Organic solvent-induced controllable crystallization of the inorganic salt Na3[Au(SO3)2] into ultralong nanobelts and hierarchical microstructures of nanowires†
Sen
Liu
a,
Jingqi
Tian
ab,
Lei
Wang
a,
Hailong
Li
ab and
Xuping
Sun
*a
aState Key Lab of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, Jilin, China. E-mail: sunxp@ciac.jl.cn; Fax: +0086-431-85262065; Tel: +0086-431-85262065
bGraduate School of the Chinese Academy of Sciences, Beijing, 100039, China
First published on 31st January 2011
Abstract
The present paper reports an organic solvent-induced controllable crystallization of a water-soluble inorganic salt Na3[Au(SO3)2] into ultralong nanobelts and hierarchical microstructures of one-dimensional (1D) nanowires. It was found that the morphology of the resulting crystals can be fine tuned by simply varying the experimental parameters, such as the ratios of water to organic solvent and gold salt to organic solvent, as well as the type of organic solvent.
In recent years, hierarchical microstructures have attracted significant attention because of their unique properties and potential applications in electronics, catalysis, electrochemistry, biology and nanotechnology.1–5 It is well known that the practical applications of hierarchical microstructures are strongly dependent on their size, shape and dimensionality6,7 and considerable attention has been paid on preparing hierarchical microstructures with various compositions such as metal, metal oxide, DNAetc.8–10 Indeed, numerous synthetic strategies have been developed.11–13 Among them, self-assembly of nanostrutures has proven to be a most promising route and has become a hot topic of scientific and technological importance.14–16 Up to now, numerous nanostructure materials such as nanoparticles, nanorods and nanobelts have been successfully assembled into two-dimensional (2D) or three-dimensional (3D) microstructures.17–19 However, such microstructures are usually prepared with the aid of soft or hard templates such as organic surfactants, polymers, and solid SiO2etc.20–25 Unfortunately, the conventional template approach always requires complex processes, such as preparation of the templates first, a post-synthesis process to remove the templates by calcination or by washing with some environmentally unfriendly solvents, which limit their applications.26,27 In addition, an extra step of synthesizing nanocomposite precursors as building blocks is also involved.28,29 To solve these problems, a “synthesis and self-assembly” strategy was developed where 1D nanostructures simultaneously assemble into hierarchical microstructures during the synthesis procedure in hydrothermal or solvothermal processes.30–35 However, such a strategy still has some drawbacks, such as the requirement of surfactants and the involvement of relatively complex experimental procedures.24 Therefore, the development of a simple and controllable construction of hierarchical microstructures from 1D nanostructures by a self-assembly technique is highly desirable. More recently, we have found that ultralong nanobelts of the water-soluble inorganic salt of Na3Au(SO3)2 can be formed via organic solvent-induced crystallization.36 In this paper, we further demonstrate our recent finding that Na3Au(SO3)2 can be crystallized to produce ultralong nanobelts and hierarchical microstructures of 1D nanowires in a controllable manner by simply varying the experimental parameters, such as the ratios of water to organic solvent and gold salt to organic solvent, as well as the type of organic solvent.
Commercial gold bath (Oromerse Part B, 0.28 M) was supplied by Technic Inc. Organic solvents, including N,N-dimethylformamide (DMF), ethanol, and isopropyl alcohol (IPA) were bought from Aldrich and used as received. The inorganic salt nanobelts and its hierarchical microstructures were prepared by adding the gold bath into various solvents or solvent mixtures. In a typical synthesis of Na[Au(SO3)2] nanobelts (sample 1), 5 μL gold bath was diluted to 300 μL by H2O, followed by adding the resulting solution into 700 μL DMF at room temperature under stirring. After that, a large quantity of precipitate was observed within several minutes. The precipitate was then collected by centrifugation and further washed with IPA several times. The precipitate thus obtained was dispersed in IPA for future use. For synthesis of hierarchical microstructures (sample 2), 40 μL gold bath was introduced into 960 μL ethanol at room temperature under stirring. Optical microscopy images were taken with a LEICA DM4000 M microscope. Scanning electron microscopy (SEM) measurements were made on a HITACHI S-3400N microscope, operated at an accelerating voltage of 10 kV. Samples for SEM examination were made by placing a drop of the dispersion of the precipitate in IPA on a glass slide and air-dried at room temperature. Powder X-ray diffraction (XRD) data were recorded on a Rigaku D/MAX 2550 diffractometer with Cu-Kα radiation (λ = 1.5418 Å).
