Isolation and analysis of ginseng: advances and challenges
Lian-Wen
Qi
*ab,
Chong-Zhi
Wang
a and
Chun-Su
Yuan
*a
aTang Center for Herbal Medicine Research and Department of Anesthesia & Critical Care, The Pritzker School of Medicine, The University of Chicago, 5841 South Maryland Avenue, Chicago, Illinois 60637, USA. E-mail: cyuan@dacc.uchicago.edu
bKey Laboratory of Modern Chinese Medicines (China Pharmaceutical University), Ministry of Education, Nanjing, 210009, China. E-mail: fleude@126.com
First published on 24th January 2011
Abstract
Covering: January 2000 to September 2010
Ginseng occupies a prominent position in the list of best-selling natural products in the world. Because of its complex constituents, multidisciplinary techniques are needed to validate the analytical methods that support ginseng's use worldwide. In the past decade, rapid development of technology has advanced many aspects of ginseng research. The aim of this review is to illustrate the recent advances in the isolation and analysis of ginseng, and to highlight new applications and challenges. Emphasis is placed on recent trends and emerging techniques.
 Lian-Wen Qi | Lian-Wen Qi is a research scholar in the Tang Center for Herbal Medicine Research at the University of Chicago. He obtained his Ph.D. in Pharmacognosy at China Pharmaceutical University. Since 2009, using multi-disciplinary approaches, he has been investigating plant fingerprinting and endogenous metabonomics for the quality control of different natural products, and characterizing the response of biological systems to a number of botanical components. Dr Qi's research interests include new technology development in phytochemistry, discovery of novel bioactive lead components, and their potential clinical applications. He has published over 50 peer-reviewed journal research papers, reviews and book chapters. |
 Chong-Zhi Wang | Chong-Zhi Wang is a Research Assistant Professor in the Department of Anesthesia and Critical Care, and serves as Associate Director of the Tang Center for Herbal Medicine Research and as Botanical Core Co-Leader of a NIH/NCCAM center project at the University of Chicago. He obtained his Ph.D. from China Pharmaceutical University and finished his postdoctoral training at the University of Chicago. Dr Wang's current work, in part supported by his NIH K01 grant, focuses on the identification of active botanical constituents and the evaluation of their pharmacological activities. He has published 73 peer-reviewed journal research articles, reviews and book chapters. |
 Chun-Su Yuan | Chun-Su Yuan is the Cyrus Tang Professor in the Department of Anesthesia & Critical Care, and Director of the Tang Center for Herbal Medicine Research, University of Chicago. Since 1994, he has conducted clinical trials in evaluating the safety and efficacy of a novel compound, methylnaltrexone, approved in 2008 for opioid-induced constipation in advanced-illness patients. Dr Yuan also has a deep interest in botanical research, and has published over 200 articles. In addition, he currently serves as the Editor-in-Chief of the American Journal of Chinese Medicine. |
1 Introduction
Plants have been the basis of traditional medicines for thousands of years, and continue to be considered valuable materials in medicines. Natural products isolated from plants provide an unparalleled source of chemical diversity for discovery of biologically active molecules. For various medical conditions, especially chronic diseases, use of medicinal plants has expanded globally and gained considerable popularity.1 Among these plants, ginseng has a long history and is today one of the world's most widely used medicinal plants. Ginseng products are commercially available in roots, tablets and capsules, liquid extracts, carbonated drinks and teas.2The name ginseng is derived from a Chinese term referring to the “man-like” shape of the root. The genus is Panax, meaning “cure all” in Greek. Panax ginseng C. A. Meyer (commonly referred to as Asian, Chinese or Korean ginseng) and Panax quinquefolius L. (also known as American or North American ginseng) are the two most recognized species around the world. Another is Panax notoginseng (Burk.) F. H. Chen. Other less known species in the ginseng genus include Panax japonicus C.A. Meyer (Japanese ginseng) and Panax vietnamensis Ha et Grushv. (Vietnamese ginseng). Traditionally the ginseng root, available in white or red form, is used. White ginseng is prepared by air-drying after harvest, and red ginseng is prepared by a steaming or heating process.3,4 The leaf, berry and other parts of ginseng are also medicinal sources.5–7
Ginseng contains complex constituents. Thus, multidisciplinary methods are required to validate the analytical methods that support ginseng's use worldwide. In the past decade, advanced technologies for isolation and analysis of plants have revolutionized ginseng research. The literature describes investigation of ginseng with pharmacognosy, phytochemistry, biosynthesis, and pharmacology.8–13 The aim of this review is to illustrate the recent advances in the isolation and analysis of ginseng, and to highlight their new applications, especially in species authentication, quality assessment, and interaction with biological systems. This review covers the literature between January 2000 and September 2010, and is organized into three major sections: (1) preparation and isolation; (2) analytical advances; and (3) applications and challenges. Recent trends and emerging techniques are emphasized rather than routine methods. Information related to biosynthesis, pharmacology and clinical aspects is beyond the scope of this article.
2 Preparation and isolation
The first challenge in isolating and analyzing ginseng is the complexity of the sample matrices. The construction and maintenance of high-quality chemical libraries in ginseng is needed, calling for state-of-the-art procedures for separation and isolation. Recent developments in this field include automated and solvent-free extraction techniques and more efficient isolation methods.2.1 Sample extraction and preparation techniques
Efficient sample preparation can improve extraction efficiency and enrich the target analytes. Ginseng saponins have been extracted from ginseng with different solvents and methods. The conventional method uses heat-reflux,14 Soxhlet,15 shaking16 or ultrasound-assisted extraction (UAE).17 Soxhlet extraction at 80–90 °C for 20–24 h efficiently extracts saponins.15 Modern ultra-pressure or ultra-temperature extraction methods have been applied such as pressurized liquid extraction (PLE),18 microwave-assisted extraction (MAE),19 high-pressure MAE,20 pressurized hot water extraction (PHWE)21 and supercritical fluid extraction (SFE).15 Compared with conventional methods, newer methods use less solvent, are easily automated, take a short time and are more efficient.22Table 1 lists the characteristics of nine different extraction techniques. Each has advantages and limitations depending on the projected use of results.| Techniquea | Core technology | Extraction efficiency | Operation | Speed | Automation | Cost | Ref. |
|---|---|---|---|---|---|---|---|
| a Abbreviations: HRE: heat reflux extraction; SAE: shaking-assisted extraction; UAE: ultrasound-assisted extraction; PLE: pressurized liquid extraction; MAE: microwave-assisted extraction; HP-MAE: high-pressure MAE; PHWE: pressurized hot- water extraction; SFE: supercritical fluid extraction. | |||||||
| HRE | Heating | Low | Simple | Low | Difficult | Very low | 14 |
| Soxhlet | Soxhlet extractor | High | Moderate | Very low | Possible | Low | 15 |
| SAE | Mechanical shaking | High | Simple | Moderate | Difficult | Low | 16 |
| UAE | Ultrasound | Low | Simple | Low | Difficult | Low | 17 |
| PLE | High pressure | Moderate | Complicated | Rapid | Easy | Moderate | 18 |
| MAE | Microwave | Moderate | Simple | Rapid | Difficult | Low | 19 |
| HP-MAE | High pressure and microwave | Moderate | Simple | Rapid | Difficult | Low | 20 |
| PHWE | High pressure and hot water | Moderate | Complicated | Moderate | Easy | Moderate | 21 |
| SFE | Supercritical fluid | Moderate | Complicated | Rapid | Easy | High | 15 |
Plant material is considered first. Small sample particles have larger specific external areas, stronger solvent penetration, and thus higher extraction yield.23 Extraction solvent and extraction volume are key in obtaining high recovery.24 Among various solvent systems, 100% methanol has better extraction efficiency than water or 70% aqueous methanol extraction;25 50% ethanol has higher efficiency than an ethanol–glycerin–water mixture or 65% glycerin.26 Adding non-ionic surfactants like Triton X-100 to solvent enhances the extraction yield.27 Various extraction methods have been compared quantitatively.18,23,28 Extraction conditions affect results, and the best conditions for each technique should be optimized in comparison. For instance, efficiency differs between Soxhlet extraction for 2 h23 and 20 h.15 Extraction efficiency also depends on the ginsenoside. Since degradation is possible during the extraction process, temperature is a factor to be considered.29
2.2 Isolation and purification methods
A high-quality chemical library of ginseng saponins is important for high-throughput screening, research into the structure–activity relationship, and investigation of the mechanisms of this group of compounds. Because ginseng has many constituents, the isolation process can be time-consuming and tedious. New technologies are therefore welcome.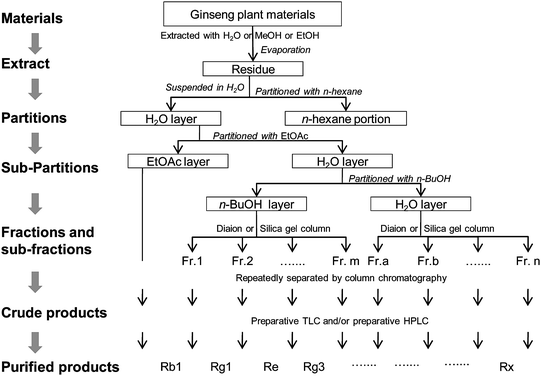 | ||
| Fig. 1 Procedures for isolation of ginseng saponins from ginseng plant materials. | ||
| Material | Solvent systema (volume ratio) | Detectionb | Obtained compound | Isolation efficiency c | Ref. |
|---|---|---|---|---|---|
| a Abbreviations: Hex: n-hexane; BuOH: butanol; CH2Cl2: dichloromethane; MeOH: methanol; NH4OAc: ammonium acetate; iPrOH: isopropanol; CHCl3: chloroform; EtOAc: ethyl acetate. b Abbreviations: TLC: thin-layer chromatography; ELSD: evaporative light scattering detection; UV: ultraviolet. c Abbreviations: RP: reversed-phase; MPLC: medium-pressure liquid chromatography. | |||||
| P. notoginseng, root | Hex–n-BuOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
TLC | Ginsenosides Rb1, Re, Rg1 and notoginsenoside R1 | 157, 13, 56, and 17 mg of Rb1, Re, Rg1 and R1 from 283 mg MeOH extract of five tablets | 38 |
| P. ginseng, root | CH2Cl2–MeOH–NH4OAc–iPrOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
ELSD | Ginsenosides Rf, Rd, Re, and Rb1 | 10.7, 11.0, 13.4 and 13.9 mg of Rf, Rd, Re and Rb1 from 480 mg enriched fraction by macroporous resin | 39 |
| P. notoginseng, root | CHCl3–MeOH–2-BuOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), EtOAc–nBuOH–H2O (1 4), EtOAc–nBuOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
ELSD | Ginsenosides Rg1, Rd, Re, Rb1 and notoginsenoside R1 | Not provided | 40 |
| Red P. ginseng, steamed root | CH2Cl2–MeOH–H2O–iPrOH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
ELSD | Ginsenosides Rg3, Rk1, Rg5 and F4 | 32.2, 26.6, 28.6 and 8.1 mg of Rg3, Rk1, Rg5 and F4 from 350 mg enriched fraction by RP-C18 column | 41 |
| P. ginseng, root | EtOAc–iPrOH–0.1% formic acid in H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
UV | Ginsenoside Ro | 61 mg Ro from 100 mg enriched sample by normal-phase MPLC | 42 |
CPC is also a liquid–liquid partition chromatography without sorbent working in a constant-gravity field. Ginsenosides Rc, Rb1, and Re were isolated from P. quinquefolius by CPC using a two-phase solvent system of ethyl acetate–n-butanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).43 Both CPC and HSCCC require the technician to be familiar with the experimental conditions and the practical separation procedure, which are unique.44
2).43 Both CPC and HSCCC require the technician to be familiar with the experimental conditions and the practical separation procedure, which are unique.44
Ginseng saponins are divided into several groups. Two major groups are the protopanaxadiol (PPD)-type saponins with sugar moieties attached to the C-3 and/or C-20, and the protopanaxatriol (PPT) group with sugar moieties at C-6 and/or at C-20. Other groups include the ocotillol-type with a five-membered epoxy ring at C-20, the oleanane-type with a nonsteroidal structure, and the dammarane type with a modified C-20 side chain.9,56 As techniques are developed for chemical purification and structural identification, novel ginseng saponins continue to be discovered. Fig. 2–4 and Table 3 show the structures of 123 new dammarane-type saponins isolated from various parts of Panax plants in the period from January 2000 to September 2010. They are placed in the order of the structure type. Compounds 4 and 91 have different structures, but both have been named ginsenoside Rh5 (Table 3). Apart from ginseng saponins, other novel compounds also have been characterized including polyacetylenes,91–95 polysaccharides,96 phenolic components,97,98 amino acids99 and alkaloids.100
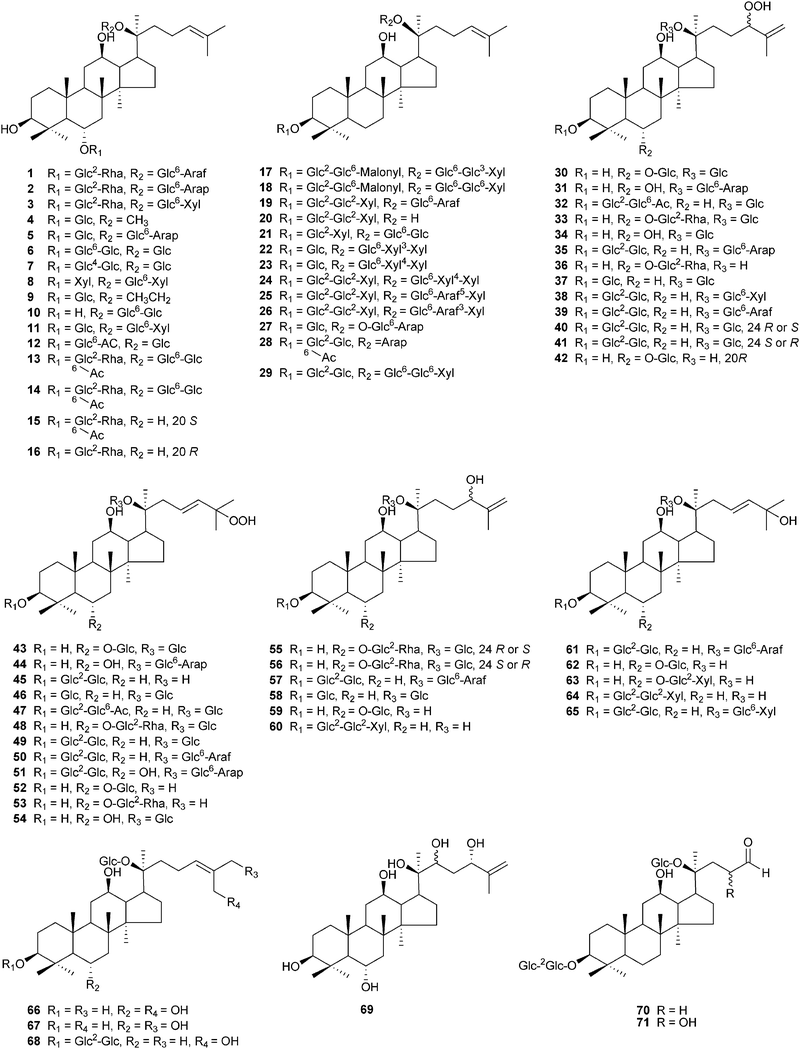 | ||
| Fig. 2 Chemical structures of recently isolated dammarane-type saponins from ginseng, compounds 1–71. | ||
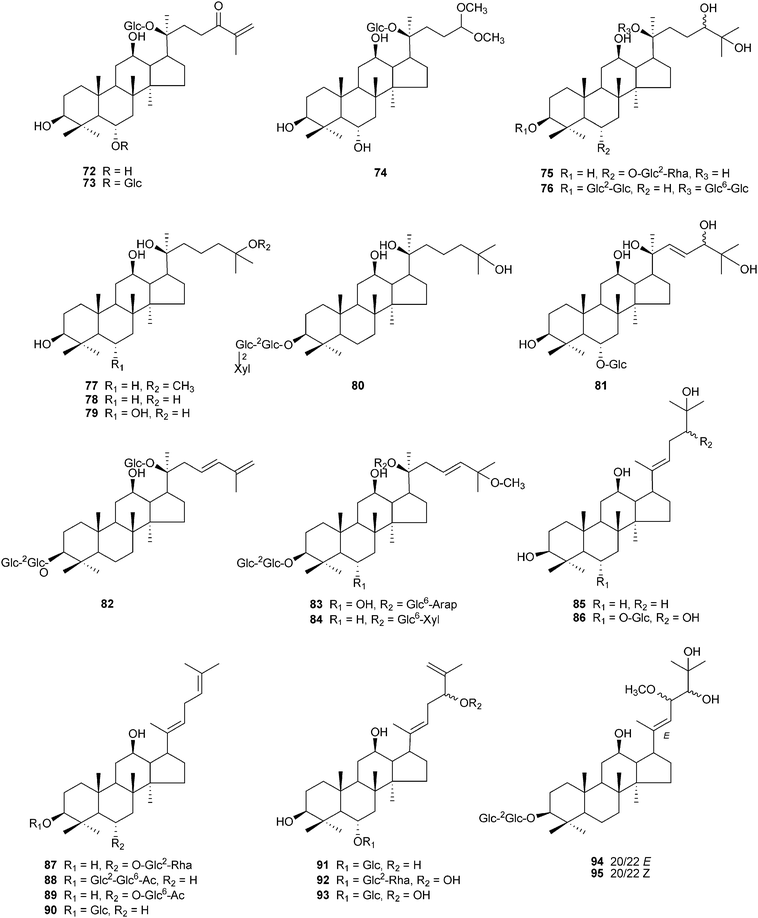 | ||
| Fig. 3 Chemical structures of recently isolated dammarane-type saponins from ginseng, compounds 72–95. | ||
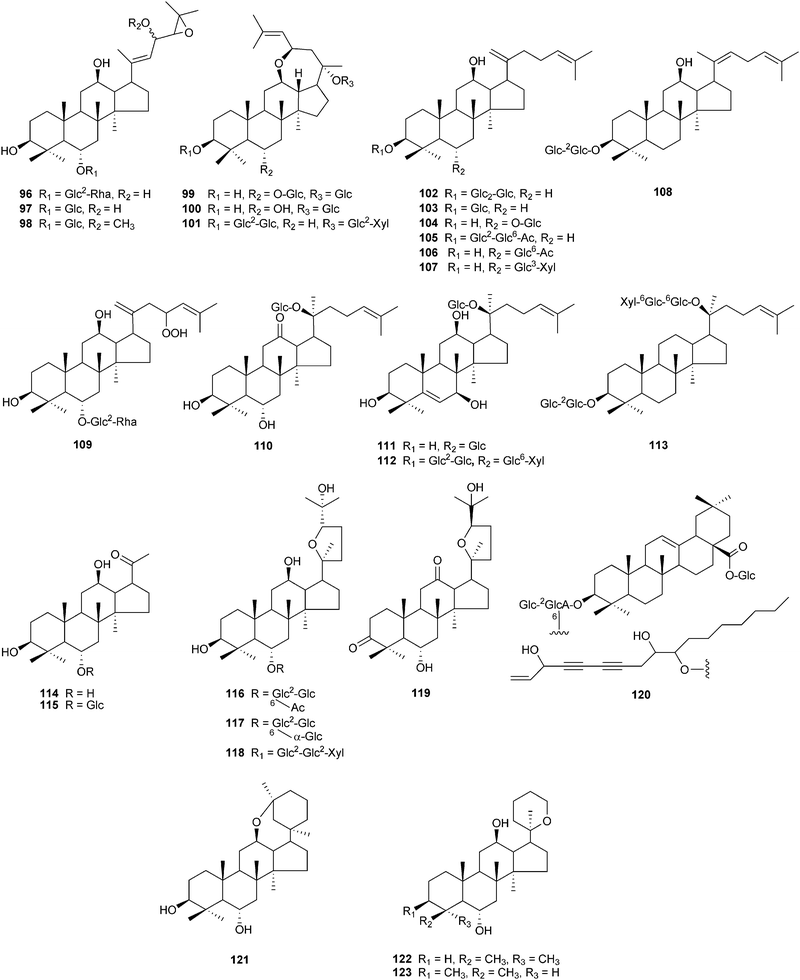 | ||
