for structure-dependent binding of Congo red to Alzheimer beta-amyloid fibrils, Neurobiol. Aging, 1998, 19, 37–40 CrossRef CAS
.
- X. B. Mao, Y. B. Wang, L. Liu, L. Niu, Y. L. Yang and C. Wang, Molecular-level evidence of the surface-induced transformation of peptide structures revealed by scanning tunneling microscopy, Langmuir, 2009, 25, 8849–8853 CrossRef CAS
.
- R. Matmour, I. De Cat, S. J. George, W. Adriaens, P. Leclere, P. H. H. Bomans, N. A. J. M. Sommerdijk, J. C. Gielen, P. C. M. Christianen, J. T. Heldens, J. C. M. van Hest, D. W. P. M. Lowik, S. De Feyter, E. W. Meijer and A. P. H. J. Schenning, Oligo(p-phenylenevinylene)-peptide conjugates: Synthesis and self-assembly in solution and at the solid-liquid interface, J. Am. Chem. Soc., 2008, 130, 14576–14583 CrossRef CAS
.
- A. Kühnle, T. R. Linderoth, B. Hammer and F. Besenbacher, Chiral recognition in dimerization of adsorbed cysteine observed by scanning tunnelling microscopy, Nature, 2002, 415, 891–893 CrossRef CAS
.
- M. Lingenfelder, G. Tomba, G. Costantini, L. C. Ciacchi, A. D. Vita and K. Kern, Tracking the chiral recognition of adsorbed dipeptides at the single-molecule level, Angew. Chem., Int. Ed., 2007, 46, 4492–4495 CrossRef CAS
.
- X. B. Mao, X. J. Ma, L. Liu, L. Niu, Y. L. Yang and C. Wang, Structural characteristics of the beta-sheet-like human and rat islet amyloid polypeptides as determined by scanning tunneling microscopy, J. Struct. Biol., 2009, 167, 209–215 CrossRef CAS
.
- X. Ma, L. Liu, X. Mao, L. Niu, K. Deng, W. Wu, Y. Li, Y. Yang and C. Wang, Amyloid-beta (1–42) folding multiplicity and single molecule binding behavior studied by STM, J. Mol. Biol., 2009, 388, 894–901 CrossRef CAS
.
- C. G. Claessens, U. Hahn and T. Torres, Phthalocyanines: From outstanding electronic properties to emerging applications, Chem. Rec., 2008, 8, 75–97 CrossRef CAS
.
- K. W. Hipps, L. Scudiero, D. E. Barlow, P. Manning and J. Cooke, A Self-organized 2-dimensional bifunctional structure formed by supramolecular design, J. Am. Chem. Soc., 2002, 124, 2126–2127 CrossRef CAS
.
- S. B. Lei, C. Wang, S. X. Yin, H. N. Wang, F. Xi, H. W. Liu, B. Xu, L. J. Wan and C. L. Bai, Surface stabilized porphyrin and phthalocyanine two-dimensional network connected by hydrogen bonds, J. Phys. Chem. B, 2001, 105, 10838–10841 CrossRef CAS
.
- B. Xu, S. Yin, C. Wang, Q. Zeng, X. Qiu and C. Bai, Identification of hydrogen bond characterizations of isomeric 4Bpy and 2Bpy by STM, Surf. Interface Anal., 2001, 32, 245–247 CrossRef CAS
.
- X. J. Ma, Y. T. Shen, K. Deng, H. Tang, S. B. Lei, C. Wang, Y. L. Yang and X. Z. Feng, Matrix-molecule induced chiral enhancement effect of binary supramolecular liquid crystals, J. Mater. Chem., 2007, 17, 4699–4704 RSC
.
- C. L. Claypool, F. Faglioni, W. A. Goddard, H. B. Gray, N. S. Lewis and R. A. Marcus, Source of image contrast in STM images of functionalized alkanes on graphite: A systematic functional group approach, J. Phys. Chem. B, 1997, 101, 5978–5995 CrossRef CAS
.
- D. Wintgens, D. G. Yablon and G. W. Flynn, Packing of HO(CH2)14COOH and HO(CH2)15COOH on graphite at the liquid-solid interface observed by scanning tunneling microscopy: Methylene unit direction of self-assembly structures, J. Phys. Chem. B, 2003, 107, 173–179 CrossRef CAS
.
- D. M. Cyr, B. Venkataraman, G. W. Flynn, A. Black and G. M. Whitesides, Functional group identification in scanning tunneling microscopy of molecular adsorbates, J. Phys. Chem., 1996, 100, 13747–13759 CrossRef CAS
.
- K. Tsemekhman, L. Goldschmidt, D. Eisenberg and D. Baker, Cooperative hydrogen bonding in amyloid formation, Protein Sci., 2007, 16, 761–764 CrossRef CAS
.