Fig. 1A shows the optical microscopy image of the precipitate of sample 1, indicating that the precipitate consists of a large amount of ultralong 1D structures about several hundred micrometres in length. A low magnification SEM image shown in Fig. 1B further confirms the formation of 1D structures. A higher SEM image reveals that such 1D structures are uniform nanobelts with widths ranging from 300 to 500 nm, as shown in Fig. 1C. Such observations are quite consistent with our previous results.36 It is very surprising to have found that the precipitate of sample 2 exclusively consists of microspheres with sizes ranging from 5 to 50 μm, which is shown by optical microscopy observations in Fig. 1D. A further SEM examination of such microspheres reveals it is hierarchical in nature and is assembled from small nanowires, as shown in Fig. 1E and Fig. 1F. The chemical composition of these structures was examined by energy-dispersed spectroscopy. Figure S1 shows the corresponding energy-dispersed spectrum (EDS) of the nanobelts from sample 1.† The observation of several peaks corresponding to Au, S, O, and Na elements (the peak of Si originated from the glass substrate used) indicates that these nanobelts are formed from gold bath salt. The crystal nature of these nanobelts was further confirmed by a bulk diffraction technique. Figure S2 presents the corresponding XRD pattern which shows several peaks with the strongest one occurring at 10.8°.† Because of a lack of standard or referenced XRD data on these 1D structures, we cannot provide detailed information on the crystal nature at the present time.
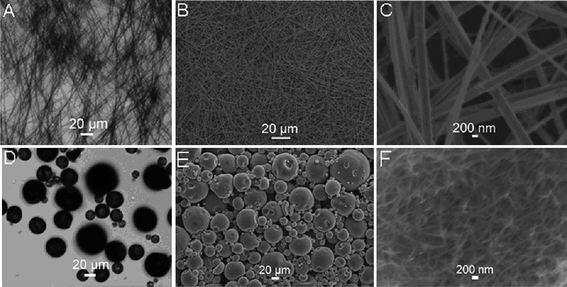 | ||
| Fig. 1 Optical microscopy images of the precipitate of sample 1 (A) and sample 2 (D), and low magnification SEM images of sample 1 (B) and sample 2 (E). C and F show the corresponding high magnification SEM images. | ||
Very interestingly, it is found that various hierarchical microstructures can be obtained by simply varying the ratios of H2O to DMF in the mixture. Fig. 2 shows SEM images of samples prepared by adding 40 μL gold bath into mixtures containing various amounts of H2O at room temperature. Notably, micrometre-scale spheres were obtained in the presence of 60 μL H2O in the mixture, as shown in Fig. 2A. The corresponding high magnification image (inset) indicates that such microsphere are assembled from 1D nanofibers with diameters ranging from 100 to 200 nm. Increasing the amount of H2O up to 235 μL also produces microspheres (Fig. 2B). Again, a local view of such microsphere reveals it is assembled from nanowires with diameters ranging from 200 to 300 nm. A further increase of H2O to 285 μL results in the formation of a large amount of nanobelts as the main product and some microspheres (Fig. 2C). A local view of such nanobelts reveal they are about 500 nm in width (inset). It should be pointed out that temperature has a significant effect on the crystallization of Na3[Au(SO3)2]. Fig. 2D shows the SEM image of the products obtained by adding 40 μL gold bath into the DMF–H2O mixture containing 60 μL H2O at 4 °C. It is clearly seen that flower-shaped hierarchical structures of nanowires are formed. The high magnification image (inset) shows that the nanowires are about 200 nm in diameter. These results suggest that hierarchical microstructures constructed by nanowires can be obtained by simply varying the content of H2O in the mixture as well as changing the reaction temperature, which can be attributed to the fact that the solubility of the inorganic salts in the mixture changed with a varied ratio of H2O to DMF and the synthesis temperature used.