| Fig. 4 Chemical structures of recently isolated dammarane-type saponins from ginseng, compounds 96–123. | ||
| No. | Name | Formula | Plant Material | Ref. |
|---|---|---|---|---|
| 1 | Floralginsenoside M | C53H90O22 | Flower buds of P. ginseng | 57 |
| 2 | Floralginsenoside N | C53H90O22 | Flower buds of P. ginseng | 57 |
| 3 | Floralquinquenoside E | C53H90O22 | Flower buds of P. quinquefolius | 35 |
| 4 | Ginsenoside Rh5 | C37H64O9 | Roots and rhizomes of P. vietnamensis | 58 |
| 5 | Notoginsenoside FP1 | C47H80O18 | Fruit pedicels of P. notoginseng | 59 |
| 6 | Notoginsenoside M | C48H82O19 | Roots of P. notoginseng | 60 |
| 7 | Notoginsenoside N | C48H82O19 | Roots of P. notoginseng | 60 |
| 8 | Notoginsenoside Rw1 | C46H78O17 | Rhizomes of P. notoginseng | 61 |
| 9 | Notoginsenoside T3 | C38H66O9 | Acid hydrolysate roots of P. notoginseng | 62 |
| 10 | Notoginsenoside U | C42H72O14 | Roots of P. notoginseng | 63 |
| 11 | Quinquenoside L17 | C47H80O18 | Leaves and stems of P. quinquefolius | 5 |
| 12 | Yesanchinoside D | C44H74O15 | Underground part of P. japonicus | 64 |
| 13 | Yesanchinoside E | C54H92O23 | Underground part of P. japonicus | 64 |
| 14 | Yesanchinoside F | C56H94O24 | Underground part of P. japonicus | 64 |
| 15 | 20(S)-acetylated Rg2 | C44H74O14 | Roots of P. quinquefolius | 65 |
| 16 | 20(R)-acetylated Rg2 | C44H74O14 | Roots of P. quinquefolius | 65 |
| 17 | Malonylginsenoside Ra3 | C62H102O30 | Fresh roots of P. ginseng | 66 |
| 18 | Malonylnotoginsenoside R4 | C62H102O30 | Roots of P. ginseng | 67 |
| 19 | Notoginsenoside FP2 | C58H98O26 | Fruit pedicels of P. notoginseng | 59 |
| 20 | Notoginsenoside FT1 | C47H80O17 | Acid hydrolysate roots of P. notoginseng | 68 |
| 21 | Notoginsenoside L | C53H90O22 | Roots of P. notoginseng | 60 |
| 22 | Notoginsenoside O | C52H88O21 | Flower buds of P. notoginseng | 69 |
| 23 | Notoginsenoside P | C52H88O21 | Flower buds of P. notoginseng | 69 |
| 24 | Notoginsenoside Q | C63H106O30 | Flower buds of P. notoginseng | 69 |
| 25 | Notoginsenoside S | C63H106O30 | Flower buds of P. notoginseng | 69 |
| 26 | Notoginsenoside T | C64H108O31 | Flower buds of P. notoginseng | 69 |
| 27 | Quinquenoside L10 | C47H80O17 | Leaves and stems of P. quinquefolius | 46 |
| 28 | Quinquenoside L14 | C47H80O17 | Leaves and stems of P. quinquefolius | 46 |
| 29 | Yesanchinoside J | C61H102O28 | Underground part of P. japonicus | 70 |
| 30 | Floralginsenoside A | C42H72O16 | Flower buds of P. ginseng | 71 |
| 31 | Floralginsenoside C | C41H70O15 | Flower buds of P. ginseng | 71 |
| 32 | Floralginsenoside H | C50H84O21 | Flower buds of P. ginseng | 47 |
| 33 | Floralginsenoside J | C48H82O20 | Flower buds of P. ginseng | 47 |
| 34 | Floralginsenoside Ka | C36H62O11 | Flower buds of P. ginseng | 31 |
| 35 | Floralginsenoside Tc | C53H90O24 | Flower buds of P. ginseng | 30 |
| 36 | Floralquinquenoside B | C42H72O15 | Flower buds of P. quinquefolius | 35 |
| 37 | Floralquinquenoside D | C42H72O15 | Flower buds of P. quinquefolius | 35 |
| 38 | Floranotoginsenoside B | C53H90O24 | Flowers of P. notoginseng | 72 |
| 39 | Floranotoginsenoside C | C53H90O24 | Flowers of P. notoginseng | 72 |
| 40 | Ginsenoside I | C48H82O20 | Flower buds of P. ginseng | 73 |
| 41 | Ginsenoside II | C48H82O20 | Flower buds of P. ginseng | 73 |
| 42 | Ginsenoside SL1 | C36H62O11 | Steamed leaves of P. ginseng | 74 |
| 43 | Floralginsenoside B | C42H72O16 | Flower buds of P. ginseng | 71 |
| 44 | Floralginsenoside D | C41H70O15 | Flower buds of P. ginseng | 71 |
| 45 | Floralginsenoside E | C42H72O15 | Flower buds of P. ginseng | 71 |
| 46 | Floralginsenoside F | C42H72O15 | Flower buds of P. ginseng | 71 |
| 47 | Floralginsenoside G | C50H84O21 | Flower buds of P. ginseng | 47 |
| 48 | Floralginsenoside I | C48H82O20 | Flower buds of P. ginseng | 47 |
| 49 | Floralginsenoside K | C48H82O21 | Flower buds of P. ginseng | 47 |
| 50 | Floralginsenoside O | C53H90O24 | Flower buds of P. ginseng | 57 |
| 51 | Floralginsenoside P | C53H90O23 | Flower buds of P. ginseng | 57 |
| 52 | Floralquinquenoside A | C36H62O11 | Flower buds of P. quinquefolius | 35 |
| 53 | Floralquinquenoside C | C42H72O15 | Flower buds of P. quinquefolius | 35 |
| 54 | Ginsenoside Rh6 | C36H62O11 | Leaves of P. ginseng | 32 |
| 55 | Floralginsenoside La | C48H82O19 | Flower buds of P. ginseng | 47 |
| 56 | Floralginsenoside Lb | C48H82O19 | Flower buds of P. ginseng | 47 |
| 57 | Floranotoginsenoside D | C53H90O23 | Flowers of P. notoginseng | 72 |
| 58 | Ginsenoside Rg7 | C36H60O9 | Leaves of P. ginseng | 32 |
| 59 | Notopanaxoside A | C36H62O10 | Roots of P. notoginseng | 75 |
| 60 | Notoginsenoside FT3 | C47H80O18 | Acid hydrolysate roots of P. notoginseng | 68 |
| 61 | Floranotoginsenoside A | C53H90O23 | Flowers of P. notoginseng | 72 |
| 62 | Ginsenoside ST2 | C36H62O10 | Steamed leaves of P. ginseng | 54 |
| 63 | Notoginsenoside Rw2 | C41H70O14 | Rhizomes of P. notoginseng | 61 |
| 64 | Notoginsenoside ST5 | C47H80O18 | Steamed roots of P. notoginseng | 33 |
| 65 | Yesanchinoside H | C53H90O23 | Underground part of P. japonicus | 70 |
| 66 | Ginsenoside Ki | C36H62O10 | Leaves of P. ginseng | 76 |
| 67 | Ginsenoside Km | C36H62O10 | Leaves of P. ginseng | 76 |
| 68 | Quinquenoside L2 | C48H82O19 | Leaves and stems of P. quinquefolius | 77 |
| 69 | Dammar-25(26)-ene-3,6,12,20,22,24-hexanol | C30H52O6 | Leaves of P. ginseng | 78 |
| 70 | Floralginsenoside Kb | C45H76O19 | Flower buds of P. ginseng | 31 |
| 71 | Floralginsenoside Kc | C45H76O20 | Flower buds of P. ginseng | 31 |
| 72 | Floralginsenoside Ta | C36H60O10 | Flower buds of P. ginseng | 30 |
| 73 | Vina-ginsenoside R25 | C42H70O15 | Roots and rhizomes of P. vietnamensis | 58 |
| 74 | Floralginsenoside Tb | C35H62O11 | Flower buds of P. ginseng | 30 |
| 75 | Quinquenoside L9 | C42H74O15 | Leaves and stems of P. quinquefolius | 79 |
| 76 | Quinquenoside L16 | C54H94O25 | Leaves and stems of P. quinquefolius | 46 |
| 77 | 25-OCH3-PPD | C31H56O4 | Leaves of P. notoginseng | 80 |
| 78 | 25-OH-PPD | C30H54O4 | Fruits of P. ginseng | 48 |
| 79 | 25-OH-PPT | C30H54O5 | Fruits of P. ginseng | 48 |
| 80 | Notoginsenoside FT2 | C47H82O18 | Acid hydrolysate roots of P. notoginseng | 68 |
| 81 | Notoginsenoside T4 | C36H62O11 | Acid hydrolysate roots of P. notoginseng | 62 |
| 82 | Quinquenoside L1 | C48H80O18 | Leaves and stems of P. quinquefolius | 77 |
| 83 | Quinquefoloside La | C54H92O23 | Leaves of P. quinquefolius | 45 |
| 84 | Quinquefoloside Lc | C54H92O23 | Leaves of P. quinquefolius | 81 |
| 85 | Dammar-(E)-20(22)-ene-3,12,25-triol | C30H52O3 | Acid hydrolysate roots of P. ginseng | 82 |
| 86 | Notoginsenoside ST1 | C36H62O10 | Steamed roots of P. notoginseng | 33 |
| 87 | Ginsenoside Rg6 | C42H70O12 | Stem-leaves of P. ginseng | 83 |
| 88 | Ginsenoside Rs4 | C42H70O12 | Steamed roots of P. notoginseng | 84 |
| 89 | Ginsenoside Rs6 | C42H70O12 | Steamed roots of P. notoginseng | 84 |
| 90 | Isoginsenoside Rh3 | C36H60O7 | Fruits of P. ginseng | 49 |
| 91 | Ginsenoside Rh5 | C36H60O9 | Leaves of P. ginseng | 32 |
| 92 | Ginsenoside SL2 | C42H70O14 | Steamed leaves of P. ginseng | 74 |
| 93 | Ginsenoside ST1 | C36H60O10 | Steamed leaves of P. ginseng | 54 |
| 94 | Notoginsenoside ST2 | C43H74O15 | Steamed roots of P. notoginseng | 33 |
| 95 | Notoginsenoside ST3 | C43H74O15 | Steamed roots of P. notoginseng | 33 |
| 96 | Ginsenoside Rg8 | C42H70O12 | Roots of P. quinquefolius | 85 |
| 97 | Notoginsenoside T1 | C36H60O10 | Acid hydrolysate roots of P. notoginseng | 62 |
| 98 | Notoginsenoside T2 | C36H62O10 | Acid hydrolysate roots of P. notoginseng | 62 |
| 99 | 12,23-Epoxyginsenoside Rg1 | C42H70O14 | Leaves of P. ginseng | 86 |
| 100 | Ginsenoside Rh9 | C36H60O9 | Leaves of P. ginseng | 32 |
| 101 | Quinquefoloside-Lb | C53H88O22 | Leaves of P. quinquefolius | 45 |
| 102 | Ginsenoside Rk1 | C42H70O12 | Processed roots of P. ginseng | 36 |
| 103 | Ginsenoside Rk2 | C36H60O7 | Processed roots of P. ginseng | 36 |
| 104 | Ginsenoside Rk3 | C36H60O8 | Processed roots of P. ginseng | 36 |
| 105 | Ginsenoside Rs5 | C38H62O9 | Steamed roots of P. notoginseng | 84 |
| 106 | Ginsenoside Rs7 | C38H62O9 | Steamed roots of P. notoginseng | 84 |
| 107 | Notoginsenoside T5 | C41H68O12 | Acid hydrolysate roots of P. notoginseng | 62 |
| 108 | Ginsenoside Rz1 | C42H70O12 | Steamed roots of P. notoginseng | 53 |
| 109 | Ginsenoside SL3 | C42H70O14 | Steamed leaves of P. ginseng | 74 |
| 110 | Ginsenoside Rh8 | C36H60O9 | Leaves of P. ginseng | 32 |
| 111 | Ginsenoside Rh7 | C36H60O9 | Leaves of P. ginseng | 32 |
| 112 | Yesanchinoside G | C53H88O23 | Underground part of P. japonicus | 70 |
| 113 | Yesanchinoside I | C59H100O26 | Underground part of P. japonicus | 70 |
| 114 | Hexanordammaran | C24H40O4 | Leaves of P. ginseng | 87 |
| 115 | Notoginsenoside R10 | C30H50O9 | Steamed leaves of P. ginseng | 88 |
| 116 | Yesanchinoside A | C44H74O16 | Underground part of P. japonicus | 64 |
| 117 | Yesanchinoside B | C48H82O20 | Underground part of P. japonicus | 64 |
| 118 | Yesanchinoside C | C47H80O19 | Underground part of P. japonicus | 64 |
| 119 | Panaxadione | C30H48O5 | Seeds of P. ginseng | 51 |
| 120 | Polyacetyleneginsenoside Ro | C65H100O21 | Roots of P. ginseng | 89 |
| 121 | Isodehydroprotopanaxatriol | C30H50O3 | Acid hydrolysate roots of P. ginseng | 90 |
| 122 | 20,25-Epoxydammaran-2-en-6,12-diol | C30H50O3 | Acid hydrolysate roots of P. ginseng | 90 |
| 123 | 3-Methyl-28-nordammaran-2-en-6,12-diol | C30H50O3 | Acid hydrolysate roots of P. ginseng | 90 |
2.3 Knockout technologies
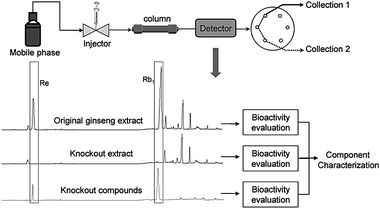
The constituents and functions of ginseng are studied by isolation-to-bioassay or bioassay-guided isolation.1 To prove whether the obtained constituents are active compounds of the extract, it is necessary to prepare an extract in which the assumed compound(s) is removed,101 called a “knockout” extract. The “knockout” concept has been widespread in genetic engineering for pharmacologic studies since 1989.102 In bioactivity comparisons, if a knockout extract has low biological activity compared with the activity of the original extract, the eliminated constituents can be considered bioactive compounds.101 Therefore, one goal of studies is to validate methods for preparing knockout extracts. Chemical chromatography and immunoaffinity chromatography have been introduced for this purpose.To improve efficiency, Liu et al.101 developed an on-line chromatography technology to prepare the knockout extract. As shown in Fig. 5, an HPLC technique was used to separate constituents in a herb extract, ensuring target compounds achieved baseline separation from adjacent peaks. Then a six-port valve-switching unit ‘cut and captured’ target compounds according to the chromatographic retention times. Ingredients were thus divided into two parts: the target compounds and others without the target. Based on valve-switching, the main bioactive and characteristic components (or a combination of them) can be obtained. Fig. 5 also shows an application example with ginseng. With HPLC, ginseng extract underwent baseline separation of its major components.104 Through the valve switching technique, the original extract, the Re and Rb1 knockout extract, and knockout compounds (Re + Rb1) can be obtained. A comparison of the bioactivities of the samples shows the overall efficacy of target peaks, the bioactive contribution of each component, and the interaction of multiple components.
Fig. 6 shows a protocol for preparing ginsenoside Rb1 knockout ginseng extract.113–115 The first step is to obtain anti-ginsenoside Rb1 MAbs from mice through synthesis of antigen conjugates, immunization, hybridization and purification. Since anti-ginsenoside Rb1 MAbs have a sugar chain in the molecule, the second step is to produce aldehyde groups through oxidative cleavage by sodium periodide (NaIO4).115,116 The oxidized product is treated with agarose gels conjugated with a hydrazine group to give hydrazone. The immunoaffinity column is produced with an affinity gel combined with MAbs.110,114 After method validation, ginseng extract is charged to the column, washed with phosphate buffer and then eluted with acetate buffer and 20% methanol to give pure ginsenoside Rb1. All washed buffer solutions are collected and deionized. The water solution is concentrated and subjected to thin-layer chromatography (TLC) or HPLC analysis. This immunoaffinity chromatographic method can establish one-step preparation of Rb1 knockout ginseng extract.
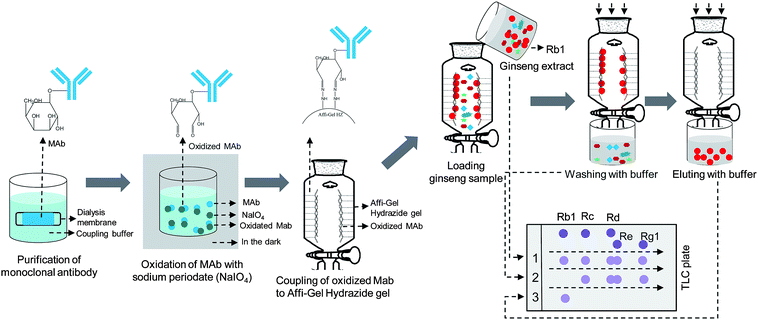 | ||
| Fig. 6 Immunoaffinity chromatographic method for preparation of Rb1 knockout extract from P. ginseng extract, modified from ref. 114. The red spots in the top right part of the graphic indicate Rb1. Lines 1, 2, and 3 in the thin-layer chromatography (TLC) plate in the bottom right part of the graphic indicate crude extract, knockout extract, and purified Rb1, respectively. | ||
Compared with chemical chromatography for knockout, immunoaffinity chromatography enhanced analytical selectivity, decreased the number of tedious steps in sample preparation, and improved sampling loading volume. Additionally, the run-time of chromatographic separations and optimal conditions is reduced. However, immunoaffinity chromatography has some limitations, namely complicated MAbs and column preparation, non-commercial availability of MAbs, and instability of the immunoaffinity columns.