- B. Y. Ma and R. Nussinov, Simulations as analytical tools to understand protein aggregation and predict amyloid conformation, Curr. Opin. Chem. Biol., 2006, 10, 445–452 CrossRef CAS
.
- J. J. W. Wiltzius, M. Landau, R. Nelson, M. R. Sawaya, M. I. Apostol, L. Goldschmidt, A. B. Soriaga, D. Cascio, K. Rajashankar and D. Eisenberg, Molecular mechanisms for protein-encoded inheritance, Nat. Struct. Mol. Biol., 2009, 16, 973–979 CrossRef CAS
.
- M. R. Sawaya, S. Sambashivan, R. Nelson, M. I. Ivanova, S. A. Sievers, M. I. Apostol, M. J. Thompson, M. Balbirnie, J. J. W. Wiltzius, H. T. McFarlane, A. O. Madsen, C. Riekel and D. Eisenberg, Atomic structures of amyloid cross-beta spines reveal varied steric zippers, Nature, 2007, 447, 453–457 CrossRef CAS
.
- B. Raman, E. Chatani, M. Kihara, T. Ban, M. Sakai, K. Hasegawa, H. Naiki, C. M. Rao and Y. Goto, Critical balance of electrostatic and hydrophobic interactions is required for beta(2)-microglobulin amyloid fibril growth and stability, Biochemistry, 2005, 44, 1288–1299 CrossRef CAS
.
- V. Chauhan and A. Chauhan, Kinetics of formation of hydrophobic domains in fibrillar amyloid beta-protein, FEBS J., 2005, 272, 70–70
.
- R. D. Hills and C. L. Brooks, Hydrophobic cooperativity as a mechanism for amyloid nucleation, J. Mol. Biol., 2007, 368, 894–901 CrossRef CAS
.
- D. E. Otzen, O. Kristensen and M. Oliveberg, Designed protein tetramer zipped together with a hydrophobic Alzheimer homology: A structural clue to amyloid assembly, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 9907–9912 CrossRef CAS
.
- A. Quist, L. Doudevski, H. Lin, R. Azimova, D. Ng, B. Frangione, B. Kagan, J. Ghiso and R. Lal, Amyloid ion channels: A common structural link for protein-misfolding disease, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10427–10432 CrossRef CAS
.
- H. B. Jang, J. Zheng, R. Lal and R. Nussinov, New structures help the modeling of toxic amyloid beta ion channels, Trends Biochem. Sci., 2008, 33, 91–100 CAS
.
- L. Polo, G. Valduga, G. Jori and E. Reddi, Low-density lipoprotein receptors in the uptake of tumour photosensitizers by human and rat transformed fibroblasts, Int. J. Biochem. Cell Biol., 2002, 34, 10–23 CrossRef CAS
.
- Y. Degani and I. Willner, Complex formation between anthraquinone-2,6-disuIfonate and a neutral zinc porphyrin. Effects of CTAB micelles on complex stability and photoinduced electron transfer, J. Phys. Chem., 1985, 89, 5685–5689 CrossRef CAS
.
- P. J. Muller and B. C. Wilson, Photodynamic therapy of brain tumors - A work in progress, Lasers Surg. Med., 2006, 38, 384–389 CrossRef
.
- B. W. Henderson and T. Dougherty, How does photodynamic therapy work, Photochem. Photobiol., 1992, 55, 145–157 CrossRef CAS
.
- T. J. Dougherty, M. T. Cooper and T. S. Mang, Cutaneous phototoxic occurrences in patients receiving photofrin, Lasers Surg. Med., 1990, 10, 485–488 CAS
.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic therapy, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS
.
- D. A. Bellnier, Y. K. Ho, R. K. Pandey, J. R. Missert and T. Dougherty, Distribution and Elimination of Photofrin-Ii in Mice, Photochem. Photobiol., 1989, 50, 221–228 CrossRef CAS
.
- C. E. Giacomelli and W. Norde, Influence of hydrophobic Teflon particles on the structure of amyloid beta-peptide, Biomacromolecules, 2003, 4, 1719–1726 CrossRef CAS
.
- T. Vermonden, C. E. Giacomelli and W. Norde, Reversibility of structural rearrangements in bovine serum albumin during homomolecular exchange from AgI particles, Langmuir, 2001, 17, 3734–3740 CrossRef CAS
.
- M. Groenning, Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils—current status, J. Chem. Biol., 2009, 3, 1–18 Search PubMed
.
-
W. L. DeLano, The PyMOL User's Manual (Delano Scientific, San Carlos, California, 2002) Search PubMed
.
- Nanorex Inc., http://nanoengineer-1.com/content/.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0nr00782j |
| This journal is © The Royal Society of Chemistry 2011 |