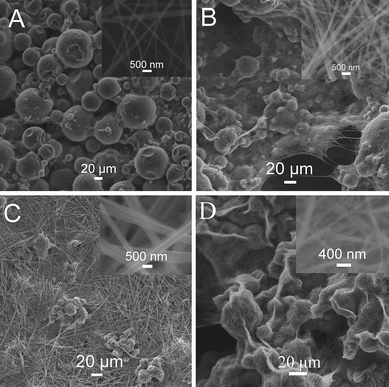 | ||
| Fig. 2 SEM images of products obtained by adding 40 μL gold bath into the DMF–H2O mixture containing (A) 60, (B) 235, and (C) 285 μL H2O at room temperature; D shows the SEM image of the products obtained by adding 40 μL gold bath into the DMF–H2O mixture containing 60 μL H2O at 4 °C (the total volume of the mixture solution for each preparation is 1 mL). | ||
We also examined the influence of the amount of gold bath on the formation of Na3[Au(SO3)2] crystals. Fig. 3 shows the SEM images of the products formed by adding (A) 5, (B) 10, (C) 20, (D) 40, (E) 100, and (F) 400 μL gold bath into DMF, where the total volume of the mixture solution is 1 mL. It is clearly seen that microspheres were formed when small amounts of gold bath are added into DMF. For example, when 5 μL gold bath was introduced into DMF, microspheres with sizes about 20–50 μm are formed (Fig. 3A). The corresponding high magnification image (inset) shows that the microsphere consists of nanowires about 400 nm in diameter. Additionally, by further increasing of the amount of gold bath up to 10, 20, and 40 μL, we still observed the formation of hierarchical microspheres (Fig. 3B–D). It should be pointed out that the size of the microspheres and the diameter of the nanowires gradually increase with the increased amount of gold bath. However, a further increasing of the amount of gold bath up to 100 μL results in the formation of microspheres and nanobelts, as shown in Fig. 3E. The use of 400 μL gold salt only gives nanobelts (Fig. 3F). These observations indicate that the amount of gold bath has heavy influence on the formation of hierarchical microstructures.
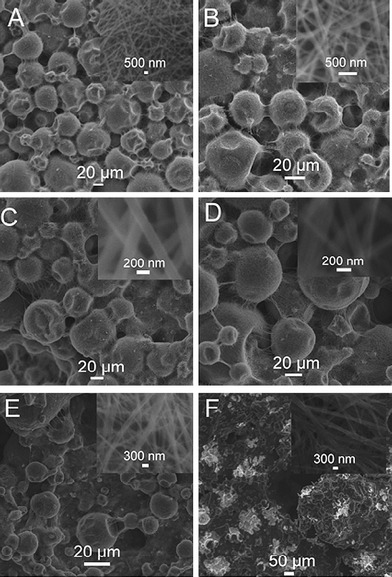 | ||
| Fig. 3 SEM images of the products formed by adding (A) 5, (B) 10, (C) 20, (D) 40, (E) 100, and (F) 400 μL gold bath into DMF, where the total volume of the mixture solution is 1 mL. Inset: a local view of the structures. | ||
It is found that various hierarchical microstructures can also be obtained by using ethanol as solvent. Fig. 4 shows the SEM images of the products formed by adding (A) 5, (B) 10, (C) 20, and (D) 40 μL gold bath into ethanol, where the total volume of the mixture solution is 1 mL. It is obviously seen that microspheres were formed by adding various amounts of gold bath into ethanol. For instance, when 5 μL gold bath was added into ethanol, microspheres with sizes in the range of 5–20 μm assembled from nanowires are formed (Fig. 4A). The corresponding high magnification image (insert) shows the nanowires have a diameter of 100 nm and a length of about 1 μm. Additionally, by increasing the amount of gold bath up to 40 μL, microspheres were still obtained, which are constructed by nanowires (Fig. 4B-D). It is also found that the size of the microspheres and the diameter of the nanowires gradually increase with an increase in the amount of gold bath (inset). These observations demonstrate that the use of a weak polar solvent can also lead to hierarchical microstructures assembled from nanowires.