3 Analytical advances
In this section, we emphasize emerging techniques, general trends, and recent advances for the analysis of ginseng. Some methods highlighted are (for separation): high-performance thin-layer chromatography (HPTLC); hydrophilic interaction chromatography (HILIC); ultrahigh-pressure liquid chromatography (UPLC); multidimensional chromatography; and on-line concentration electrokinetic chromatography; and (for detection) advanced mass spectrometry (MS), i.e. quadrupole (Q)-MS, ion trap (IT)-MS, time-of-flight (TOF)-MS instruments, and more recently, Q-IT, IT-TOF and Q-TOF.3.1 Direct profiling technologies
With the development of chemometrics and informatics in multivariate statistical analysis, spectrometric and spectroscopic methods are frequently applied to the profiling of crude ginseng extracts without prior separation. Profiling without hyphenation technologies is useful for rapid evaluation of the composition, authentication of the ginseng plant species, quality control, and metabolomics.117 The major techniques are nuclear magnetic resonance (NMR), infrared (IR) and Raman spectroscopy.There are several reports on the discrimination of ginsengs by NMR profiling. Sugar and methyl signals in ginseng saponins obtained from 1H-NMR with principal component analysis (PCA) and cluster analysis can differentiate geographical origins and ages of P. ginseng roots.120,121 Ginseng saponins, saccharides, amino acids, fumaric acid and inositol were screened as important markers by two-dimensional (2-D) NMR with PCA to discriminate between P. ginseng and P. quinquefolius preparations and white and red ginseng root.122
Direct NMR profiling is powerful for recording changes in composition within a ginseng mixture. One of the main drawbacks of this method is its low sensitivity – it is not well suited for detecting minor changes in composition.
Based on a unique IR spectral fingerprint in the 2000–600 cm−1 region, Yap et al. described a “2-6PC rule” for categorizing ginseng products to authenticate them from morphological adulterations123 and to distinguish among P. ginseng, P. quinquefolius and P. notoginseng.124 Near-infrared spectroscopy was used to determine the total sugar content in ginsengs.125 Fourier transform infrared (FTIR) spectroscopy with support vector machine and wavelet transform technology identified the cultivation area of ginsengs126 and differentiate P. quinquefolius from P. ginseng.127 In a recent study, 2-D FTIR and 2-D IR correlation spectroscopy provided colorful maps and dynamic structural information about chemical components to authenticate P. ginseng, P. quinquefolius and P. notoginseng,128 to differentiate cultivated from wild ginseng,129 to discriminate between the different geographical sources of P. quinquefolius,130 and to evaluate the different ages of ginseng samples.131
Raman spectroscopy with pattern recognition has been used to classify ginsengs according to species and processing method132 and to investigate polyacetylenes in P. quinquefolius roots.133
3.2 Separation-based methods
One emerging trend has been combining techniques, which have been useful for profiling the composition of ginseng samples. Two major steps are involved – separation and detection. Separation of ginseng saponins is performed by TLC or HPTLC; HPLC, HILIC or UPLC; gas chromatography (GC); multidimensional chromatography; or electrophoresis (CE). Among these techniques, liquid chromatography is used most frequently. A comparison of different separation modes for ginseng analysis is given in Table 4.| Method | Advantages | Limitations | Ref. |
|---|---|---|---|
| a Abbreviations: HPTLC: high-performance thin layer chromatography; GC: gas chromatography; HPLC: high-performance liquid chromatography; HILIC: hydrophilic interaction liquid chromatography; UPLC: ultra-performance liquid chromatography; CE: electrophoresis; 2-D: two-dimensional; ESI: electrospray ionization; MS: mass spectrometry. | |||
| TLC/HPTLC | Easy to use | Low efficiency in separation | 134,135 |
| Versatility | Low reproducibility | ||
| Low cost | Low accuracy in quantification | ||
| GC | High sensitivity | Limited to volatile compounds | 91,136–138 |
| High resolution | Time consumption in derivation | ||
| High speed | Thermal instability | ||
| HPLC | Widely available | Extended analytical time | 28,74,104,121,139–141 |
| Relatively easy to use | Relatively high cost | ||
| High accuracy and precision | |||
| HILIC | Suitable for hydrophilic molecules | Peak broadening | 142–145 |
| Beneficial to ESI process | Short column lifetime | ||
| Weak interaction with stationary phase | |||
| UPLC | Highly efficient separation | Back-pressure increase | 146–148 |
| Reduced analytical time | Dedicated instrumentation | ||
| Reduced solvent consumption | |||
| CE | Short analytical time | Low reproducibility | 149–152 |
| Easy to automate | Insufficient detection sensitivity | ||
| Environmentally friendly | Complicated interfacing to MS | ||
| 2-D chromatography | Powerful separation ability | Possibility of artifact formation | 136,153 |
| High peak capacity | Extended analysis time | ||
| Excellent detection sensitivity | Limitations in mobile phase | ||
Many TLC procedures have been carried out under high-performance conditions (HPTLC). In HPTLC, the plates are precoated for the stationary phase with a mean particle size of 5 μm for better separation and reproducibility. HPTLC was developed for differentiation of the ginseng species. A fingerprint pattern with HPTLC discriminates white and red P. ginseng, P. quinquefolius and P. notoginseng.154,155 With the introduction of densitometry, TLC also has become a useful tool for the quantitative analysis of ginseng saponins. A typical application was performed by Vanhaelen-Fastré et al.,156 who used HPTLC for simultaneous quantification of six major ginseng saponins in P. ginseng. Kevers et al.157 developed an automatic HPTLC sampler and scanner that determines ginseng saponins and identifies the different species and sources. The major disadvantages of TLC and HPTLC are low accuracy and reproducibility.
GC has been used for analysis of volatile organic compounds (e.g., oils).136,158 Sample preparation is the crucial first step in GC analysis of volatile compounds in ginseng. Novel sample preparation techniques have been established to extract volatile compounds from the matrix, including headspace solid-phase microextraction137 and solvent-free solid injection based on direct vaporization.158 Another important use of GC has been to analyze multiresidue pesticides and their metabolites in ginseng samples. There are reports of methods that describe the screening of pesticides in dried ginseng powders, extracts and preparations.151,159,160 To make methods faster, automated, and cost-efficient, GC has been combined with high resolution time-of-flight (TOF) MS.160
GC can also determine other active ingredients. There are methods for measuring semi-volatile components such as sesquiterpenes and polyacetylenes91,153 and for quantifying phenolic constituents after derivatization to their trimethylsilyl derivatives.161 GC is also a powerful tool to test endogenous metabonomics in biological samples for ginseng's therapeutic effects and toxicity.138
As has been the general trend for a number of years, GC is decreasing in popularity, as reflected by the number of new procedures being reported. Since the complex patterns of the different types of ginseng saponins cannot be evaluated with GC, it is rarely used in ginseng studies.
HILIC has been gaining interest in the last few years as an alternative to HPLC.142–145 HILIC is especially useful for separation of polar and hydrophilic analytes as well as polar metabolites in biological samples. Separations are carried out in the hydrophilic stationary phase using isocratic hydro-organic eluents that contain high levels of the organic component. Retention times increase with the hydrophilicity of the analytes. When combined with MS, high organic content in the mobile phase benefits the electrospray ionization process. The influence of column temperature and the choice of eluent components such as buffer are key to separation efficiency and selectivity.145 In HILIC, a silica column (50 × 2.1 mm, 3 μm) was used to analyze dencichine (a neuro-excitatory non-protein amino acid) in different ginseng species with a run time of approximately 5 min. Dencichine was not retained by any of the reversed-phase packing.142 HILIC using diols,143 or polyvinyl alcohol-bonded,145,162 or polyamine-bonded144 stationary phases was developed for the separation and quantitative determination of ginseng saponins in ginseng preparations. Shorter analysis time (15–30 min compared with more than 60 min in RP-HPLC) and better resolution were achieved. The elution window for the analysis of compounds that are difficult to separate can be broadened by the combination of HILIC with conventional HPLC.163
UPLC, which uses short columns packed with particles less than 2 μm in size, has emerged as a powerful method in many laboratories. Chromatographic separation is 5–10 times faster than HPLC, without a reduction in resolution.146,164,165 Existing HPLC conditions can be directly transferred to UPLC. Combining UPLC with an MS detector fulfils key requirements in terms of rapidity, sensitivity, selectivity, and peak-assignment certainty for the analysis of analytes at low concentrations in complex matrices.166 UPLC has been popular for rapid separation of ginseng saponins in metabolomic fingerprinting,146–148 quality control,167 and biological samples.168,169 A comparison was made of the performance of UPLC on a 100 × 2.1 mm, 1.7 μm column and of HPLC on a 250 × 4.6 mm, 5.0 μm column. Twenty-five ginseng saponins were identified within a 20-min run time with UPLC, while fewer markers were recognized by HPLC within 80 min.148 At present, the major limitation of UPLC is the cost of dedicated equipment and consumables, keeping the instrument more of a research than a routine tool. To provide a more economical alternative, a simplified approach was recently introduced, in which a modified conventional HPLC system was connected directly with short columns to reduce time for analysis of complex herbal medicines.170,171 Direct connection offers opportunities for laboratories looking to improve separation without upgrading an existing HPLC system to an UPLC system.
In general, analysis of ginseng saponins with CE can be optimized by sampling and concentrating the ginseng samples, buffer additives, surface modifications and detections.174 The concentration sensitivity of MEKC and MEEKC is poor because the best path is short and only a small volume of sample can be injected. To improve the sensitivity of MEKC, on-line concentration techniques with stacking procedures have been developed. Wang et al.175 developed a field-enhanced sample injection with reverse migrating micelles to separate and concentrate ten ginseng saponins in P. notoginseng on-line. Ginseng saponins achieved separation within 35 min, shorter than the 65 min by HPLC. Sensitivity was one magnitude higher than with the method by Glöckl et al.,149 enabling two minor ginsenosides, Rh1 and Rg2, to be determined.
Cao et al.152 used on-line dual sweeping based on borate complexation and enhancement of the organic solvent field enhancement for the preconcentration of ginsenosides Rf, Rg1, and Re in MEKC with nonionic Brij-35 micelles. Subsequently, they established a complex formation and acetonitrile sweeping technique in non-ionic MEEKC for ginsenosides Rf, Rb2 and Re using a Brij-35 microemulsion.151 Pressure and electrokinetic injections of long sample plugs were also presented for simultaneous stacking of notoginsenoside R1, and ginsenosides Rg1, Rf, Rh1, Rd, Rg3 in MEEKC.150 Improvements in sensitivity were in the range 50- to 200-fold.
In spite of novel CE techniques, the applications of CE for analysis of ginseng are limited. One example uses a CE microchip coupled with a polymerase chain reaction (PCR) analysis of short tandem repeats for rapid authentication of ginseng species.174 Most applications are for protein analysis to decipher ginseng's biological mechanisms.
Shellie et al.153 developed comprehensive 2-D GC (GC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GC) to identify qualitative differences between Panax species extracts. GC
GC) to identify qualitative differences between Panax species extracts. GC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GC revealed the presence of numerous common components and possible species-specific components. Qiu et al.136 described a comprehensive GC
GC revealed the presence of numerous common components and possible species-specific components. Qiu et al.136 described a comprehensive GC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GC technique to analyze the chemical composition of volatile oil in P. ginseng at different ages. This method for PCA classifies and differentiates ginseng samples at different ages.
GC technique to analyze the chemical composition of volatile oil in P. ginseng at different ages. This method for PCA classifies and differentiates ginseng samples at different ages.
With 2-D chromatography, artifacts may form, polar and nonvolatile solvents may be unavailable in the mobile phase, and analysis times are long.
3.3 Hyphenated detection-based methods
After sample separation, the next step is to detect the target compounds. Hyphenated detection-based methods include ultraviolet (UV) or diode-array detection (DAD), evaporative light scattering detection (ELSD), charged aerosol detection (CAD), MS and ELISA. Different detectors can be combined to obtain complementary information in one run, such as DAD-ELSD and DAD-MS. Table 5 compares the advantages and limitations of the various methods.| Methoda | Establishment | Sensitivity | Structure | Linear range | Operation | Precision | Accuracy | Cost |
|---|---|---|---|---|---|---|---|---|
| a Abbreviations: UV/DAD: ultraviolet or diode-array detection; ELSD: evaporative light scattering detection; CAD: charged aerosol detection; MS: mass spectrometry; ELISA: enzyme-linked immunosorbent assay. | ||||||||
| UV/DAD | Very easy | Moderate | Possible | Very wide | Simple | Fairly good | Very good | Very low |
| ELSD | Easy | Low | No | Narrow | Fair | Good | Good | Low |
| CAD | Easy | Moderate | No | Wide | Fair | Good | Good | Low |
| MS | Not easy | High | Powerful | Narrow | Not simple | Low | Low | High |
| ELISA | Very complex | Very high | Possible | Very narrow | Simple | Low | Low | Low |
An HPLC–ELSD method has been developed for simultaneous monitoring of 12 ginseng saponins in different parts of P. quinquefolius,28 14 saponins in red P. ginseng,180 and 19 saponins in black ginseng.17 Wan et al.18 described the chemical characteristics of three medicinal plants of the Panax genus by HPLC–ELSD. Quantitative evaluation of ginseng saponins using both UV and ELSD in P. quinquefolius showed similar results, except that UV could not be applied to detect pseudoginsenoside F11.140,181 However, in light of its better sensitivity, easier handling and greater availability, UV is still recommended for the routine analysis of ginseng samples.
Bai et al.183 established an HPLC–CAD method for quantitative analysis of notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rg2, Rh1, and Rd in 30 batches of P. notoginseng samples. The sensitivity of CAD was higher than that of ELSD or UV. Wang et al.184 compared LC–CAD, LC–ELSD and LC–UV methods to determine ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3 and Rd in P. ginseng. CAD had higher sensitivity than UV and ELSD, and better linearity and reproducibility than ELSD.
The limitations of CAD are the need for volatile buffers, non-volatility of analytes, and dependence on the changes in the eluent composition for a response.
Four types of mass analyzer are used for analysis of ginseng and ginseng-related samples, i.e., quadrupole (Q),160 ion trap (IT),194 time-of-flight (TOF),147,148,164,195–197 and Fourier transform ion cyclotron resonance (FT-ICR).198,199 Quadrupoles tend to be the simplest and least expensive mass analyzers. Quadrupole mass analyzers can operate in scanning mode or selected ion monitoring (SIM) mode. Because of the scan mode's low sensitivity, the SIM mode is used for quantifying and monitoring target compounds. The SIM mode, which provides information on a few ions, is not suited for screening unknown compounds. Ion traps are also relatively inexpensive. Compared with losses with quadrupole, sensitivity losses during the full-scan mode can be avoided. An advantage of ion traps is that multiple stages of mass spectrometry can be performed without additional mass analyzers. Time-of-flight mass analyzers measure mass accurately and have high resolution and full-scan mass range. Accurate mass measurement, which gives the elemental composition of obtained ions, facilitates non-target component identification. Compared with quadrupole and ion trap, TOF is more expensive and has a narrower linear range for quantification. Like ion traps, FT-ICR mass analyzers can process mass reaction in multiple stages without additional analyzers. Like TOF, FT-ICR analyzers also have a wide mass range and a high mass resolution. They are, however, the most expensive of the mass analyzers.
Multi-stage MS (also called tandem MS or MS/MS or MSn) combines the different designs of mass analyzers. MS/MS gives advanced structural information and has the sensitivity, specificity, and versatility. Triple quadrupole (QqQ),200 quadrupole ion trap (Q-IT),194,201 quadrupole time-of-fight (Q-TOF),164,196 or ion trap time-of-flight (IT-TOF)202,203 instruments are used for ginseng analysis. Hybrid systems such as Q-TOF-MS and IT-TOF-MS, which provide fragmentation information together with accurate mass measurements of product ions, are powerful tools for structural analysis. In MS/MS, collision-induced dissociation (CID) between analyte ions and neutral molecules forms fragment ions. Voltages are adjusted to increase collision energy to produce abundant fragmentation for predicting the structure of compounds. Another advantage of multi-stage MS is that nonanalyte ions are discarded in the first stage and thus sample cleanup is less. Multi-stage MS, however, is extremely expensive, which makes it impractical for most laboratories.
CID can also be achieved in single-stage mass spectrometers (commonly called in-source CID). The advantage of performing CID in single-stage instruments is simplicity and relatively low cost.204 One application is in-source CID in single-stage TOF-MS.205 The fragmentor in TOF-MS is crucial for efficient transmission of the ions to obtain the best balance between sensitivity and fragmentation.206,207 With dynamic adjustment of the fragmentor from gentle ionization to intense fragmentation, molecular ions and abundant fragment ions are readily observed for unambiguous identification with similar or better performance than tandem MS.170,171 The disadvantage of CID in single-stage MS is that a specific precursor ion cannot be selected, so the source of product ions cannot be determined.
One limitation of MS-based methods is the inherent lack of ionization of certain components. Another is that LC–MS ionization is instrument-dependent, making it difficult to construct standard mass spectrum libraries for ginseng saponins. Equipment, especially for TOF-MS, must be calibrated accurately and regularly. As with most advanced technologies, generating useful and reliable data depend on the experience and the skill of the operators.
ELISA has been applied for single or simultaneous quantification of ginsenosides Re, Rb1, Rg1, Rd and Rc in various ginseng extracts.109,110,116,208,209 Eastern blotting has been used to identify chikusetsusaponins III and IV in P. japonicus.114 ELISA suffers from the complex preparation of MAbs, strong cross-reactivity caused by some structurally similar constituents, and narrow dynamic linear range for quantification.