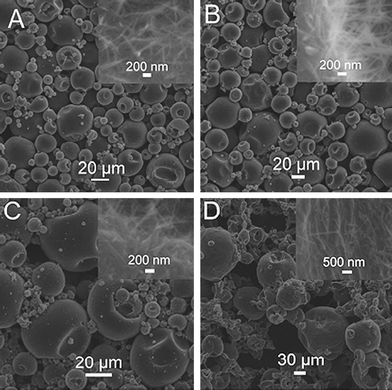 | ||
| Fig. 4 SEM images of the products formed by adding (A) 5, (B) 10, (C) 20, and (D) 40 μL gold bath into ethanol, where the total volume of the mixture solution is 1 mL. | ||
We also investigated using IPA as the solvent to synthesize Na3[Au(SO3)2] crystals. Fig. 5 shows the images of the products formed by adding (A) 5, (10), (C) 20 and (D) 40 μL gold bath into IPA, where the total volume of the mixture solution is 1 mL. It was found that hierarchical microstructures were also obtained by adding various amounts of gold bath into IPA. Clearly, microspheres with sizes of 10–30 μm assembled from nanowires are formed by adding 5 μL gold bath into IPA (Fig. 5A), and the corresponding high magnification image (inset) shows that these nanowires have a diameter of 200 nm. By varying the amount of gold bath from 5 to 40 μL, microspheres assembled from nanowires are still obtained (Fig. 5B-D), while the diameters of nanowires gradually changed (inset). Very interestingly, the use of 40 μL gold bath produces echinus-like microspheres with numerous nanowires extended out of the surface (Fig. 5D). A local view of such nanowires reveals they are about 200 nm in diameter (inset). As far as we known, this is the first example demonstrating the formation of echinus-like hierarchical microstructures of inorganic salt crystals via an organic solvent-induced crystallization route.
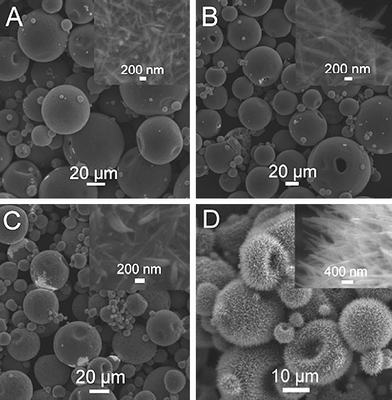 | ||
| Fig. 5 SEM images of the products formed by adding (A) 5, (B) 10, (C) 20, and (D) 40 μL gold bath into IPA, where the total volume of the mixture solution is 1 mL. | ||
As we know, a solvent-induced crystallization route is very effective for synthesizing 1D inorganic nanostructures and has also been widely used for the fabrication of 1D organic nanostructures.37–40 In the present study, there are two major factors for morphology controllable synthesis of Na3[Au(SO3)2] crystals: the ion concentration of gold salt and the polarity of the solvent used. Well-separated nanobelts are obtained with a low ion concentration of gold salt in a strong polar solvent (sample 1), where the polarity values of H2O and DMF are 10.2 and 6.4, respectively. Hierarchical microspheres assembled from nanowires are also formed by adding a large amount of gold bath into a strong polar solvent. In contrast, hierarchical microspheres assembled from nanowires are formed with various concentrations of gold salt in a weak polar solvent such as ethanol and IPA (the polarity values for both are 4.3). Scheme 1 presents a schematic to illustrate the organic solvent-induced controllable crystallization of Na3[Au(SO3)2] into ultralong microbelts and hierarchical microstructures of nanowires.
![A scheme to illustrate the organic solvent-induced controllable crystallization of Na3[Au(SO3)2] into ultralong microbelts and hierarchical microstructures of nanowires.](/image/article/2011/NR/c0nr00690d/c0nr00690d-s1.gif) | ||
| Scheme 1 A scheme to illustrate the organic solvent-induced controllable crystallization of Na3[Au(SO3)2] into ultralong microbelts and hierarchical microstructures of nanowires. | ||
In summary, organic solvent-induced controllable crystallization of water-soluble inorganic salt Na3[Au(SO3)2] into 1D nanobelts and hierarchical microstructures of nanowires has been successfully demonstrated for the first time. The synthesised structures may hold great promise as easily decomposable templates for the fabrication of tubular structures and hierarchical microstructures of other materials for a variety of applications.
Acknowledgements
This work was supported by National Basic Research Program of China (No. 2011CB935800).References
- Z. Y. Yuan, T. Z. Ren, A. Azioune, J. J. Pireaux and B. L. Su, Chem. Mater., 2006, 18, 1753 CrossRef CAS.
- G. J.de A. Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093 CrossRef.
- A. Corma, P. Atienzar, H. Garcia and J.-Y. Chane-Ching, Nat. Mater., 2004, 3, 394 CrossRef CAS.
- K. Valle, P. Belleville, F. Pereira and C. Sanchez, Nat. Mater., 2006, 5, 107 CrossRef CAS.
- F.-S. Xiao, L. Wang, C. Yin, K. Lin, Y. Di, J. Li, R. Xu, D. S. Su, R. Schlögl, T. Yokoi and T. Tatsumi, Angew. Chem., Int. Ed., 2006, 45, 3090 CrossRef CAS.
- Y. Xia, Y. Yin, Y. Lu and J. McLellan, Adv. Funct. Mater., 2003, 13, 907 CrossRef CAS.
- J. Hu, T. W. Odom and C. M. Lieber, Acc. Chem. Res., 1999, 32, 435 CrossRef CAS.
- S. Guo, L. Wang and E. Wang, Chem. Commun., 2007, 3163 RSC.
- M. Yu, H. Wang, X. Zhou, P. Yuan and C. Yu, J. Am. Chem. Soc., 2007, 129, 14576 CrossRef CAS.
- Y. He, T. Ye, M. Su, C. Zhang, A. E. Ribbe, W. Jiang and C. Mao, Nature, 2008, 452, 198 CrossRef CAS.
- J. H. Moon and S. Yang, Chem. Rev., 2010, 100, 547 CrossRef.
- X. Sun and M. Hanger, Langmuir, 2007, 23, 9147 CrossRef CAS.
- X. Zhang, Z. Ai, F. Jia and L. Zhang, J. Phys. Chem. C, 2008, 112, 747 CrossRef CAS.
- H. Zhang, Q. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766 CrossRef CAS.
- J.-S. Hu, L.-L. Ren, Y.-G. Guo, H.-P. Liang, A.-M. Cao, L.-J. Wan and C.-L. Bai, Angew. Chem., Int. Ed., 2005, 44, 1269 CrossRef CAS.
- M. Yada, C. Taniguchi, T. Torikai, T. Watari, S. Furuta and H. Katsuki, Adv. Mater., 2004, 16, 1448 CrossRef CAS.
- N. Du, H. Zhang, B. Chen, X. Ma, Z. Liu, J. Wu and D. Yang, Adv. Mater., 2007, 19, 1641 CrossRef CAS.