4 Applications and challenges
As one of the most widely studied medicinal plants and herbs during the last decade, a great number of scientists have contributed to developing methods for analyzing ginseng. Advances in isolation and analysis technology have improved analysis times, efficiency, and method development. Application of these methods has been made to various aspects of ginseng and ginseng-related samples. In this section, we review species differentiation, quality assessment, and interaction with biological systems. Challenges in studying ginseng are also included.4.1 Species authentication
Ginseng grows or is cultivated in different geographical regions. Although ginsengs are closely related taxonomically, and contain similar chemical constituents, they differ in their effects on symptoms in people. In the herbal market, adulterants are sometimes part of samples.128,210 Because the roots of ginsengs are similar in appearance and many commercial ginseng products are sold as powder or shredded slices, identification of the origins of a product is not easy. Some new methods have been developed to differentiate ginseng species, mainly through chemical profiling or molecular markers.The total ginsenoside content of P. notoginseng is higher than that of P. quinquefolius and much higher than that of P. ginseng. One difference between the three species is the unique presence of ginsenoside Rf in P. ginseng.213 Ginsenoside Rb2 is detected in abundance in P. ginseng but in trace amounts in the other two species.18 Notoginsenoside R1 is characteristic of P. notoginseng, while pseudoginsenoside F11 is a marker compound in P. quinquefolius. F11 possesses the same molecular weight and has similar retention times as Rf.141 F11 without chromophores cannot be detected by UV; HPLC–ELSD140 or HPLC–MS164 are capable of detecting both F11 and Rf. A UPLC/Q-TOF-MS was developed for 2-min analyses of Rf and F11 in P. ginseng-adulterated P. quinquefolius.164 Establishing the ratios of Rg1/Re, Rg1/Rb1 and Rb2/Rb1 is also useful. Ratios less than 0.4 indicate P. quinquefolius; higher ratios are characteristic of P. ginseng.140,164 One exception is the high Rg1/Rb1 ratio found in wild P. quinquefolius.214 Other spectrometric methods have been reported for effective differentiation, such as 2-D correlation FTIR spectroscopy128 and 2-D NMR spectroscopy.122
Zhu et al.223 revealed the phylogenetic relationships between thirteen Panax taxa by DNA sequence analysis on the chloroplast trnK gene and nuclear 18S rRNA gene. Subsequently, they developed a multiplex amplification refractory mutation system for the identification of five commonly used Panax species, i.e., P. ginseng, P. japonicus, P. quinquefolius, P. notoginseng and P. vietnamensis.224 Qin et al.174 developed a microchip electrophoresis method coupled with a PCR–short tandem repeats technique to discriminate between P. quinquefolius and P. ginseng and also between cultivated and wild P. quinquefolius. Diao et al.225 developed a PCR-RFLP and amplification refractory mutation system for identifying P. ginseng from its adulterants. P. ginseng was successfully identified in single herbal medicines or preparations containing diverse components. Sasaki et al.226 developed a loop-mediated isothermal amplification PCR (LAMP) method that could be conducted under isothermal conditions. Lu et al.227 established nested PCR and DNA sequencing methods to identify P. ginseng in preparations. With this method, analytical time and costs were reduced. Table 6 summarizes the advantages and limitations of molecular biology methods.
| Methoda | Principle | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| a Abbreviations: RAPD: random amplified polymorphic DNA; SCAR: sequence-characterized amplified region; RFLP: restriction fragment length polymorphism; AFLP: amplified fragment length polymorphism; DALP: direct amplification of length polymorphism; LAMP: loop-mediated isothermal amplification of polymerase chain reaction (PCR). | ||||
| RAPD | Use of small arbitrary primers to produce multiple DNA fragments | Technical simplicity, no sequence information required, polymorphism identification | Low reproducibility, extra amplified fragments | 228–232 |
| SCAR | Extension of RAPD primers based on polymorphic fragment sequences | Reduced competition between primer binding sites and high reproducibility | Low sensitivity | 218 |
| RFLP | Comparison of band profiles after restriction enzyme digestion of target DNA. | High reliability, no sequence information required | Large DNA amounts required, low polymorphism levels | 70,217,225,233 |
| AFLP | Polymorphism based on gain or loss of restriction site, or selective bases | High reproducibility, no prior sequence knowledge required | Difficulty in identifying homologous markers | 220,234,235 |
| DALP | Use of an arbitrarily primed-PCR to produce genomic fingerprints and to enable sequencing of DNA polymorphisms | High resolution fingerprint, new genetic markers characterization | Low reproducibility | 222 |
| DNA sequencing | Measurement of every base in the DNA sequence | Commonly used, application to primer designation | Time-consuming | 227 |
| Microsatellite | Determination of the short tandem repeats at a location | Highly polymorphic and codominant | High cost and highly time-consuming | 220,236,237 |
| LAMP | A single-tube technique for the amplification of DNA | Reduced reaction time, high sensitivity | Low specificity | 226 |
4.2 Quality assessment
As global market demands for ginseng increase, quality control has become important. Unlike chemical drugs with a single entity, ginseng, like fruits and vegetables, has many constituents. Natural variations in soil and climate and preparation procedures affect the safety and batch-to-batch uniformity of ginseng products. Monitoring for quality should be done at selected steps for the dynamic coordination of all links. Good ginseng materials and manufacturing processes depend on good agricultural practices (GAPs) and good manufacturing practices (GMPs). Independent quality evaluation is an integral part of ginseng production. To standardize a ginseng extract, metabolomic fingerprinting is performed or marker compound(s) determined.Table 7 lists some examples of ginseng analysis using the metabolomic fingerprinting technique. The analytical tools include MS-based chromatography and NMR-based spectroscopy.118 Various constituents can be identified and quantified at low concentrations, making MS a sensitive analytical tool for metabolic fingerprinting.136,146,148 Abundant fragment ions by multi-stage MS and high mass accuracy by TOF-MS offer structural information and the possibility of identifying unknown components. In NMR-based fingerprinting, the 1H NMR spectrum provides a wealth of chemical information on ginseng.212,238 NMR has the highest reliability in metabolomics.118 Apart from ginseng saponins, other important metabolites in ginseng such as carbohydrates, amino acids and organic acids can be elucidated based on their chemical shifts in the 1H NMR spectra. The concentration of each metabolite in the sample can be easily calculated from the integration of the signals in the spectra. There is no requirement for calibration curves to convert signal intensity into concentration. When the spectral complexity and signal overlap in the 1H NMR spectra are high, the chemical structures of metabolites can be confirmed by diverse 2D-NMR spectroscopic methods.122
| Instrumenta | Chemometricsb | Material | Result | Ref. |
|---|---|---|---|---|
| a Abbreviations: HPTLC: high-performance thin layer chromatography; 2-D NMR: two-dimensional nuclear magnetic resonance; GC–TOF-MS: gas chromatography time-of-flight mass spectrometry; UPLC–ESI-Q-TOF-MS: ultra-performance liquid chromatography electrospray ionization quadrupole-TOF-MS. b Abbreviations: CASE: computer-aided software engineering; PCA: principal component analysis; PLS-DA: partial least squares-discriminant analysis. | ||||
| HPTLC fluorescent | CASE software | P. ginseng, P. quinquefolius, P. notoginseng | Species authentication and stability of ginseng preparations achieved | 155,211 |
| 1H-NMR | PCA | Fresh ginseng roots of different ages | Effective authentication and quality control | 238 |
| 1H-NMR | PCA and cluster analysis | P. ginseng roots of different origins | Sugar signals and methyl signals separate, showing origin-dependent and age-transitions | 120,121 |
| 1H-NMR | PCA and PLS-DA | P. ginseng, P. quinquefolius of different origins, and Korean ginseng products | Glucose, fumarate, and various amino acids serve as biomarkers for quality assurance | 212 |
| 2-D J-resolved 1H-NMR | PCA | Different ages of P. ginseng and commercial ginseng capsules | Alanine, arginine, fumaric acid, inositol and ginseng saponins are important metabolites for differentiation | 122 |
| GC–GC–TOF-MS | PCA | Radix ginseng volatile oils of different ages | α-Cadinol, α-bisabolol, thujopsene, and n-hexadecanoic acid increase with age of the sample | 136 |
| UPLC–ESI-Q-TOF-MS | PCA | Different parts of P. notoginseng | Slight variations discerned due to different geographical locations, cultivations and collection times | 146 |
| UPLC–ESI-Q-TOF-MS | PCA and PLS-DA | P. ginseng, P. quinquefolius, and P. notoginseng | Chikusetsusaponin IVa, Ro, Rc, Rb1, Rb2 and Rg2 account for variations among ginseng species | 147 |
| UPLC–ESI-Q-TOF-MS | PCA | P. ginseng from China and Korea, P. quinquefolius, P. notoginseng, and P. japonicus | Rf, F11, malonyl-Rb1, and Rb2 account for variations in species | 148 |
When the data obtained are analyzed by statistical methods, all relevant information must be extracted from a complex data set. Multivariate data analysis is used to recognize patterns and find discriminating signals. Among the multivariate data analysis, PCA146,238 and partial least squares-discriminant analysis (PLS-DA)148,212 have become the routine processing steps for raw analytical data. Marker signals for differentiation can then be identified, and the upper and lower limits of the signals are defined for quality control of ginseng.
For a comprehensive evaluation, laboratory methods use as many markers as possible. Kim et al.180 evaluated white and red P. ginseng through simultaneous determination of 14 ginseng saponins by HPLC–ELSD. Sun et al.17 studied Korean white, red and black P. ginseng by measuring 19 ginseng saponins. Qian et al.239 used HPLC–DAD and MS for simultaneous determination of flavonoid, saponins and polyacetylenes in ginseng samples. Comparisons were made of ginseng for the effects of different origins, ages, cultivation methods, plant parts, and commercial manufacturer.3,104,139,214,240,241 Individual and total ginsenoside contents varied in different commercial ginseng products.139 Ginsenoside profiles and content also varied depending on the plant parts tested – in decreasing order of ginsenoside content, they were: leaf > root-hair > rhizome > root > stem.28
To quantify marker compound(s), high-quality ginsenoside libraries are required. Lack of reference standards, in spite of improved methods for determining compounds, makes it difficult to evaluate ginseng products. Some reference compounds are commercially available, but their cost and limited supply cannot meet ginseng demand worldwide. Since most chemical compounds in a herb have a similar skeleton, researchers have introduced a relative quantification correction standard that uses a universal compound for calibration against other structurally similar components. In one study, a commercially available compound, verticinone, was used to quantify twenty-six other steroidal alkaloids.192 Compared with absolute quantitative determinations, an average accuracy of 98.5% was obtained. We have attempted to use Rb1 and Rg1 to determine PPD-type and PPT-type ginseng saponins, respectively, and method validation and robustness tests are still under investigation.
Because the active compounds are unknown, the common practice is to select one or more major ginsenosides such as Rb1 and Rg1 as active or markers. The drawback is less of a link between quality and activity. A combined method of quantifying marker compounds with profiling metabolomic fingerprinting might be a better way to link metabolic profiles with activity and to assure batch-to-batch consistency.
Fig. 7 shows one method for rapid identification of targeted and untargeted ginseng saponins. Available reference compound(s) are used to obtain characteristic fragmentation pathways and diagnostic ions of ginseng saponins. The diagnostic ions and pathways represent the mother nucleus and some substituent groups of saponins. For instance, our ongoing investigations revealed that all PPD-type ginseng saponins produce three common skeleton ions by TOF-MS, i.e., m/z at 407.37, 425.37 and 443.38. Sugar types, numbers and linkage sequences also can be determined by simultaneous or successive losses of sugar moieties and sugar cross-ring cleavages.189
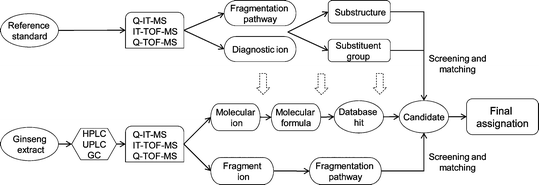 | ||
| Fig. 7 General strategy for identification of ginseng saponins in ginseng samples by mass spectrometry (MSn and accurate mass experiments). | ||
Following this analysis, the complex ginseng extract is tested. Chromatographic separation by LC is important for subsequent identification. Diagnostic ions and fragmentation mechanisms from reference compounds are a basic requirement for screening known and unknown compounds in ginseng extract. Target compounds can be unequivocally identified by comparison of accurate retention times, molecular ions, and characteristic fragment ions with those of the reference compounds. Identification of untargeted ginseng saponins is complex. The first step is to determine the molecular ions of chromatographic peaks in the ginseng extract. Then, several criteria are used to calculate the accurate molecular formula of each peak: the acceptable accuracy threshold, the general rule of the number of nitrogen atoms, the dibasic ester (DBE) index, and the “show isotopic pattern” function. With the obtained molecular formula, screening for a hit is subsequently performed against various chemical databases. From the candidates collected, the characterized diagnostic ions can then be used for locating the candidates that contain such a substructure and/or substituent groups. Finally, the most likely structure can be determined from these candidates by fragmentation screening and matching.
Online structure characterization has been used to identify 152 saponins from P. notoginseng by LC–ESI-MSn;188 while 35 ginseng saponins in P. ginseng187 and 30 ginseng saponins in P. quinquefolius186 were identified by LC–APCI-MS.
Malonyl ginseng saponins, also called acidic ginseng saponins, are compounds with a malonyl group attached at the 6′′-position of the glucosyl moiety. As early as 1983, four malonyl ginseng saponins, namely the malonyl-ginsenosides Rb1, Rc, Rb2 and Rd, were isolated from P. ginseng245 and later from P. quinquefolius.246 Malonyl-ginsenoside Ra372 and malonyl-notoginsenoside R473 were recently isolated from the fresh roots of P. ginseng. Approximately fifteen malonyl ginseng saponins were found in ginseng extract by HPLC–MS/MS.186,187,194,244 Malonyl ginseng saponins, more polar and water-soluble than neutral ginseng saponins, are extremely unstable and susceptible to thermal degradation during drying, storage, heating and extraction.29,247,248 In dried ginseng roots, malonyl ginseng saponins decreased as the air temperature increased.29 Malonyl ginseng saponins are absent (or found in trace amounts) in red ginseng prepared by a steaming or heating process.248 Cold alcohol is recommended to extract malonyl ginseng saponins, and 40% ethanol has maximum extraction efficiency.29 Malonyl ginseng saponins are thus not often found in commercial products prepared by hot extraction processes.190
Malonyl-ginseng saponins make up much of the total saponins in ginseng. Their concentration accounts for approximately one-third of the total ginsenoside content in P. quinquefolius, but relatively little in P. ginseng and P. notoginseng.249,250 Malonyl-ginsenoside Rb1 is the most abundant.11 The profiles of malonyl-ginseng saponins differ among different species.194,249 Because of their instability and the lack of suitable reference compounds, determining their profiles presents a challenge. Since they are usually invisible on HPLC–UV chromatograms, standard assay procedures may underestimate ginsenoside content by ignoring malonyl ginsenosides. Use of phosphate buffer is important for resolution of malonylated and other esterified ginseng saponins.247,251 If ELSD or MS is used for detection, ammonium acetate,244 ammonium formate,190 and acetic acid194 are recommended instead of phosphate. An alternative strategy is to determine malonyl ginseng saponins by an indirect two-step method that hydrolyzes these acidic saponins into their respective neutral saponins with KOH. However, the results may give only a rough estimate because other derivatives also might be transformed. Both APCI-MS186,187 and ESI-MS16,194,244 are useful for characterization of malonyl-ginsenosides in ginseng extracts. Fig. 8 shows the MS characterization of malonyl-ginsenoside Rb1 by tandem MS in negative ion mode. Fig. 8A lists the sequential mass spectra of deprotonated malonyl-ginsenoside Rb1, and Fig. 8B suggests the fragmentation pathways. The presence of a free carboxylic acid group means that malonyl ginseng saponins can be rapidly identified as they display loss of the neutral units of mass 44 Da (CO2), 42 Da (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO) and 60 Da (CH3COOH).
CO) and 60 Da (CH3COOH).
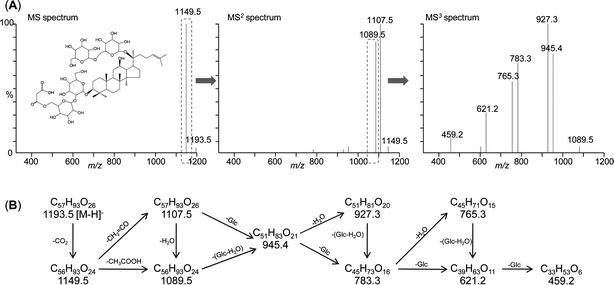 | ||
| Fig. 8 Mass spectrometry (MS) characterization of malonyl-ginsenoside Rb1 by tandem MS in negative ion mode. (A) Sequential mass spectrometric analysis of deprotonated malonyl-ginsenoside Rb1. (B) Interpretation of the fragmentation pattern. Experimental conditions are given in ref. 194. | ||
Neutral ginseng saponins, usually stable during drying and storage conditions,252 degrade to new compounds under intensive heating or steaming. The conversion means that ginsenoside profiles of unprocessed and processed ginseng are different.52,55,253,254 As shown in Fig. 9, during steaming or heating, the polar ginseng saponins decreased, and less polar ginseng saponins increased. The structural changes in ginsenosides during the process are elimination of sugar chains and isomerization of the hydroxyl configuration at C-20 of the aglycones.52 The protopanaxadiol (PPD)-type ginsenosides selectively eliminate the C-20 sugar chain to produce Rg3.255,256 Rg3 is further transformed to Rk1 and Rg5 by dehydration.3 A small amount of Rh2 observed in red ginseng implies that the elimination of C-3 sugar residue is relatively difficult in processing.55 The protopanaxatriol (PPT)-type ginsenosides first lose the C-20 sugar residue and subsequently their terminal sugar unit at C-6 to form Rg2 and/or Rh1.248 Rh1 is further converted to Rk3 and Rh4 by dehydration at C-20. In summary, the elimination of sugar chains at C-20, then at C-6 or at C-3, and a subsequent dehydration reaction at C-20 are the effects of steaming.
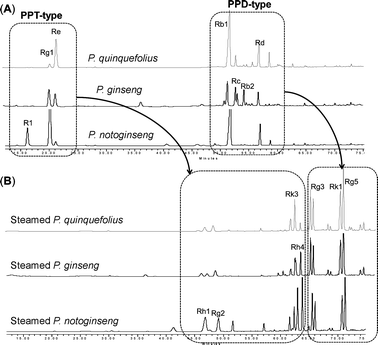 | ||
| Fig. 9 HPLC–UV chromatograms of ginseng roots and chemical degradation in steaming process, modified from ref. 256. (A) Ethanol extract of roots of P. quinquefolius, P. ginseng and P. notoginseng. (B) Ethanol extract of steamed ginseng roots at 120 °C for 4 h. Chromatographic separation was performed on a Prodigy ODS column. Detection wavelength was set at 202 nm. The mobile phase used acetonitrile and water for a gradient elution. | ||
The steps in pesticide analysis are extraction, cleanup, separation and detection.259 Ginseng samples are blended with organic solvent alone, solvent mixtures, or water. Acetone,159,260 acetonitrile,159,261 methanol,262 and ethyl acetate160 are used. Pesticide extraction methods are Soxhlet extraction,257,263 solid-phase extraction (SPE),159,261 matrix solid-phase dispersion,61,196 and SFE.264,265 Traditional cleanup procedures fractionate extracts based on polarity, as in liquid–liquid partitioning257,258 and column chromatography, or adsorption chromatography using florisil196,260,266,267 and neutral alumina.260,268 SPE265,268 and gel permeation chromatography160 have become popular. GC is used for separation of large-scale multiresidue pesticides.159,160,266 Because some pesticides are highly polar, have low volatility and/or are thermo-labile, LC is used for analysis.61 Element-selective electron capture,258,261,263,267 nitrogen-phosphorus analysis,258,261 and flame photometrics are used.193 The detectors are sometimes connected to combine results in a single run.261,263,267 MSn and TOF-MS are the most commonly used detection techniques.159,160,267,268
In a survey of P. ginseng and P. quinquefolius extracts by Durgnat et al.,269 13 out of 30 samples contained levels of pesticides greater than the maximum residue limits. When Hayward et al.159,160 analyzed 170 organohalogen and organophosphorous pesticides and their isomers and metabolites in dried ginseng roots, seven products contained combinations of pesticides.
4.3 Interaction with biological systems
| Materiala | Administrationb (species) | Analytical method, LLOQ c (ng ml−1) | T max (h) | T 1/2 (h) | F (%) | Ref. |
|---|---|---|---|---|---|---|
| a PPD: 20(S)-protopanaxadiol; IH-901 (or compound K): 20-O-(β-D-glucopyranosyl)-20(S)-protopanaxadiol; “Shenmai”: a prescription prepared from Radix Ginseng and Radix Ophiopogonis. b i.v.: intravenous; i.g.: intragastrical; i.m.: intramuscular. c LLOQ: lower limit of quantification; UPLC: ultra-performance liquid chromatography; ESI-MS: electrospray ionization-mass spectrometry; APCI: atmospheric pressure chemical ionization; Q-TOF: quadrupole time-of-fight; SSIM: segmental and selected ion monitoring. d T max: time to reach the maximum plasma concentration after i.g. or oral administration. e T 1/2: elimination half-life. f F: oral bioavailability; ND: not detected in plasma after oral administration. | ||||||
| P. ginseng berry and Re | i.v. and i.g. (rat) | UPLC–ESI-MS, 2.5 | 0.4 ± 0.2 | 0.2–0.5 | 0.19–0.28 | 270 |
| Rd | i.g. and i.v. (dog) | LC–ESI-MS, 5 | 3.0 | 24.2 ± 2.85 for i.g., 39.4 ± 12.0 for i.v. | 0.26 | 271 |
| Rd | i.v. (human) | LC–ESI-MS-MS, 3 | 0.5 ± 1.0 | 19.3 ± 3.4 | — | 272,273 |
| Rg1 | i.v. (rat) | LC–ESI-MS, 2–10 | — | — | 1.33 | 274 |
| Rh1 | i.g. and i.v. (rat) | LC–ESI-MS, 5 | 1.00 ± 0.00 | 0.43 ± 0.08 for i.g., 0.41 ± 0.05 for i.v. | 1.01 ± 0.03 | 275 |
| 20(S)- and 20(R)-Rg2 | i.v. (rat) | LC–UV, 3900–7800 | — | 0.64 ± 0.02 for 20S, 1.19 ± 0.06 for 20R | — | 276 |
| 20(S)-Rg2 | i.g. and i.v. (rat) | LC–ESI-MS, 5 | — | 0.53 ± 0.34 | ND | 277 |
| 20(S)-Rg3 | i.g. and i.v. (rat) | LC–ESI-MS, 2–10 | — | 0.23 | 2.63 | 278,279 |
| 20(S)-Rg3 | i.m. (human) | LC–ESI-MS/MS, 2.0–2.5 | — | — | — | 280 |
| 20(R)-Rg3 | i.g. and i.v. (rat) | LC–ESI-MS, 30 | — | 0.31 for i.v. | ND | 281 |
| 20(R)-Rh2 | i.g. and i.v. (rat) | LC–ESI-Q-TOF-MS | — | 0.27 | ND | 282 |
| IH-901 | i.g. and i.v. (rat) | LC–ESI-MS/MS, 5 | 0.63 ± 0.18 | 3.70 ± 1.38 for i.v. | 4.54 ± 0.38 | 283,284 |
| PPD | i.g. and i.v. (rat) | LC–APCI-MS, 1 | 2.50 ± 1.23 | 1.47 ± 0.30 for i.g., 2.73 ± 0.85 for i.v. | 36.8 ± 12.4 | 285 |
| PPD | oral (human) | LC–ESI-MS/MS, 10 | 1.28 ± 0.49 | 4.77 ± 2.05 | — | 286 |
| 25-OH-PPD | i.g. and i.v. (rat) | LC–ESI-MS/MS, 10 | 5.50 ± 4.70 | 3.9 ± 2.1 for i.g., 4.5 ± 2.6 for i.v. | 64.8 ± 14.3 | 287 |
| P. notoginseng extract | i.g. and i.v. (rat) | LC–UV, 1200–4000 | — | 18.57 for Rb1, 14.13 for Rg1 | 4.35 for Rb1, 18.4 for Rg1 | 288 |
| P. notoginseng extract | i.g. and i.v. (rat) | LC–ESI-MS, 2.8–4.0 | 0.71 ± 0.19 for R1, 0.75 ± 0.00 for Rg1, 0.88 ± 0.14 for Rd, 0.79 ± 0.10 for Re, 0.83 ± 0.13 for Rb1 | 1.11 ± 0.51 for R1, 5.01 ± 2.09 for Rg1, 18.15 ± 9.14 for Rd, 1.01 ± 1.35 for Re, 20.15 ± 6.27 for Rb1 | 9.29 for R1, 6.06 for Rg1, 2.36 for Rd, 7.06 for Re, 1.18 for Rb1 | 289 |
| Ginseng saponins | i.g. (rat) | LC–ESI-MS, 8–50 | — | — | — | 290 |
| ‘Shenmai’ | i.v. (human) | LC–ESI-MS-MS, 1 | — | 2.09 ± 1.89 for Rg1 | — | 291 |
| ‘Shenmai’ | i.v. (rat) | UPLC–SSIM-MS, 1–10 | — | — | — | 292 |
Sample pretreatment and analysis is key in PK studies. A simple sample pretreatment uses protein precipitation and liquid–liquid extraction. Another sample pretreatment method uses SPE to remove interference and matrix effects. SPE suffers from lower extraction recoveries than liquid–liquid extraction. For analysis, LC–UV and LC–MS have been developed to assay ginseng saponins in biological samples. LC–UV is convenient but has low sensitivity with a lower limit of quantification (LLOQ) greater than 1000 ng ml−1. LC–MS and MS-MS are more sensitive (LLOQ, 1–10 ng ml−1) and specific. Segmental and SIM have better sensitivity and wider dynamic ranges than SIM alone.292 PPD and PPT aglycone have weak ionization efficiency in MS compared with saponins, which possess sugar moieties.290 Digoxin is generally selected as the internal standard for MS quantification.
The oral bioavailability of PPD-type saponins (ginsenosides Ra3,293 Rb1,289 Rd,271 Rg3,278 and Rh2294) and PPT-type saponins (ginsenosides Rg1,274 Re,270 Rh1,275 and notoginsenoside R1289) is less than 5%.295 Excretion of ginseng saponins at 0.2–1.2% can be expected in human urine.296 Extensive metabolism in the gastrointestinal tract,279,297 poor membrane permeability,293 and lowered solubility of deglycosylated products298 limit intestinal absorption of ginseng saponins. Higher oral doses might saturate metabolism and increase the bioavailability.284,288 PPT-type saponins have better bioavailability than that of PPD group,288,289 perhaps because PPD-type saponins degrade faster than the PPT-type. IH-901 or compound K, an intestinal bacterial metabolite of PPD-type saponins, contributes to low bioavailability because of biliary excretion and hepatic metabolism via esterification with fatty acids.283,284 Changing the pharmaceutical formulation may improve bioavailability. For instance, micronized Rh2 has a doubled bioavailability;298 the inclusion complex of IH901 and the water-soluble agent β-cyclodextrin offers 1.9-fold higher bioavailability than pure IH901 powder.299 The bioavailability of PPD aglycone and 25-OH-PPD is improved because of less metabolism and favorable membrane permeability.285–287
The time to reach maximum concentration (Tmax) in plasma and tissues is generally less than 2 h, indicating that ginseng saponins are rapidly absorbed and readily distributed in the tissues.276,300 Levels of a ginseng saponin in the system depend on its elimination half-life (T1/2). Most ginseng saponins and their deglycosylated products were excreted by the biliary system through active transport, which affected their T1/2 values and systemic exposure.293 Time curves of ginseng saponins exhibited distinct multiple peaks after oral administration, indicating the involvement of enterohepatic recirculation.284
Tissue disposition demonstrated that liver and bile are responsible for systemic clearance of ginseng saponins from the circulation.283,284,300 Hepatic cytochrome P450 catalyzed ginsenoside metabolism has been described, and CYP3A4-catalyzed oxygenation metabolism played an important role in the hepatic disposition of ginsenosides. Table 8 shows that Ra3, Rb1, Rc and Rd have significantly longer T1/2 values (18.57–39.4 h) than other ginseng saponins (0.2–4.77 h). Attachment of more sugar moieties in the PPD-type ginsenosides Ra3, Rb1, Rc and Rd blocks their access to biliary transporters and slows biliary excretion.293 Some PK parameters shown in Table 8 are far from the mean values, such as Tmax and T1/2 of 25-OH-PPD and Rd. Whether this variation is caused by different factors or experimental conditions requires further evaluation.
![Proposed in vivo metabolic pathways of ginseng saponins. (A) protopanaxadiol-type saponins. (B) protopanaxatriol-type saponins. “” denotes major pathways; “” denotes additional pathways. [P]: metabolites detected in plasma; [U]: metabolites in urine; [F]: metabolites in feces; [B]: metabolites in bile. ig: metabolites after oral administration; iv: metabolites after intravenous injection.](/image/article/2011/NP/c0np00057d/c0np00057d-f10.gif) | ||
Fig. 10 Proposed in vivo metabolic pathways of ginseng saponins. (A) protopanaxadiol-type saponins. (B) protopanaxatriol-type saponins. “ ” denotes major pathways; “ ” denotes major pathways; “ ” denotes additional pathways. [P]: metabolites detected in plasma; [U]: metabolites in urine; [F]: metabolites in feces; [B]: metabolites in bile. ig: metabolites after oral administration; iv: metabolites after intravenous injection. ” denotes additional pathways. [P]: metabolites detected in plasma; [U]: metabolites in urine; [F]: metabolites in feces; [B]: metabolites in bile. ig: metabolites after oral administration; iv: metabolites after intravenous injection. | ||
The conversion of ginseng saponins in the gastrointestinal tract also has been studied using in vitro models such as acid conditions, enzymes, intestinal bacteria and microsomes.198,301 Because of high ginsenoside concentration used in the in vitro studies, the metabolic profiles may differ from in vivo results.302 The main metabolic pathways in vivo include deglycosylation reactions in ginseng saponins by intestinal bacteria via stepwise cleavage of the sugar moieties.293,295,297 Oxygenation by intestinal and hepatic CYP450 enzymes is another metabolic pathway.275,281,303 Oxygenation generally occurred on the ‘top-right’ aliphatic chain.282 Parental metabolites that could be further esterified with fatty acids in liver and tissues sustain fatty acid conjugates longer in the body.304 After oral administration of P. notoginseng extract to rats, the most abundant metabolites in rat feces were IH-901, Rg1 and F1.293 PPD and PPT aglycones were detected in low quantities in feces and not detected in plasma, bile and urine.293 After human volunteers ingested Ginsana G115 capsules (4% ginseng saponins), IH-901, Rh1 and F1 were the major metabolites found in the systemic circulation and excreted in urine.297 In some publications, 20(S)- and 20(R)-ginsenosides were not differentiated.278–281 Only a few studies have compared the metabolism and pharmacokinetics of 20(S)- and 20(R)-ginsenosides.276
Because of competitive absorption and metabolism between the various components, differences can be observed between the administration of a single ginsenoside and of ginseng extract. The metabolic profile of a ginsenoside can also vary with different methods of administration. For instance, Rb1 is the dominant metabolite after intravenous injection of Rd, and Rg3 is the major metabolite after oral administration of Rd.273
The principal analytical techniques for metabonomic studies are based on NMR spectroscopy and mass spectrometry. All metabonomic studies result in complex, multivariate datasets that require visualization software and chemometric and bioinformatic methods for interpretation. Wang et al.138 reported metabolic regulatory network alterations in response to acute cold stress in the rat and changes after ginsenoside intervention. Urinary metabolite profiling using GC–MS with multivariate statistical techniques revealed biochemical changes of these metabolites and demonstrated the protective effect of total ginseng saponins. Several metabolic pathways were identified in metabolic regulation and compensation to restore homeostasis. Wang et al.309 performed a metabonomics study on the effects of the ginsenoside Rg3 on liver-tumor-bearing rats. They combined column-switching with the orthogonal selectivity of hydrophilic interaction chromatography and reversed-phase chromatography. The administration of a single, high dose of Rg3 changed the metabolic pattern in cancer rats during the three days studied, and 17 biomarker candidates were identified.
For such studies, complex data must be interpreted and combined with analytical methods. One problem is that the number of metabolites produced by a given system is difficult to predict. Given the potential interest in this field, metabonomics might be useful for a comprehensive evaluation of the systemic clinical efficacy, safety, and mechanism of action of ginseng.
Zou et al.314,315 proposed biological fingerprinting to investigate the binding characteristics of natural products with target biomacromolecules that are free or immobilized in affinity chromatography. Bioactive compounds that specifically bind target biomacromolecules can be screened from complex herbal medicines. Li et al.1 developed holistic methods and integrated evaluation models to investigate biomacromolecule-binding compounds in ginseng preparations. Crude extracts are divided into two major fractions, namely, fractions unrelated to bioactivities and fractions related to bioactivities. The latter fraction is then characterized by on-line HPLC–MS. Structural information and binding parameters of each captured compound can be determined. A good example is the recent development of 2-D turbulent flow chromatography (TFC) coupled on-line to LC–MS for solution-based ligand screening against multiple proteins, shown in Fig. 11.313 This technique is an efficient tool for high-throughput screening of library ligands with low-to-high binding affinities and for evaluating the structure–activity relationship.
 | ||
| Fig. 11 Schematic diagram of the two-dimensional turbulent flow column-switching LC–MS apparatus, modified from ref. 313. Ginseng extract or ginseng saponin is incubated with the target biomacromolecule. The first turbulent flow chromatography (TFC), which only retains low-molecular-weight analytes, is used to separate free compounds and the protein–ginsenoside complex. The captured complex is broken up in the reaction wire by a dissociation solution. The compounds released are separated and enriched from biomacromolecules by the second TFC. On-line LC–MS is used to determine the structural information and affinity parameters of the binding compounds. | ||
Wang et al.316 screened anti-platelet aggregation agents from P. notoginseng using human platelet extraction and HPLC–DAD-ESI-MS/MS analysis. Five compounds that bind with human platelets were detected, and four were identified as adenosine, guanosine, ginsenoside Rh1 and F1. Liu et al.317 studied the interaction of total saponins of P. notoginseng with human serum albumin (HSA) by FTIR spectroscopy. Ginseng saponins were shown to combine with HSA through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups of polypeptide chain. The ginsenoside–protein combination caused a significant loss of α-helix structure and changes of the tyrosine residues in protein. Dasgupta et al.318 compared the effects of five different ginseng species on serum digoxin measurement by immunoassays. Digibind, an antidigoxin antibody, can bind free digoxin-like immunoreactive components of ginseng. Thus, the ginsenoside–biomacromolecule interaction reveals complex interconnected biological networks, which may help with discovery of new protein targets and drug candidates.
N groups of polypeptide chain. The ginsenoside–protein combination caused a significant loss of α-helix structure and changes of the tyrosine residues in protein. Dasgupta et al.318 compared the effects of five different ginseng species on serum digoxin measurement by immunoassays. Digibind, an antidigoxin antibody, can bind free digoxin-like immunoreactive components of ginseng. Thus, the ginsenoside–biomacromolecule interaction reveals complex interconnected biological networks, which may help with discovery of new protein targets and drug candidates.
5 Concluding remarks and perspectives
Compounds isolated from ginseng are an abundant source of chemical diversity from which to discover active molecules. Synthesis or biosynthesis is the preferred way to produce novel compounds for pharmacological activities. Synthesis also helps us understand structure–activity relationships. Besides sample preparation and purification steps, current isolation protocols often comprise in vitro assays, which are frequently coupled on-line to HPLC or MS systems.A major challenge of ginseng research is the complexity of chemical constituents, especially polar macromolecules. However, technical advances in analytical tools are promising. Chromatography shows a trend towards faster and more comprehensive separations, indicated by wide applications of UPLC, HILIC and multidimensional chromatography. Recent innovations have made mass spectrometers more sensitive and selective, and MS and tandem MS are now applied in numerous analytical fields of ginseng research.
One approach to investigating ginseng is to view the extract as an indivisible whole, a self-organized and interactive multicomponent entity. Plant metabolomic fingerprinting and metabonomics of ginseng-treated biological samples are holistic approaches to quality control and activity evaluation, respectively. Other better integral analyses are needed to measure complex ginseng constituents, their metabolites and other related macromolecules.
Ginseng analysis requires expertise in chemistry, chemometrics, biology, and bioinformatics. With the exponentially increasing amount of data from ginseng plant and ginseng-treated biological samples, collaboration among chemists, biologists, and computer scientists is important. We believe that there are good prospects for obtaining new insights into drug discovery and clinical utility by the continued study of ginseng.
6 Acknowledgements
Special thanks go to Dr Lorena Tomas Laudo for encouraging us to prepare this review. We also thank Sally Kozlik for editing the manuscript. This work was supported in part by the NIH grants P01 AT004418 and K01 AT005362.7 References
- P. Li, L. W. Qi, E. H. Liu, J. L. Zhou and X. D. Wen, TrAC, Trends Anal. Chem., 2008, 27, 66–77 CrossRef CAS.
- L. Jia and Y. Q. Zhao, Curr. Med. Chem., 2009, 16, 2475–2484 CrossRef CAS.
- C. Z. Wang, H. H. Aung, M. Ni, J. A. Wu, R. B. Tong, S. Wicks, T. C. He and C. S. Yuan, Planta Med., 2007, 73, 669–674 CrossRef CAS.
- A. B. Fishbein, C. Z. Wang, X. L. Li, S. R. Mehendale, S. Sun, H. H. Aung and C. S. Yuan, Arch. Pharmacal Res., 2009, 32, 505–513 Search PubMed.
- G. Y. Li, Y. M. Zeng, H. Meng, X. Li and J. H. Wang, Chin. Chem. Lett., 2009, 20, 1207–1210 CrossRef CAS.
- J. T. Xie, C. Z. Wang, B. Zhang, S. R. Mehendale, X. L. Li, S. Sun, A. H. Han, W. Du, T. C. He and C. S. Yuan, Biol. Pharm. Bull., 2009, 32, 1552–1558 CrossRef CAS.
- J. T. Xie, S. R. Mehendale, A. B. Wang, A. H. Han, J. A. Wu, J. Osinski and C. S. Yuan, Pharmacol. Res., 2004, 49, 113–117 CrossRef CAS.
- T. K. Yun, Lancet Oncol., 2001, 2, 49–55 CrossRef CAS.
- L. W. Qi, C. Z. Wang and C. S. Yuan, Biochem. Pharmacol., 2010, 80, 947–954 CrossRef CAS.
- L. Jia, Y. Zhao and X. J. Liang, Curr. Med. Chem., 2009, 16, 2924–2942 CrossRef CAS.
- L. P. Christensen, Adv. Food Nutr. Res., 2009, 55, 1–99 Search PubMed.
- N. Angelova, H. W. Kong, R. Van Der Heijden, S. Y. Yang, Y. H. Choi, H. K. Kim, M. Wang, T. Hankemeier, J. Van Der Greef, G. Xu and R. Verpoorte, Phytochem. Anal., 2008, 19, 2–16 CrossRef CAS.