- R. Fan, Y. Wu, D. Li, M. Yue, A. Majumdar and P. Yang, J. Am. Chem. Soc., 2003, 125, 5254 CrossRef CAS.
- H. Ogohara, M. Sadakane, Y. Nodasaka and W. Ueda, Chem. Mater., 2006, 18, 4981 CrossRef CAS.
- W.-C. Li, A.-H. Lu, R. Palkovits, W. Schmidt, B. Spliethoff and F. Schüth, J. Am. Chem. Soc., 2005, 127, 12595 CrossRef CAS.
- D. Kuang, T. Brezesinski and B. Smarsly, J. Am. Chem. Soc., 2004, 126, 10534 CrossRef CAS.
- W. A. Lopes and H. M. Jaeger, Nature, 2001, 414, 735 CrossRef CAS.
- F. Carn, A. Colin, M.-F. Achard, H. Deleuze, E. Sellier, M. Birot and R. Backov, J. Mater. Chem., 2004, 14, 1370 RSC.
- A. Fisxher, Y.-S. Jun, A. Thomas and M. Antonnietti, Chem. Mater., 2008, 20, 7383 CrossRef CAS.
- P. Bai, P. Wu, Z. Yan, J. Zhou and X. S. Zhao, J. Phys. Chem. C, 2007, 111, 9729 CrossRef CAS.
- X. Wu, Y. Tian, Y. Cui, L. Wei, Q. Wang and Y. Chen, J. Phys. Chem. C, 2007, 111, 9704 CrossRef CAS.
- S. Dai, M. C. Burleigh, Y. H. Ju, H. J. Gao, J. S. Lin, S. J. Pennycook, C. E. Barnes and Z. L. Xue, J. Am. Chem. Soc., 2000, 122, 992 CrossRef CAS.
- J. Polleux, J. Ba, M. Antonitti and M. Niederberger, Adv. Mater., 2005, 17, 2509 CrossRef CAS.
- M. Kim, G. H. Jeong, K. Y. Lee, K. Kwon and S. W. Han, J. Mater. Chem., 2008, 18, 2208 RSC.
- C.-H. Kuo and M. H. Huang, J. Am. Chem. Soc., 2008, 130, 12815 CrossRef CAS.
- J. Zhu and R. C. Hayward, Angew. Chem., Int. Ed., 2008, 47, 2113 CrossRef CAS.
- P.-P. Wang, B. Bai, S. Hu, J. Zhuang and X. Wang, J. Am. Chem. Soc., 2009, 131, 16953 CrossRef CAS.
- Y. Li, J. Liu, X. Huang and G. Li, Cryst. Growth Des., 2007, 7, 1350 CrossRef CAS.
- J. Yu, L. Zhang, B. Cheng and Y. Su, J. Phys. Chem. C, 2007, 111, 10582 CrossRef CAS.
- Z. Zhuang, Q. Peng, B. Zhang and Y. Li, J. Am. Chem. Soc., 2008, 130, 10482 CrossRef CAS.
- X. Sun and M. Hagner, Chem. Mater., 2008, 20, 2869 CrossRef CAS.
- H. Ouyang, W.-H. Lee, W. Ouyang, S.-T. Shiue and T.-M. Wu, Macromolecules, 2004, 37, 7719 CrossRef CAS.
- W. Huang, C. Luo, B. Li and Y. Han, Macromolecules, 2006, 39, 8075 CrossRef CAS.
- B. Ray, S. Elhasri, A. Thierry, P. Marie and J.-M. Guenet, Macromolecules, 2002, 35, 9730 CrossRef CAS.
- W. R. Moore and R. P. Sheldon, Polymer, 1961, 2, 315 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: EDS and XRD analysis of nanobelts. See DOI: 10.1039/c0nr00690d |
| This journal is © The Royal Society of Chemistry 2011 |