- N. Fuzzati, J. Chromatogr., B, 2004, 812, 119–133 CrossRef CAS.
- S. J. Kim, H. N. Murthy, E. J. Hahn, H. L. Lee and K. Y. Paek, Sep. Purif. Technol., 2007, 56, 401–406 CrossRef CAS.
- J. A. Wood, M. A. Bernards, W. K. Wan and P. A. Charpentier, J. Supercrit. Fluids, 2006, 39, 40–47 CrossRef CAS.
- T. Ligor, A. Ludwiczuk, T. Wolski and B. Buszewski, Anal. Bioanal. Chem., 2005, 383, 1098–1105 CrossRef CAS.
- B. S. Sun, L. J. Gu, Z. M. Fang, C. Y. Wang, Z. Wang, M. R. Lee, Z. Li, J. J. Li and C. K. Sung, J. Pharm. Biomed. Anal., 2009, 50, 15–22 CrossRef CAS.
- J. B. Wan, S. P. Li, J. M. Chen and Y. T. Wang, J. Sep. Sci., 2007, 30, 825–832 CrossRef CAS.
- J. H. Kwon, J. M. Belanger and J. R. Pare, J. Agric. Food Chem., 2003, 51, 1807–1810 CrossRef CAS.
- Y. T. Wang, J. Y. You, Y. Yu, C. F. Qu, H. R. Zhang, L. Ding, H. Q. Zhang and X. W. Li, Food Chem., 2008, 110, 161–167 CrossRef CAS.
- A. S. Engelberth, E. C. Clausen and D. J. Carrier, Sep. Purif. Technol., 2010, 72, 1–6 CrossRef CAS.
- J. Wu, L. Lin and F. T. Chau, Ultrason. Sonochem., 2001, 8, 347–352 CrossRef CAS.
- Y. Yang, L. Chen, X. X. Zhang and Z. K. Guo, J. Liq. Chromatogr. Relat. Technol., 2004, 27, 3203–3211 CrossRef CAS.
- T. T. Dong, K. J. Zhao, W. Z. Huang, K. W. Leung and K. W. Tsim, Phytother. Res., 2005, 19, 684–688 CrossRef CAS.
- R. M. Corbit, J. F. S. Ferreira, S. D. Ebbs and L. L. Murphy, J. Agric. Food Chem., 2005, 53, 9867–9873 CrossRef CAS.
- S. Gafner, C. Bergeron, M. M. McCollom, L. M. Cooper, K. L. McPhail, W. H. Gerwick and C. K. Angerhofer, J. Agric. Food Chem., 2004, 52, 1546–1550 CrossRef CAS.
- M. P. K. Choi, K. K. C. Chan, H. W. Leung and C. W. Huie, J. Chromatogr., A, 2003, 983, 153–162 CrossRef CAS.
- C. L. Qu, Y. P. Bai, X. Q. Jin, Y. T. Wang, K. Zhang, J. Y. You and H. Q. Zhang, Food Chem., 2009, 115, 340–346 CrossRef CAS.
- X. W. Du, R. B. H. Wills and D. L. Stuart, Food Chem., 2004, 86, 155–159 CrossRef CAS.
- H. T. Nguyen, G. Y. Song, J. A. Kim, J. H. Hyun, H. K. Kang and Y. H. Kim, Bioorg. Med. Chem. Lett., 20, pp. 309–314 Search PubMed.
- N. H. Tung, G. Y. Song, N. X. Nhiem, Y. Ding, B. H. Tai, L. G. Jin, C. M. Lim, J. W. Hyun, C. J. Park, H. K. Kang and Y. H. Kim, J. Agric. Food Chem., 58, pp. 868–874 Search PubMed.
- D. Q. Dou, Y. J. Chen, L. H. Liang, F. G. Pang, N. Shimizu and T. Takeda, Chem. Pharm. Bull., 2001, 49, 442–446 CrossRef CAS.
- P. Y. Liao, D. Wang, Y. J. Zhang and C. R. Yang, J. Agric. Food Chem., 2008, 56, 1751–1756 CrossRef CAS.
- N. H. Tung, K. Cho, J. A. Kim, G. Y. Song and Y. H. Kim, Bull. Korean Chem. Soc., 2010, 31, 1381–1384 CrossRef CAS.
- S. Nakamura, S. Sugimoto, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2007, 55, 1342–1348 CrossRef CAS.
- I. H. Park, N. Y. Kim, S. B. Han, J. M. Kim, S. W. Kwon, H. J. Kim, M. K. Park and J. H. Park, Arch. Pharmacal Res., 2002, 25, 428–432 Search PubMed.
- O. Sticher, Nat. Prod. Rep., 2008, 25, 517–554 RSC.
- Q. Z. Du, G. Jerz, R. Waibel and P. Winterhalter, J. Chromatogr., A, 2003, 1008, 173–180 CrossRef CAS.
- X. C. Qi, S. Ignatova, G. A. Luo, Q. L. Liang, F. W. Jun, Y. M. Wang and I. Sutherland, J. Chromatogr., A, 2010, 1217, 1995–2001 CrossRef CAS.
- X. L. Cao, Y. Tian, T. Y. Zhang, Q. H. Liu, L. J. Jia and Y. Ito, J. Liq. Chromatogr. Relat. Technol., 2003, 26, 1579–1591 CrossRef CAS.
- Y. W. Ha, S. S. Lim, I. J. Ha, Y. C. Na, J. J. Seo, H. Shin, S. H. Son and Y. S. Kim, J. Chromatogr., A, 2007, 1151, 37–44 CrossRef CAS.
- Y. J. Cheng, Q. L. Liang, P. Hu, Y. M. Wang, F. W. Jun and G. A. Luo, Sep. Purif. Technol., 2010, 73, 397–402 CrossRef CAS.
- J. Wang, H. L. Bai, C. M. Liu and L. Li, Chromatographia, 2010, 71, 267–271 CrossRef CAS.
- Y. Ito, J. Chromatogr., A, 2005, 1065, 145–168 CrossRef CAS.
- H. P. Jiang, Y. K. Qiu, D. R. Cheng, T. G. Kang and D. Q. Dou, Magn. Reson. Chem., 2008, 46, 786–790 CrossRef CAS.
- J. Chen, R. Zhao, Y. M. Zeng, H. Meng, W. J. Zuo, X. Li and J. H. Wang, J. Asian Nat. Prod. Res., 2009, 11, 195–201 CrossRef CAS.
- S. Nakamura, S. Sugimoto, H. Matsuda and M. Yoshikawa, Heterocycles, 2007, 71, 577–588 CrossRef CAS.
- W. Wang, Y. Q. Zhao, E. R. Rayburn, D. L. Hill, H. Wang and R. W. Zhang, Cancer Chemother. Pharmacol., 2007, 59, 589–601 CrossRef CAS.
- J. Y. Wang, X. G. Li, Y. N. Zheng and X. W. Yang, J. Asian Nat. Prod. Res., 2004, 6, 289–293 CrossRef CAS.
- C. Z. Wang, J. A. Wu, E. McEntee and C. S. Yuan, J. Agric. Food Chem., 2006, 54, 2261–2266 CrossRef CAS.
- S. Sugimoto, S. Nakamura, H. Matsuda, N. Kitagawa and M. Yoshikawa, Chem. Pharm. Bull., 2009, 57, 283–287 CrossRef CAS.
- S. Sun, C. Z. Wang, R. Tong, X. L. Li, A. Fishbein, Q. Wang, T. C. He, W. Du and C. S. Yuan, Food Chem., 2010, 118, 307–314 CrossRef CAS.
- S. M. Lee, H. J. Shon, C. S. Choi, T. M. Hung, B. S. Min and K. Bae, Chem. Pharm. Bull., 2009, 57, 92–94 CrossRef CAS.
- H. T. Nguyen, G. Y. Song, H. K. Kang and Y. H. Kim, Bull. Korean Chem. Soc., 2010, 31, 2094–2096 CrossRef.
- C. Z. Wang, B. Zhang, W. X. Song, A. B. Wang, M. Ni, X. J. Luo, H. H. Aung, J. T. Xie, R. Tong, T. C. He and C. S. Yuan, J. Agric. Food Chem., 2006, 54, 9936–9942 CrossRef CAS.
- M. Yoshikawa, T. Murakami, K. Yashiro, J. Yamahara, H. Matsuda, R. Saijoh and O. Tanaka, Chem. Pharm. Bull., 1998, 46, 647–654 CAS.
- M. Yoshikawa, S. Sugimoto, S. Nakamura, H. Sakumae and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 1034–1038 CrossRef CAS.
- Q. Le Tran, I. K. Adnyana, Y. Tezuka, T. Nagaoka, Q. K. Tran and S. Kadota, J. Nat. Prod., 2001, 64, 456–461 CrossRef CAS.
- X. Y. Wang, D. Wang, X. X. Ma, Y. J. Zhang and C. R. Yang, Helv. Chim. Acta, 2008, 91, 60–66 CrossRef CAS.
- M. Yoshikawa, T. Morikawa, K. Yashiro, T. Murakami and H. Matsuda, Chem. Pharm. Bull., 2001, 49, 1452–1456 CrossRef CAS.
- X. M. Cui, Z. Y. Jiang, J. Zeng, J. M. Zhou, J. J. Chen, X. M. Zhang, L. S. Xu and Q. Wang, J. Asian Nat. Prod. Res., 2008, 10, 845–849 CrossRef CAS.
- R. W. Teng, H. Z. Li, D. Z. Wang and C. R. Yang, Helv. Chim. Acta, 2004, 87, 1270–1278 CrossRef CAS.
- H. Sun, Y. Ye and Y. Pan, Chem. Biodiversity, 2005, 2, 510–515 CrossRef CAS.
- K. Zou, S. Zhu, C. Tohda, S. Q. Cai and K. Komatsu, J. Nat. Prod., 2002, 65, 346–351 CrossRef CAS.
- J. M. Jia, Z. Q. Wang and L. J. Wu, Chin. Chem. Lett., 2008, 19, 1099–1102 CrossRef CAS.
- C. C. Ruan, Z. Liu, X. Li, X. Liu, L. J. Wang, H. Y. Pan, Y. N. Zheng, G. Z. Sun, Y. S. Zhang and L. X. Zhang, Molecules, 2010, 15, 2319–2325 Search PubMed.
- Y. N. Meng, X. G. Li, Y. N. Meng, J. Y. Wang, Y. N. Zheng and X. W. Yang, Chem. J. Chin. Univ., 2007, 28, 1316–1318.
- J. T. Chen, H. Z. Li, D. Wang, Y. J. Zhang and C. R. Yang, Helv. Chim. Acta, 2006, 89, 1442–1448 CrossRef CAS.
- M. Yoshikawa, T. Morikawa, Y. Kashima, K. Ninomiya and H. Matsuda, J. Nat. Prod., 2003, 66, 922–927 CrossRef CAS.
- K. Zou, S. Zhu, M. R. Meselhy, C. Tohda, S. Q. Cai and K. Komatsu, J. Nat. Prod., 2002, 65, 1288–1292 CrossRef CAS.
- M. Yoshikawa, S. Sugimoto, S. Nakamura and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 571–576 CrossRef CAS.
- J. R. Wang, Y. Yamasaki, T. Tanaka, I. Kouno and Z. H. Jiang, Molecules, 2009, 14, 2087–2094 Search PubMed.
- F. Qiu, Z. Z. Ma, S. X. Xu, X. S. Yao, C. T. Che and Y. J. Chen, J. Asian Nat. Prod. Res., 2001, 3, 235–240 CrossRef CAS.
- H. T. Nguyen, G. Y. Song, V. M. Chau, V. K. Phan, L. G. Jin, H. J. Boo, H. K. Kang and Y. H. Kim, Chem. Pharm. Bull., 2010, 58, 1111–1115 CrossRef.
- N. Komakine, M. Okasaka, Y. Takaishi, K. Kawazoe, K. Murakami and Y. Yamada, J. Nat. Med., 2006, 60, 135–137 Search PubMed.
- N. H. Tung, G. Y. Song, Y. J. Park and Y. H. Kim, Chem. Pharm. Bull., 2009, 57, 1412–1414 CrossRef.
- J. H. Wang, W. Li, Y. Sha, Y. Tezuka, S. Kadota and X. Li, J. Asian Nat. Prod. Res., 2001, 3, 123–130 CrossRef CAS.
- J. Huang, X. H. Tang, T. Ikejima, X. J. Sun, X. B. Wang, R. G. Xi and L. J. Wu, Arch. Pharmacal Res., 2008, 31, 323–329 Search PubMed.
- J. Wang, Y. Sha, W. Li, Y. Tezuka, S. Kadota and X. Li, J. Asian Nat. Prod. Res., 2001, 3, 293–297 CrossRef CAS.
- Y. K. Qiu, D. Q. Dou, L. P. Cai, H. P. Jiang, T. G. Kang, B. Y. Yang, H. X. Kuang and M. Z. C. Li, Fitoterapia, 2009, 80, 219–222 CrossRef CAS.
- Y. Zhao, W. Wang, L. Han, E. R. Rayburn, D. L. Hill, H. Wang and R. Zhang, Med. Chem., 2007, 3, 51–60 CrossRef CAS.
- L. N. Tao, Q. Meng, J. Y. Yin, R. Xing and H. R. Guo, Chin. Chem. Lett., 2009, 20, 687–689 CrossRef CAS.
- X. W. Yang, L. Y. Li, J. M. Tian, Z. W. Zhang, J. M. Ye and W. F. Gu, Chin. Chem. Lett., 2000, 11, 909–912 CAS.
- I. H. Park, S. B. Han, J. M. Kim, L. Z. Piao, S. W. Kwon, N. Y. Kim, T. L. Kang, M. K. Park and J. H. Park, Arch. Pharmacal Res., 2002, 25, 837–841 Search PubMed.
- D. Dou, W. Li, N. Guo, R. Fu, Y. Pei, K. Koike and T. Nikaido, Chem. Pharm. Bull., 2006, 54, 751–753 CrossRef CAS.
- L. B. Wang, Z. H. Wu, H. Y. Gao, J. Huang, B. H. Sun and L. J. Wu, Chin. Chem. Lett., 2008, 19, 837–840 CrossRef CAS.
- L. J. Wu, L. B. Wang, H. Y. Gao, B. Wu, X. M. Song and Z. S. Tang, Fitoterapia, 2007, 78, 556–560 CrossRef CAS.
- H. Z. Li, R. W. Teng and C. R. Yang, Chin. Chem. Lett., 2001, 12, 59–62 CAS.
- H. J. Zhang, Z. Z. Lu, G. T. Tan, S. X. Qiu, N. R. Farnsworth, J. M. Pezzuto and H. H. S. Fong, Tetrahedron Lett., 2002, 43, 973–977 CrossRef CAS.
- Y. Wei, C. M. Ma and M. Hattori, Phytochem. Lett., 2009, 2, 63–66 Search PubMed.
- J. H. Liu, C. S. Lee, K. M. Leung, Z. K. Yan, B. H. Shen, Z. Z. Zhao and Z. H. Jiang, J. Agric. Food Chem., 2007, 55, 8830–8835 CrossRef CAS.
- M. C. Yang, H. C. Kwon, Y. J. Kim, K. R. Lee and H. O. Yang, J. Nat. Prod., 2010, 73, 801–805 CrossRef CAS.
- H. H. Chan, H. D. Sun, M. V. B. Reddy and T. S. Wu, Phytochemistry, 2010, 71, 1360–1364 CrossRef CAS.
- S. W. Lee, K. Kim, M. C. Rho, M. Y. Chung, Y. H. Kim, S. Lee, H. S. Lee and Y. K. Kim, Planta Med., 2004, 70, 197–200 CrossRef CAS.
- K. Hirakura, K. Sugama, M. Morita, K. Nakajima, H. Sasaki, M. Okada and H. Sato, Heterocycles, 2000, 53, 2451–2457 CrossRef CAS.
- K. Rittenbach, R. Ilarraza, Y. Wu, F. Davoine and D. J. Adamko, Pharm. Biol., 2009, 47, 17–17.
- R. C. Y. Choi, J. T. T. Zhu, K. W. Leung, G. K. Y. Chu, H. Q. Xie, V. P. Chen, K. Y. Z. Zheng, D. T. W. Lau, T. T. X. Dong, P. C. Y. Chow, Y. F. Han, Z. T. Wang and K. W. K. Tsim, J. Alzheimers Dis., 2010, 19, 795–811 CAS.
- C. D. Liu, J. Chen and J. H. Wang, Chem. Nat. Compd., 2009, 45, 808–810 CrossRef CAS.
- M. V. Naval, M. P. Gomez-Serranillos, M. E. Carretero and C. De Arce, J. Chromatogr., A, 2006, 1121, 242–247 CrossRef CAS.
- J. Y. Wang, X. G. Li and X. W. Yang, J. Asian Nat. Prod. Res., 2006, 8, 605–608 CrossRef CAS.
- Y. Liu, J. L. Zhou, P. Liu, S. Sun and P. Li, J. Chromatogr. A, 1217, pp. 5239–5245 Search PubMed.
- D. Y. Hui, J. Nutr., 1998, 128, 2052–2057 CAS.
- J. T. Xie, H. H. Aung, J. A. Wu, A. S. Attele and C. S. Yuan, Am. J. Chin. Med., 2002, 30, 187–194 Search PubMed.
- A. B. Wang, C. Z. Wang, J. A. Wu, J. Osinski and C. S. Yuan, Phytochem. Anal., 2005, 16, 272–277 CrossRef CAS.
- X. H. Zhang, H. L. Liu and J. M. Chen, J. Chromatogr. Sci., 2005, 43, 47–51 CAS.
- S. P. Ip and C. T. Che, J. Chromatogr., A, 2006, 1135, 241–244 CrossRef CAS.
- N. Fukuda, H. Tanaka and Y. Shoyama, Biol. Pharm. Bull., 1999, 22, 219–220 CAS.
- N. Fukuda, H. Tanaka and Y. Shoyama, Cytotechnology, 2000, 34, 197–204 CrossRef CAS.
- O. Morinaga, N. Fukuda, H. Tanaka and Y. Shoyama, Glycobiology, 2005, 15, 1061–1066 CrossRef CAS.
- O. Morinaga, H. Tanaka and Y. Shoyama, J. Chromatogr., B, 2006, 830, 100–104 CrossRef CAS.
- S. J. Shan, H. Tanaka and Y. Shoyama, Anal. Chem., 2001, 73, 5784–5790 CrossRef CAS.
- N. Fukuda, H. Tanaka and Y. Shoyama, Analyst, 2000, 125, 1425–1429 RSC.
- H. Tanaka, N. Fukuda and Y. Shoyama, Phytochem. Anal., 2006, 17, 46–55 CrossRef CAS.
- H. Tanaka, N. Fukuda and Y. Shoyama, J. Agric. Food Chem., 2007, 55, 3783–3787 CrossRef CAS.
- H. Tanaka, N. Fukuda, S. Yahara, S. Isoda, C. S. Yuan and Y. Shoyama, Phytother. Res., 2005, 19, 255–258 CrossRef CAS.
- W. Putalun, N. Fukuda, H. Tanaka and Y. Shoyama, Anal. Bioanal. Chem., 2004, 378, 1338–1341 CrossRef CAS.
- C. Seger and S. Sturm, J. Proteome Res., 2007, 6, 480–497 CrossRef CAS.
- F. van der Kooy, F. Maltese, Y. H. Choi, H. K. Kim and R. Verpoorte, Planta Med., 2009, 75, 763–775 CrossRef CAS.
- B. Rasmussen, O. Cloarec, H. R. Tang, D. Staerk and J. W. Jaroszewski, Planta Med., 2006, 72, 556–563 CrossRef CAS.
- J. Kang, S. Lee, S. Kang, H. N. Kwon, J. H. Park, S. W. Kwon and S. Park, Arch. Pharmacal Res., 2008, 31, 330–336 Search PubMed.
- W. N. Lin, H. Y. Lu, M. S. Lee, S. Y. Yang, H. J. Chen, Y. S. Chang and W. T. Chang, Am. J. Chin. Med., 2010, 38, 205–218 Search PubMed.
- S. Y. Yang, H. K. Kim, A. W. M. Lefeber, C. Erkelens, N. Angelova, Y. H. Choi and R. Verpoorte, Planta Med., 2006, 72, 364–369 CrossRef CAS.
- K. Y. L. Yap, S. Y. Chan and C. S. Lim, J. Biomed. Sci., 2007, 14, 265–273 CrossRef CAS.
- K. Y. L. Yap, T. K. H. Lai, S. Y. Chan and C. S. Lim, J. AOAC Int., 2009, 92, 672–679 CAS.
- Y. J. Lu, Y. L. Qu, Z. Q. Cao and M. Song, Spectrosc. Spect. Anal., 2006, 26, 1457–1459 Search PubMed.
- X. J. Jin, Y. Zhang, Y. F. Xie, Q. Cong and B. Zhao, Spectrosc. Spect. Anal., 2009, 29, 656–660 Search PubMed.
- D. T. Li, C. G. Cheng, Z. X. Du, Y. Q. He and L. C. Kong, Spectrosc. Spect. Anal., 2006, 26, 2186–2189 Search PubMed.
- G. H. Lu, Q. Zhou, S. Q. Sun, K. S. Y. Leung, H. Zhang and Z. Z. Zhao, J. Mol. Struct., 2008, 883, 91–98 CrossRef.
- D. Liu, Y. G. Li, H. Xu, S. Q. Sun and Z. T. Wang, J. Mol. Struct., 2008, 883, 228–235 CrossRef.
- Y. M. Li, S. Q. Sun, Q. Zhou, Z. Qin, J. X. Tao, J. Wang and X. Fang, Vib. Spectrosc., 2004, 36, 227–232 CrossRef CAS.
- Y. L. Zhang, J. B. Chen, Y. Lei, Q. Zhou, S. Q. Sun and I. Noda, J. Mol. Struct., 2010, 974, 94–102 CrossRef CAS.
- J. J. Mao and J. W. Xu, Spectrochim. Acta, Part A, 2006, 65, 497–500 CrossRef.
- M. Baranska, H. Schulz and L. P. Christensen, J. Agric. Food Chem., 2006, 54, 3629–3635 CrossRef CAS.
- A. Ludwiczuk, T. Wolski and S. Berbec, J. Planar Chromatogr.–Mod. TLC, 2002, 15, 147–150 CrossRef CAS.
- T. Ohno, E. Mikami and H. Oka, J. Nat. Med., 2006, 60, 141–145 Search PubMed.
- Y. Q. Qiu, X. Lu, T. Pang, C. F. Ma, X. Li and G. W. Xu, J. Sep. Sci., 2008, 31, 3451–3457 CrossRef CAS.
- X. Di, R. A. Shellie, P. J. Marriott and C. W. Huie, J. Sep. Sci., 2004, 27, 451–458 CrossRef CAS.
- X. Y. Wang, M. M. Su, Y. P. Qiu, Y. Ni, T. Zhao, M. M. Zhou, A. H. Zhao, S. L. Yang, L. P. Zhao and W. Jia, J. Proteome Res., 2007, 6, 3449–3455 CrossRef CAS.
- W. Lim, K. W. Mudge and F. Vermeylen, J. Agric. Food Chem., 2005, 53, 8498–8505 CrossRef CAS.
- W. K. Li and J. F. Fitzloff, J. Liq. Chromatogr. Relat. Technol., 2002, 25, 17–27 CrossRef CAS.
- T. W. D. Chan, P. P. H. But, S. W. Cheng, I. M. Y. Kwok, F. W. Lau and H. X. Xu, Anal. Chem., 2000, 72, 1281–1287 CrossRef CAS.
- H. L. Koh, A. J. Lau and E. C. Y. Chan, Rapid Commun. Mass Spectrom., 2005, 19, 1237–1244 CrossRef CAS.
- D. W. Lou, Y. Saito, P. K. Zarzycki, M. Ogawa and K. Jinno, Anal. Bioanal. Chem., 2006, 385, 96–104 CrossRef CAS.
- N. S. Quiming, N. L. Denola, Y. Saito and K. Jinno, J. Sep. Sci., 2008, 31, 1550–1563 CrossRef CAS.
- N. S. Quiming, N. L. Denola, A. B. Soliev, Y. Saito and K. Jinno, Anal. Bioanal. Chem., 2007, 389, 1477–1488 CrossRef CAS.
- M. Dan, M. M. Su, X. F. Gao, T. Zhao, A. H. Zhao, G. X. Xie, Y. P. Qiu, M. M. Zhou, Z. Liu and W. Jia, Phytochemistry, 2008, 69, 2237–2244 CrossRef CAS.
- G. X. Xie, Y. Ni, M. M. Su, Y. Y. Zhang, A. H. Zhao, X. F. Gao, Z. Liu, P. G. Xiao and W. Jia, Metabolomics, 2008, 4, 248–260 CrossRef CAS.
- G. X. Xie, R. Plumb, M. M. Su, Z. H. Xu, A. H. Zhao, M. F. Qiu, X. B. Long, Z. Liu and W. Jia, J. Sep. Sci., 2008, 31, 1015–1026 CrossRef CAS.
- I. Glöckl, M. Veit and G. Blaschke, Planta Med., 2002, 68, 158–161 CrossRef CAS.
- J. Cao, L. W. Qi, J. Chen and P. Li, Electrophoresis, 2008, 29, 4422–4430 CrossRef CAS.
- J. Cao, L. Yi, P. Li and Y. X. Chang, J. Chromatogr., A, 2009, 1216, 5608–5613 CrossRef CAS.
- J. Cao, B. Li, Y. X. Chang and P. Li, Electrophoresis, 2009, 30, 1372–1379 CrossRef CAS.
- R. A. Shellie, P. J. Marriott and C. W. Huie, J. Sep. Sci., 2003, 26, 1185–1192 CrossRef CAS.
- E. Reich, A. Schibli and A. DeBatt, J. AOAC Int., 2008, 91, 13–20 CAS.
- P. Xie, S. Chen, Y. Z. Liang, X. Wang, R. Tian and R. Upton, J. Chromatogr., A, 2006, 1112, 171–180 CrossRef CAS.
- R. J. Vanhaelen-Fastre, M. L. Faes and M. H. Vanhaelen, J. Chromatogr., A, 2000, 868, 269–276 CrossRef CAS.
- C. Kevers, P. Jacques, T. Gaspar, P. Thonart and J. Dommes, J. Chromatogr. Sci., 2004, 42, 554–558 CAS.
- A. M. A. El-Aty, I. K. Kim, M. R. Kim, C. Lee and J. H. Shim, Biomed. Chromatogr., 2008, 22, 556–562 CrossRef.
- J. W. Wong, K. Zhang, K. Tech, D. G. Hayward, A. J. Krynitsky, I. Cassias, F. J. Schenck, K. Banerjee, S. Dasgupta and D. Brown, J. Agric. Food Chem., 2010, 58, 5884–5896 CrossRef CAS.
- D. G. Hayward and J. W. Wong, Anal. Chem., 2009, 81, 5716–5723 CrossRef CAS.
- X. Y. Sun, Y. P. Zheng, D. H. Lin, H. Zhang, F. Zhao and C. S. Yuan, Am. J. Chin. Med., 2009, 37, 589–596 Search PubMed.
- N. S. Quiming, N. L. Denola, A. B. Soliev, Y. Saito and K. Jinno, Chromatographia, 2007, 66, 5–11 CrossRef CAS.
- S. Louw, A. S. Pereira, F. Lynen, M. Hanna-Brown and P. Sandra, J. Chromatogr., A, 2008, 1208, 90–94 CrossRef CAS.
- L. Li, G. A. Luo, Q. L. Liang, P. Hu and Y. M. Wang, J. Pharm. Biomed. Anal., 2010, 52, 66–72 CrossRef.
- D. Muth, E. Marsden-Edwards, P. Kachlicki and M. Stobiecki, Phytochem. Anal., 2008, 19, 444–452 CrossRef CAS.
- D. Guillarme, J. Schappler, S. Rudaz and J. L. Veuthey, TrAC, Trends Anal. Chem., 2010, 29, 15–27 CrossRef CAS.
- J. Guan, C. M. Lai and S. P. Li, J. Pharm. Biomed. Anal., 2007, 44, 996–1000 CrossRef CAS.
- G. F. Deng, D. L. Wang, M. X. Meng, F. Hu and T. W. Yao, J. Chromatogr., B, 2009, 877, 2113–2122 CrossRef CAS.
- X. Y. Wang, T. Zhao, X. F. Gao, M. Dan, M. M. Zhou and W. Jia, Anal. Chim. Acta, 2007, 594, 265–273 CrossRef CAS.
- L. W. Qi, C. Y. Chen and P. Li, Rapid Commun. Mass Spectrom., 2009, 23, 3227–3242 CrossRef CAS.
- E. H. Liu, L. W. Qi, B. Li, Y. B. Peng, P. Li, C. Y. Li and J. Cao, Rapid Commun. Mass Spectrom., 2009, 23, 119–130 CrossRef CAS.
- T. J. Pappas, M. Gayton-Ely and L. A. Holland, Electrophoresis, 2005, 26, 719–734 CrossRef CAS.
- C. W. Huie, Electrophoresis, 2006, 27, 60–75 CrossRef CAS.
- J. H. Qin, F. C. Leung, Y. S. Fung, D. R. Zhu and B. C. Lin, Anal. Bioanal. Chem., 2005, 381, 812–819 CrossRef CAS.
- S. F. Wang, S. Ye and Y. Y. Cheng, J. Chromatogr., A, 2006, 1109, 279–284 CrossRef CAS.
- L. Ciesla and M. Waksmundzka-Hajnos, J. Chromatogr., A, 2009, 1216, 1035–1052 CrossRef CAS.
- R. K. Gilpin and C. S. Gilpin, Anal. Chem., 2009, 81, 4679–4694 CrossRef CAS.
- L. W. Qi, Q. T. Yu, P. Li, S. L. Li, Y. X. Wang, L. H. Sheng and L. Yi, J. Chromatogr., A, 2006, 1134, 162–169 CrossRef CAS.
- J. Cao, Y. J. Wei, L. W. Qi, P. Li, Z. M. Qian, H. W. Luo, J. Chen and J. Zhao, Biomed. Chromatogr., 2008, 22, 164–172 CrossRef CAS.
- S. N. Kim, Y. W. Ha, H. Shin, S. H. Son, S. J. Wu and Y. S. Kim, J. Pharm. Biomed. Anal., 2007, 45, 164–170 CrossRef CAS.
- W. K. Li and J. F. Fitzloff, J. Liq. Chromatogr. Relat. Technol., 2002, 25, 29–41 CrossRef CAS.
- T. Vehovec and A. Obreza, J. Chromatogr., A, 2010, 1217, 1549–1556 CrossRef CAS.
- C. C. Bai, S. Y. Han, X. Y. Chai, Y. Jiang, P. Li and P. F. Tu, J. Liq. Chromatogr. Relat. Technol., 2009, 32, 242–260 CrossRef CAS.
- L. Wang, W. S. He, H. X. Yan, Y. Jiang, K. S. Bi and P. F. Tu, Chromatographia, 2009, 70, 603–608 CrossRef CAS.
- K. S. Y. Leung, K. Chan, A. Bensoussan and M. J. Munroe, Phytochem. Anal., 2007, 18, 146–150 CrossRef CAS.
- X. Ma, H. B. Xiao and X. M. Liang, Chromatographia, 2006, 64, 31–36 CrossRef.
- X. Q. Ma, X. M. Liang, Q. Xu, X. Z. Zhang and H. B. Xiao, Phytochem. Anal., 2005, 16, 181–187 CrossRef CAS.
- Y. Liu, J. Li, J. He, Z. Abliz, J. Qu, S. Yu, S. Ma, J. Liu and D. Du, Rapid Commun. Mass Spectrom., 2009, 23, 667–679 CrossRef CAS.
- S. Liu, M. Cui, Z. Liu, F. Song and W. Mo, J. Am. Soc. Mass Spectrom., 2004, 15, 133–141 CrossRef CAS.
- B. D. Sloley, Y. C. J. Lin, D. Ridgway, H. A. Semple, Y. K. Tam, R. T. Coutts, R. Lobenberg and N. Tam-Zaman, J. AOAC Int., 2006, 89, 16–21 CAS.
- C. H. Xia, G. J. Wang, R. G. Sun, H. P. Hao, Y. Q. Xiong, S. H. Gu, L. L. Shang and C. N. Zheng, J. Chromatogr., B, 2008, 862, 72–78 CrossRef CAS.
- J. L. Zhou, P. Li, H. J. Li, Y. Jiang, M. T. Ren and Y. Liu, J. Chromatogr., A, 2008, 1177, 126–137 CrossRef CAS.
- J. W. Wong, M. K. Hennessy, D. G. Hayward, A. J. Krynitsky, I. Cassias and F. J. Schenck, J. Agric. Food Chem., 2007, 55, 1117–1128 CrossRef CAS.
- G. C. Kite, M. J. R. Howes, C. J. Leon and M. S. J. Simmonds, Rapid Commun. Mass Spectrom., 2003, 17, 238–244 CrossRef CAS.
- W. C. S. Cho, T. T. Yip, W. S. Chung, S. K. W. Lee, A. W. N. Leung, C. H. K. Cheng and K. K. M. Yue, J. Ethnopharmacol., 2006, 108, 272–279 CrossRef CAS.
- S. L. Li, S. F. Lai, J. Z. Song, C. F. Qiao, X. Liu, Y. Zhou, H. Cai, B. C. Cai and H. X. Xu, J. Pharm. Biomed. Anal., 2010, 53, 946–957 CrossRef CAS.
- L. Xia, H. L. Liu, P. Li, J. L. Zhou, L. W. Qi, L. Yi and J. Chen, J. Sep. Sci., 2008, 31, 3156–3169 CrossRef CAS.
- H. Kong, M. Wang, K. Venema, A. Maathuis, R. van der Heijden, J. van der Greef, G. Xu and T. Hankemeier, J. Chromatogr., A, 2009, 1216, 2195–2203 CrossRef CAS.
- K. Tanaka, M. Kubota, S. Zhu, U. Sankawa and K. Komatsu, Nat. Prod. Commun., 2007, 2, 625–632 CAS.
- Y. W. Ha, K. S. Ahn, J. C. Lee, S. H. Kim, B. C. Chung and M. H. Choi, Anal. BioAnal. Chem., 396, pp. 3017–3025 Search PubMed.
- Q. C. Ji, M. R. Harkey, G. L. Henderson, M. E. Gershwin, J. S. Stern and R. M. Hackman, Phytochem. Anal., 2001, 12, 320–326 CrossRef CAS.
- H. Hao, N. Cui, G. Wang, B. Xiang, Y. Liang, X. Xu, H. Zhang, J. Yang, C. Zheng, L. Wu, P. Gong and W. Wang, Anal. Chem., 2008, 80, 8187–8194 CrossRef CAS.
- C. Zheng, H. Hao, X. Wang, X. Wu, G. Wang, G. Sang, Y. Liang, L. Xie, C. Xia and X. Yao, J. Mass Spectrom., 2009, 44, 230–244 CrossRef CAS.
- Q. Wu, Q. Yuan, E. H. Liu, L. W. Qi, Z. M. Bi and P. Li, Biomed. Chromatogr., 2009 Search PubMed.
- R. Hu, J. Zhao, L. W. Qi, P. Li, S. L. Jing and H. J. Li, Rapid Commun. Mass Spectrom., 2009, 23, 1619–1635 CrossRef CAS.
- L. W. Qi, X. D. Wen, J. Cao, C. Y. Li, P. Li, L. Yi, Y. X. Wang, X. L. Cheng and X. X. Ge, Rapid Commun. Mass Spectrom., 2008, 22, 2493–2509 CrossRef CAS.
- L. W. Qi, J. Cao, P. Li, Q. T. Yu, X. D. Wen, Y. X. Wang, C. Y. Li, K. D. Bao, X. X. Ge and X. L. Cheng, J. Chromatogr., A, 2008, 1203, 27–35 CrossRef CAS.
- B. Sritularak, O. Morinaga, C. S. Yuan, Y. Shoyama and H. Tanaka, J. Nat. Med., 2009, 63, 360–363 Search PubMed.
- O. Morinaga, T. Uto, C. S. Yuan, H. Tanaka and Y. Shoyama, Fitoterapia, 2010, 81, 284–288 CrossRef CAS.
- C. Z. Wang, M. Ni, S. Sun, X. L. Li, H. He, S. R. Mehendale and C. S. Yuan, J. Agric. Food Chem., 2009, 57, 2363–2367 CrossRef CAS.
- P. S. Xie and A. Y. Leung, J. Chromatogr., A, 2009, 1216, 1933–1940 CrossRef CAS.
- E. J. Lee, R. Shaykhutdinov, A. M. Weljie, H. J. Vogel, P. J. Facchini, S. U. Park, Y. K. Kim and T. J. Yang, J. Agric. Food Chem., 2009, 57, 7513–7522 CrossRef CAS.
- W. K. Li, C. G. Gu, H. J. Zhang, D. V. C. Awang, J. F. Fitzloff, H. H. S. Fong and R. B. van Breemen, Anal. Chem., 2000, 72, 5417–5422 CrossRef CAS.
- E. M. Schlag and M. S. McIntosh, Phytochemistry, 2006, 67, 1510–1519 CrossRef CAS.
- C. C. Hon, Y. C. Chow, F. Y. Zeng and F. C. C. Leung, Acta Pharmacol. Sin., 2003, 24, 841–846 Search PubMed.
- K. S. Cheung, H. S. Kwan, P. P. H. But and P. C. Shaw, J. Ethnopharmacol., 1994, 42, 67–69 CrossRef CAS.
- J. Y. Um, H. S. Chung, M. S. Kim, H. J. Na, H. J. Kwon, J. J. Kim, K. M. Lee, S. J. Lee, J. P. Lim, K. R. Do, W. J. Hwang, Y. S. Lyu, N. H. An and H. M. Kim, Biol. Pharm. Bull., 2001, 24, 872–875 CrossRef CAS.
- J. Wang, W. Y. Ha, F. N. Ngan, P. P. H. But and P. C. Shaw, Planta Med., 2001, 67, 781–783 CrossRef CAS.
- I. S. H. Ho and F. C. Leung, Mol. Genet. Genomics, 2002, 266, 951–961 CrossRef CAS.
- W. Y. Ha, P. C. Shaw, J. Liu, F. C. F. Yau and J. Wang, J. Agric. Food Chem., 2002, 50, 1871–1875 CrossRef CAS.
- H. Fushimi, K. Komatsu, M. Isobe and T. Namba, Biol. Pharm. Bull., 1997, 20, 765–769 CAS.
- W. Y. Ha, F. C. F. Yau, P. P. H. But, J. Wang and P. C. Shaw, Planta Med., 2001, 67, 587–589 CrossRef CAS.
- S. Zhu, H. Fushimi, S. Q. Cai and K. Komatsu, Planta Med., 2003, 69, 647–653 CrossRef CAS.
- S. Zhu, H. Fushimi, S. Cai and K. Komatsu, Planta Med., 2004, 70, 189–192 CrossRef CAS.
- Y. Diao, X. M. Lin, C. L. Liao, C. Z. Tang, Z. J. Chen and Z. L. Hu, Planta Med., 2009, 75, 557–560 CrossRef CAS.
- Y. Sasaki, K. Komatsu and S. Nagumo, Biol. Pharm. Bull., 2008, 31, 1806–1808 CrossRef CAS.
- K. T. Lu, H. C. Lee, F. S. Liu, C. F. Lo and J. H. Lin, J. Food Drug Anal., 2010, 18, 58–63 Search PubMed.
- X. M. Cui, C. K. Lo, K. L. Yip, T. T. X. Dong and K. W. K. Tsim, Planta Med., 2003, 69, 584–586 CrossRef CAS.
- A. Mathur, A. K. Mathur, R. S. Sangwan, A. Gangwar and G. C. Uniyal, Genet. Resour. Crop Evol., 2003, 50, 245–252 Search PubMed.
- D. P. Bai, J. Brandle and R. Reeleder, Genome, 1997, 40, 111–115 CrossRef CAS.
- W. Lim and K. Mudgez, Hortscience, 2004, 39, 889–889 Search PubMed.
- W. Lim, K. W. Mudge and L. A. Weston, Planta Med., 2007, 73, 71–76 CrossRef CAS.
- C. Z. Wang, P. Li, J. Y. Ding, G. Q. Jin and C. S. Yuan, Planta Med., 2005, 71, 384–386 CrossRef CAS.
- Y. E. Choi, C. H. Ahn, B. B. Kim and E. S. Yoon, Biol. Pharm. Bull., 2008, 31, 135–138 CrossRef CAS.
- G. D. Reunova, I. L. Kats, T. I. Muzarok and Y. N. Zhuravlev, Russ. J. Genet., 2010, 46, 938–947 Search PubMed.
- N. V. Dan, N. Ramchiary, S. R. Choi, T. S. Uhm, T. J. Yang, I. O. Ahn and Y. P. Lim, Conserv. Genet., 2010, 11, 1223–1225 Search PubMed.
- K. H. Ma, A. Dixit, Y. C. Kim, D. Y. Lee, T. S. Kim, E. G. Cho and Y. J. Park, Conserv. Genet., 2007, 8, 1507–1509 Search PubMed.
- Y. S. Shin, K. H. Bang, D. S. In, O. T. Kim, D. Y. Hyun, I. O. Ahn, B. C. Ku, S. W. Kim, N. S. Seong, S. W. Cha, D. Lee and H. K. Choi, Arch. Pharmacal Res., 2007, 30, 1625–1628 Search PubMed.
- Z. M. Qian, J. Lu, Q. P. Gao and S. P. Li, J. Chromatogr., A, 2009, 1216, 3825–3830 CrossRef CAS.
- V. A. Assinewe, B. R. Baum, D. Gagnon and J. T. Arnason, J. Agric. Food Chem., 2003, 51, 4549–4553 CrossRef CAS.
- Y. Wang, J. Y. Pan, X. Y. Xiao, R. C. Lin and Y. Y. Cheng, Phytochem. Anal., 2006, 17, 424–430 CrossRef CAS.
- S. Perez and D. Barcelo, TrAC, Trends Anal. Chem., 2007, 26, 494–514 CrossRef CAS.
- L. W. Qi, X. J. Gu, P. Li, Y. Liang, H. Hao and G. Wang, Rapid Commun. Mass Spectrom., 2009, 23, 2151–2160 CrossRef CAS.
- N. Fuzzati, B. Gabetta, K. Jayakar, R. Pace and F. Peterlongo, J. Chromatogr., A, 1999, 854, 69–79 CrossRef CAS.
- I. Kitagawa, T. Taniyama, T. Hayashi and M. Yoshikawa, Chem. Pharm. Bull., 1983, 31, 3353–3356 CAS.
- H. Yamaguchi, R. Kasai, H. Matsuura, O. Tanaka and T. Fuwa, Chem. Pharm. Bull., 1988, 36, 3468–3473 CAS.
- G. X. Ren and F. Chen, J. Agric. Food Chem., 1999, 47, 1501–1505 CrossRef CAS.
- Y. H. Chang and P. K. W. Ng, J. Agric. Food Chem., 2009, 57, 2356–2362 CrossRef CAS.
- W. C. Chuang, H. K. Wu, S. J. Sheu, S. H. Chiou, H. C. Chang and Y. P. Chen, Planta Med., 1995, 61, 459–465 CAS.
- W. A. Court, J. G. Hendel and J. Elmi, J. Chromatogr., A, 1996, 755, 11–17 CrossRef CAS.
- L. P. Christensen, M. Jensen and U. Kidmose, J. Agric. Food Chem., 2006, 54, 8995–9003 CrossRef CAS.
- V. J. Davidson, X. Li and R. B. Brown, J. Food Eng., 2004, 63, 369–373 CrossRef.
- A. J. Lau, S. O. Woo and H. L. Koh, J. Chromatogr., A, 2003, 1011, 77–87 CrossRef CAS.
- A. J. Lau, D. F. Toh, T. K. Chua, Y. K. Pang, S. O. Woo and H. L. Koh, J. Ethnopharmacol., 2009, 125, 380–386 CrossRef CAS.
- Y. J. Lee, H. Y. Kim, K. S. Kang, J. G. Lee, T. Yokozawa and J. H. Park, Bioorg. Med. Chem. Lett., 2008, 18, 4515–4520 CrossRef CAS.
- S. Su, L. W. Qi, G. J. Du, S. R. Mehendale, C. Z. Wang and C. S. Yuan, Food Chem., 2011, 125, 1299–1305 CrossRef.
- S. Chan, M. F. Kong, Y. C. Wong, S. K. Wong and D. W. Sin, J. Agric. Food Chem., 2007, 55, 3339–3345 CrossRef CAS.
- S. H. Sohn, S. K. Kim, H. G. Kang and J. J. Wee, J. Chromatogr., A, 2004, 1042, 163–168 CrossRef CAS.
- F. E. Ahmed, TrAC, Trends Anal. Chem., 2001, 20, 649–661 CrossRef CAS.
- L. W. Chu, J. C. Yan, D. Chen, Z. G. Hou and Z. X. Fan, Chin. J. Anal. Chem., 2006, 34, 1482–1486 CAS.
- Y. S. Park, A. M. Abd El-Aty, J. H. Choi, S. K. Cho, D. H. Shin and J. H. Shim, Biomed. Chromatogr., 2007, 21, 29–39 CrossRef CAS.
- H. W. Wen, M. F. Hsieh, Y. T. Wang, H. P. Chung, P. C. Hsieh, I. H. Lin and F. I. Chou, J. Hazard. Mater., 2010, 176, 280–287 CrossRef CAS.
- L. Quan, S. F. Li, S. J. Tian, H. Xu, A. Q. Lin and L. Gu, Chromatographia, 2004, 59, 89–93.
- C. Quan, Y. G. Shang, S. F. Li, S. K. Tang, T. Huang and X. Fang, J. Taiwan Inst. Chem. Eng., 2010, 41, 44–48 Search PubMed.
- C. Quan, S. F. Li, S. J. Tian, H. Xu, A. Q. Lin and L. Gu, J. Supercrit. Fluids, 2004, 31, 149–157 CrossRef CAS.
- F. S. Dong, X. G. Liu, Y. Q. Zheng, J. Li, W. G. Zhang, X. Z. Zhang and C. J. Li, Chin. J Anal. Chem., 2008, 36, 1716–1720 CAS.
- L. J. Han, W. Li, L. K. Han, J. N. Hu, Z. Wang, W. C. Liu and Y. N. Zheng, Chem. Anal. (Warsaw), 2009, 54, 1433–1443 CAS.
- J. Li, F. S. Dong, X. G. Liu, Y. Q. Zheng, J. R. Yao and C. P. Zhang, Chromatographia, 2009, 69, 1113–1117 CAS.
- J. M. Durgnat, J. Heuser, D. Andrey and C. Perrin, Food Addit. Contam., Part A, 2005, 22, 1224–1230 Search PubMed.
- K. M. Joo, J. H. Lee, H. Y. Jeon, C. W. Park, D. K. Hong, H. J. Jeong, S. J. Lee, S. Y. Lee and K. M. Lim, J. Pharm. Biomed. Anal., 2010, 51, 278–283 CrossRef CAS.
- W. Wang, G. J. Wang, H. T. Xie, J. G. Sun, S. Zhao, X. L. Jiang, H. Li, H. Lv, M. J. Xu and R. Wang, J. Chromatogr., B, 2007, 852, 8–14 CrossRef CAS.
- X. Zeng, Y. H. Deng, Y. Feng, Y. M. Liu, L. Yang, Y. Huang, J. Sun, W. X. Liang and Y. Y. Guan, J. Clin. Pharmacol., 2010, 50, 285–292 CrossRef CAS.
- L. Yang, Y. H. Deng, S. J. Xu and X. Zeng, J. Chromatogr., B, 2007, 854, 77–84 CrossRef CAS.
- J. Sun, G. Wang, X. Haitang, L. Hao, P. Guoyu and I. Tucker, J. Pharm. Biomed. Anal., 2005, 38, 126–132 CAS.
- L. Lai, H. P. Hao, Y. T. Liu, C. N. Zheng, Q. Wang, G. J. Wang and X. J. Chen, Planta Med., 2009, 75, 797–802 CrossRef CAS.
- F. J. Gui, X. W. Yang, L. Y. Li and J. M. Tian, J. Chromatogr., B, 2007, 850, 1–6 CrossRef CAS.
- M. J. Xu, G. J. Wang, H. T. Xie, R. Wang, W. Wang, X. Y. Li and J. G. Sun, Anal. Lett., 2006, 39, 113–126 CrossRef CAS.
- H. T. Xie, G. J. Wang, J. G. Sun, I. Tucker, X. C. Zhao, Y. Y. Xie, H. Li, X. L. Jiang, R. Wang, M. J. Xu and W. Wang, J. Chromatogr., B, 2005, 818, 167–173 CrossRef CAS.
- Z. W. Cai, T. X. Qian, R. N. S. Wong and Z. H. Jiang, Anal. Chim. Acta, 2003, 492, 283–293 CrossRef CAS.
- Q. A. Zhao, X. Zheng, J. Jiang, H. Zhou and P. Hu, J. Chromatogr., B, 2010, 878, 2266–2273 CrossRef CAS.
- T. X. Qian, Z. W. Cai, R. N. S. Wong, N. K. Mak and Z. H. Jiang, J. Chromatogr., B, 2005, 816, 223–232 CrossRef CAS.
- T. X. Oian, Z. W. Cai, R. N. S. Wong and Z. H. Jiang, Rapid Commun. Mass Spectrom., 2005, 19, 3549–3554 CrossRef CAS.
- P. S. Lee, T. W. Song, J. H. Sung, D. C. Moon, S. Song and Y. B. Chung, Planta Med., 2006, 72, 204–210 CrossRef CAS.
- I. P. Paek, Y. Moon, J. Kim, H. Y. Ji, S. A. Kim, D. H. Sohn, J. B. Kim and H. S. Lee, Biopharm. Drug Dispos., 2006, 27, 39–45 CrossRef CAS.
- H. C. Ren, J. G. Sun, G. J. Wang, J. Y. A., H. T. Xie, W. B. Zha, B. Yan, F. Z. Sun, H. P. Hao, S. H. Gu, L. S. Sheng, F. Shao, J. Shi and F. Zhou, J. Pharm. Biomed. Anal., 2008, 48, 1476–1480 CrossRef CAS.
- D. Zhang, Y. W. Wang, J. B. Han, W. S. Yu, L. L. Deng, J. P. Fawcett, Z. Y. Liu and J. K. Gu, J. Chromatogr., B, 2009, 877, 581–585 CrossRef CAS.
- X. Zhang, D. Zhang, J. Xu, J. Gu and Y. Zhao, J. Chromatogr., B, 2007, 858, 65–70 CrossRef CAS.
- Q. F. Xu, X. L. Fang and D. F. Chen, J. Ethnopharmacol., 2003, 84, 187–192 CrossRef CAS.
- X. Y. Li, G. J. Wang, H. G. Sun, H. P. Hao, Y. Q. Xiong, B. Yan, Y. T. Zheng and L. S. Sheng, Biol. Pharm. Bull., 2007, 30, 847–851 CrossRef CAS.
- X. Wang, T. Zhao, X. Gao, M. Dan, M. Zhou and W. Jia, Anal. Chim. Acta, 2007, 594, 265–273 CrossRef CAS.
- L. Yang, S. J. Xu, Z. F. Wu, Y. M. Liu and X. Zeng, Phytother. Res., 2009, 23, 65–71 CrossRef CAS.
- Y. Liang, A. Kang, T. Xie, X. Zheng, C. Dai, H. Hao, J. A, L. Sheng, L. Xie and G. J. Wang, J. Chromatogr. A, 1217, pp. 4501–4506 Search PubMed.
- H. F. Liu, J. L. Yang, F. F. Du, X. M. Gao, X. T. Ma, Y. H. Huang, F. Xu, W. Niu, F. Q. Wang, Y. Mao, Y. Sun, T. Lu, C. X. Liu, B. L. Zhang and C. Li, Drug Metab. Dispos., 2009, 37, 2290–2298 CrossRef CAS.
- X. T. Duan, X. Y. Chen, M. H. Li, B. Song and D. F. Zhong, Drug Metab. Rev., 2006, 38, 109–109.
- H. Hasegawa, J. Pharmacol. Sci., 2004, 95, 153–157 CAS.
- J. F. Cui, I. Bjorkhem and P. Eneroth, J. Chromatogr., B, 1997, 689, 349–355 CrossRef CAS.
- M. A. Tawab, U. Bahr, M. Karas, M. Wurglics and M. Schubert-Zsilavecz, Drug Metab. Dispos., 2003, 31, 1065–1071 CrossRef.
- Y. Gu, G. J. Wang, J. G. Sun, Y. W. Jia, W. Wang, M. J. Xu, T. Lv, Y. T. Zheng and Y. Sai, Food Chem. Toxicol., 2009, 47, 2257–2268 CrossRef CAS.
- P. S. Lee, J. Y. Han, T. W. Song, J. H. Sung, O. S. Kwon, S. Song and Y. B. Chung, Int. J. Pharm., 2006, 316, 29–36 CrossRef CAS.
- L. Li, Y. X. Sheng, J. L. Zhang, S. S. Wang and D. A. Guo, Biomed. Chromatogr., 2006, 20, 327–335 CrossRef CAS.
- J. Q. Ruan, W. I. Leong, R. Yan and Y. T. Wang, J. Agric. Food Chem., 2010, 58, 5770–5776 CrossRef CAS.
- G. T. Chen, M. Yang, Y. Song, Z. Q. Lu, J. Q. Zhang, H. L. Huang, S. H. Guan, L. J. Wu and D. A. Guo, Biomed. Chromatogr., 2008, 22, 779–785 CrossRef CAS.
- L. Yang, S. J. Xu, C. J. Liu and Z. J. Su, Anal. Bioanal. Chem., 2009, 395, 1441–1451 CrossRef CAS.
- H. Hasegawa, R. Suzuki, T. Nagaoka, Y. Tezuka, S. Kadota and I. Saiki, Biol. Pharm. Bull., 2002, 25, 861–866 CrossRef CAS.
- S. Rochfort, J. Nat. Prod., 2005, 68, 1813–1820 CrossRef CAS.
- M. J. Chen, M. M. Su, L. P. Zhao, J. Jiang, P. Liu, J. Y. Cheng, Y. J. Lai, Y. M. Liu and W. Jia, J. Proteome Res., 2006, 5, 995–1002 CrossRef CAS.
- M. J. Chen, L. P. Zhao and W. Jia, J. Proteome Res., 2005, 4, 2391–2396 CrossRef CAS.
- G. X. Xie, X. J. Zheng, X. Qi, Y. Cao, Y. Chi, M. M. Su, Y. Ni, Y. P. Qiu, Y. M. Liu, H. K. Li, A. H. Zhao and W. Jia, J. Proteome Res., 2010, 9, 125–133 CrossRef CAS.
- Y. Wang, J. Wang, M. Yao, X. Zhao, J. Fritsche, P. Schmitt-Kopplin, Z. Cai, D. Wan, X. Lu, S. Yang, J. Gu, H. U. Haring, E. D. Schleicher, R. Lehmann and G. Xu, Anal. Chem., 2008, 80, 4680–4688 CrossRef CAS.
- R. J. Tian, S. Y. Xu, X. Y. Lei, W. H. Jin, M. L. Ye and H. F. Zou, TrAC, Trends Anal. Chem., 2005, 24, 810–825 CrossRef CAS.
- Y. Duan, J. Zheng, V. Law and R. Nicholson, Eur. J. Pharmacol., 2006, 530, 9–14 CrossRef CAS.
- R. Nicholson, Y. Duan, J. Zheng and V. Law, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2006, 143, S117–S117 CrossRef.
- J. L. Zhou, J. J. An, P. Li, H. J. Li, Y. Jiang and J. F. Cheng, J. Chromatogr., A, 2009, 1216, 2394–2403 CrossRef CAS.
- X. Y. Su, L. Kong, X. Y. Lei, L. H. Hu, M. L. Ye and H. F. Zou, Mini-Rev. Med. Chem., 2007, 7, 87–98 CrossRef CAS.
- X. D. Huang, L. A. Kong, X. Li, X. G. Chen, M. Guo and H. F. Zou, J. Chromatogr., B, 2004, 812, 71–84 CrossRef CAS.
- J. Wang, Z. G. Huang, H. Cao, Y. T. Wang, P. Hui, C. Hoo and S. P. Li, J. Sep. Sci., 2008, 31, 1173–1180 CrossRef CAS.
- Y. Liu, M. X. Xie, J. Kang and D. Zheng, Spectrochim. Acta, Part A, 2003, 59, 2747–2758 CrossRef.
- A. Dasgupta and M. A. Reyes, Am. J. Clin. Pathol., 2005, 124, 229–236 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |