DOI:
10.1039/C1MD00098E
(Concise Article)
Med. Chem. Commun., 2011,
2, 909-917
Design and syntheses of some iminosugar derivatives as potential immunosuppressants†
Received
8th April 2011
, Accepted 10th June 2011
First published on 8th July 2011
Abstract
Eleven new iminosugar derivatives were synthesized and the effects of these synthetic iminosugars on proliferation of the mouse splenocytes were evaluated by the cell counting kit-8 (CCK-8) assay. The preliminary structure–activity relationships were deduced from the experimental data. It was found that iminosugar 14 (IC50 = 22 μM) and its C-5 epimer 15 (IC50 = 45 μM) displayed the strongest inhibitory effects, and might hold potential as immunosuppressive agents.
Introduction
Iminosugars, in which the ring oxygen has been replaced by nitrogen,1,2 were firstly isolated from Streptomyces roseochromogenes R-468 in 1965,3 and since then a large number of iminosugars have been isolated or synthesized.4 It was found that iminosugars hold great potential as therapeutic agents in many diseases such as cancer,5,6 diabetes,7,8 viral infections9 (including HIV,10 hepatitis B,11 and C virus12), and lysosomal storage disorders13,14 (including Gaucher's disease15 and Fabry disease16). Therefore, iminosugars have attracted much attention from both synthetic and biological chemists.17 However, the inhibition effects of iminosugars on immune system responses and these compounds as immunosuppressive agents have been less explored. Our previous results disclosed that some synthetic iminosugars (i.e. compounds 1–5 in Fig. 1) exhibited immunosuppressive activities.18–20 Encouraged by these works, and to further understand the structure–activity relationships of this type of compound and to find more potent immunosuppressants, we report herein the syntheses of iminosugar derivatives 6–16 (Fig. 2) and the evaluation of their immunosuppressive activities by the CCK-8 assay.
 |
| | Fig. 1 Structures of iminosugars 1–5. | |
 |
| | Fig. 2 Structures of designed iminosugar derivatives 6–16. | |
Since it was shown that extending the N-side chain may improve the immunosuppressive activity,20 compounds 6–9 were designed to check the inhibitory effect resulting from the carbon chain extension at the C-1 position. Our previous results disclosed that the configuration of the C-1 substituents had little effect on the secretion of IL-4.18 In addition, β-C-glycosides were easier to prepare,21 so compound 10 was designed to investigate whether the 2-OH group in the ring is necessary for the inhibition. On the other hand, it is well-known that one of the current clinically used small-molecule immunosuppressive agents, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclosporin A (CSA), is made up of eleven hydrophobic amino acids.22 To increase the hydrophobic property of the target molecules, one chlorine atom or cyano group was introduced, thus compounds 11–12 were designed. Considering that the existence of an N-side chain can enhance both the hydrophobic property and the immunosuppressive activity, compounds 13–15 were designed. Compound 16, as the isomer of 4,18 was designed to evaluate how the configuration of the C-1 substituents can influence the biological activity.
Results and discussion
Chemistry
The preparation of iminosugar derivatives 6 and 8 is shown in Scheme 1. Starting from the aldehyde 17,18,23 a Wittig reaction24 was performed to provide compound 19 as a chromatographically inseparable mixture of Z- and E- isomers in excellent yield (92%). The double bond in 19 was selectively reduced by 4% NiCl2·6H2O solution in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol in the presence of NaBH4,25 affording the saturated ester 21. The subsequent reduction of compound 21 by LiAlH4 produced the alcohol 23 in high yield (86%). In a similar way, alcohol 24 was prepared from the corresponding 5-epimer 18.18,23 It is noteworthy that the resolution of 1H NMR spectra of compounds 20–24 was rather low at room temperature, however this problem was successfully solved by increasing the temperature of NMR experiments to 60 °C. Finally, the full deprotection of 23 under hydrogen atmosphere over Pd/C provided the target compound 6 in 94% yield, whereas compound 8 was obtained in 84% yield using BCl3 solution.26
 |
| | Scheme 1 Reagents and conditions: a) Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOMe, THF; b) NiCl2·6H2O, NaBH4, CH2Cl2, COMPOUND LINKS CHCOOMe, THF; b) NiCl2·6H2O, NaBH4, CH2Cl2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH, 0 °C; c) LiAlH4, THF; d) Pd/C, H2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH, 1.25 M HCl; e) BCl3, CH2Cl2, 0 °C. | |
The preparation of iminosugars 7 and 9 is shown in Scheme 2. The methyl ester 21 was treated with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundNaOH aqueous solution at 60 °C, which was followed by catalytic hydrogenolysis to yield the target compound 7. In the same way, iminosugar 9 was prepared smoothly from the corresponding 5-epimer 22.
The preparation of iminosugar 10 also started from the aldehyde 17. As shown in Scheme 3, the base-promoted elimination reaction of compound 17 was carried out. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSodium hydride (NaH) was used as the base. It was found that the amount of NaH was very important to the reaction (Table 1). The amount of NaH should be no more than 0.5 equivalent, otherwise no desired product 25 is obtained. When t-BuOK was used as the base, the yield was low (<50%). The structure of 25 was unambiguously identified by its 1D (1H, 13C, DEPT)- and 2D (COSY, HMBC)- NMR analyses.
Table 1 The formation of 25via the elimination reaction of 17
| Entry |
Base
|
Product |
Yield |
| 1 |
NaH (4.0 eq) |
25
|
0 |
| 2 |
NaH (2.0 eq) |
25
|
0 |
| 3 |
NaH (1.5 eq) |
25
|
0 |
| 4 |
NaH (0.5 eq) |
25
|
83% |
| 5 |
t-BuOK (1.0 eq) |
25
|
<50% |
With compound 25 in hand, the next step was the reduction reaction. Various reducing reagents or solvents were screened (Table 2). The desired product 26 was obtained in 60% isolated yield when NaBH4 was used as the reductant (entry 5). When NaBH4 (1.5 equivalents), which was firstly dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH, was added into the flask dropwise, the yield was further improved (74%, entry 6). Interestingly, when LiAlH4 was used as the reductant, by-product 27 (Fig. 3) was collected (entry 1). Again, the final deprotection of 26viacatalytic hydrogenolysis provided the target compound 10. The structure of 10 was confirmed by its 1D (1H, 13C)- and 2D (COSY, HSQC, NOESY)- NMR analyses.
Table 2 The reduction reaction of unsaturated aldehyde 25
 |
| | Fig. 3 The structure of by-product 27. | |
The preparation of iminosugar derivative 11 is shown in Scheme 4. The starting material 28 was obtained according to the procedure described previously.20Alcohol 28 was converted to tosylate 29 in satisfactory yield (90%). Treatment of 29 with TBACN afforded compound 30 in moderate yield (63%), and at the same time by-product 31 (Fig. 4) was detected. The structure of 31 was identified based on the analysis of its 1D (1H, 13C)- and 2D (COSY, HSQC, HMBC)- NMR spectra. As displayed in Fig. 4, the nitrogen atom and the cyano group would competitively attack the carbon to undergo substitution reaction. When the reaction was performed at lower than 60 °C, compound 30 was the major product (path I). Otherwise, compound 31 became the dominant product (path II). Finally, compound 30 was deprotected using BCl3 thus affording the target compound 11.
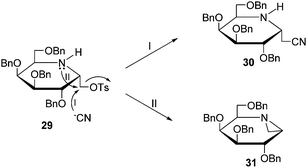 |
| | Fig. 4 The formation of compounds 30 and 31. | |
The preparation of iminosugar derivatives 12–15 is shown in Scheme 5. After treatment of 29 with DBU, aziridine 31 was obtained in 82% isolated yield. Compound 31 was subjected to de-benzylation and aziridine ring opening in the presence of BCl3 affording the target molecule 12 in high yield. The N-alkyl substituted iminosugar derivatives 32, 33, and 36 were obtained according to the procedure described previously.20 Compound 32 was reacted with MsCl in the presence of Et3N and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMAP, providing 34 in good yield (94%). Full deprotection of 34 under hydrogen atmosphere over Pd–C provided the target compound 13 in 78% isolated yield. In the same manner, iminosugars 14 and 15 were prepared starting from 33 and 36, respectively.
![Reagents and conditions: a) DBU, toluene, 80 °C; b) BCl3, CH2Cl2, 0 °C; c) MsCl, Et3N, DMAP; d) Pd/C, H2, MeOH, 4 N HCl. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene.](/image/article/2011/MD/c1md00098e/c1md00098e-s5.gif) |
| | Scheme 5 Reagents and conditions: a) DBU, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtoluene, 80 °C; b) BCl3, CH2Cl2, 0 °C; c) MsCl, Et3N, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMAP; d) Pd/C, H2, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH, 4 N HCl. DBU = COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1,8-diazabicyclo[5.4.0]undec-7-ene. | |
The preparation of iminosugar derivative 16 is shown in Scheme 6. The alcohol 3818 was treated with TEMPO/BAIB to give the crude aldehyde 39. Oxidation of 39 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium chlorite provided the acid 40 in 75% isolated yield. Full deprotection of 40 under hydrogen atmosphere over Pd/C led to the target compound 16 in quantitative yield.
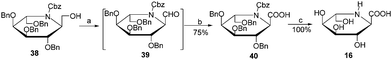 |
| | Scheme 6 Reagents and conditions: a) TEMPO, BAIB, CH2Cl2, 40 °C; b) NaH2PO4, 30% H2O2, NaClO2, CH3CN; c) Pd/C, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COMPOUND LINKS COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HOAc = 4 HOAc = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy free radical, BAIB = COMPOUND LINKS 1. TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy free radical, BAIB = COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound(diacetoxyiodo)benzene | |
Biological assay
The synthetic compounds 6–16 and 1–5 were evaluated in vitro for the effects on concanavalin A (Con A)-induced proliferation of splenocytes in mouse by the cell counting kit-8 (CCK-8) assay.20 The splenocytes were induced by 2.5 μg mL−1 of concanavalin A with 30 μM concentration of the iminosugars at 37 °C, 5% CO2 for 48 h, using the Con A-treated splenocytes as the experimental control and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclosporin A (CSA) as the positive control. Compared with the control, the levels of cell proliferation were reduced by 7.8%, 7.2%, 9.7%, no inhibition, 25.5%, 14.6%, 4.9%, 19.3%, 6.4%, 22.3%, 27.9%, 20.8%, 38.9%, 54.1%, 43.6%, and 21.2% when including 30 μM of compounds 1–16, respectively (Fig. 5). Among these compounds, iminosugar 14 (IC50 = 22 μM) and its C-5 epimer 15 (IC50 = 45 μM) displayed the strongest inhibition effects (Fig. 6). It is noteworthy that the immunosuppressive effects of 14 and 15 were increased by 6.9 and 4.5 folds (comparing compounds 14 with 1, 15 with 3). Comparing 6 (14.6%) with 1 (7.8%) and 8 (19.3%) with 3 (9.7%), it seemed that extending the carbon atom number of the terminal group can increase the inhibitory activity. Based on the fact that compound 10 (22.3%) exhibited better inhibition than compound 1 (7.8%), it is clear that a 2-OH in the ring may be not necessary for the inhibition. Comparing 11 (27.9%) with 1 (7.8%), it can be suggested that introducing the cyano group can improve the activity because of the polarity decrease of the compound. Comparing 12 (20.8%) with 1 (7.8%) and 13 (38.9%) with 5 (25.5%), it seemed that bringing one chlorine atom into the structure can increase the inhibition. This suggests that hydrophobic character may be important to the immunosuppressive activity. In addition, the configuration of the C-1 terminal group may have some effects on biological activity based on the inhibition data of compound 16 (21.2%) and 4 (no inhibition at 30 μM). Comparing 14 (54.1%) and 13 (38.9%) with 12 (20.8%), it was found that the N-alkylation is important for the improvement of the inhibitory activity, which agrees with the result previously reported by our group.20 The detailed immunosuppressive mechanisms of these synthetic iminosugars are not well-known.
 |
| | Fig. 5 Effects of compounds 1–16 on concanavalin A induced mouse splenocytes proliferation were assessed by the CCK-8 assay. Concentration of CSA was 1μM and concentration of 1–16 was 30 μM. Values are mean ± SEM. | |
 |
| | Fig. 6 Inhibition of 14 and 15 for the cell proliferation. I = inhibition, C = concentration of inhibitor. | |
The cytotoxicity of compounds 14 and 15 was also assayed by the CCK-8 method using the cells without the Con A-induced proliferation. The experimental data showed that compounds 14 and 15 were low toxic when their concentration was below 110 μM (Fig. 7).
 |
| | Fig. 7 Influence on cell viability of 14 and 15 at different concentrations. | |
Conclusion
In summary, to gather more information on the structure–activity relationships and to search for more potent immunosuppressants, we designed and synthesized 11 new iminosugar derivatives in high yield using concise synthetic routes. All synthetic compounds were evaluated in vitro for the immunosuppressive activities. The experimental data demonstrated that iminosugar 14 (IC50 = 22 μM) and its C-5 epimer 15 (IC50 = 45 μM) displayed the strongest inhibition effects. The preliminary structure–activity relationships were discussed. It was found that the immunosuppressive activities of iminosugars can be improved by decreasing the polarity or increasing the hydrophobicity. In addition, it was disclosed that the 2-OH group in the iminosugar ring may be not necessary for the inhibition. These results may benefit the discovery of new iminosugar derivatives as potent immunosuppressive agents.
Experimental
General
All chemicals were purchased and used without further purification. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundTetrahydrofuran (THF) was distilled over sodium/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzophenone, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethylene chloride (CH2Cl2) over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcalcium hydride. Reactions were monitored with analytical TLC on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel 60-F254 precoated aluminium plates and visualized under UV (254 nm) and/or by staining with acidic ceric ammonium molybdate. Column chromatography was performed on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (35–75 μm). NMR spectra were recorded on a Varian VXR-300M or Varian INOVA-500M spectrometer. Mass spectra were recorded using a PE SCLEX QSTAR spectrometer. Elemental analysis data were recorded on PE-2400C elemental analyzer. Concanavalin A and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclosporin A were purchased from Sigma. Other reagents were from commercial sources.
(2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitolyl)methyl acrylate (19)
To a solution of 17 (170 mg, 0.25 mmol) in dry THF (5.0 mL) was added Ph3P = CHCOOMe (165 mg, 0.50 mmol). The reaction mixture was stirred overnight at room temperature. The reaction was quenched by NH4Cl aqueous solution, and the mixture was extracted with EtOAc (3 × 50 mL) and washed with saturated NaHCO3 aqueous solution (20 mL) and brine (20 mL). The organic phases were combined, dried (Na2SO4), filtered, and concentrated. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 13
1 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 19 (170 mg, 92%) as a colorless oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 19 (170 mg, 92%) as a colorless oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 3.47 (dd, J = 3.0, 9.0 Hz, 1H), 3.73 (s, 3H), 3.80–3.88 (m, 1H), 4.00–4.07 (m, 3H), 4.19–4.57 (m, 4H), 4.59–4.65 (m, 1H), 4.68 (d, J = 11.5 Hz, 2H), 4.72 (d, J = 12.0 Hz, 2H), 4.78 (d, J = 11.5 Hz, 1H), 4.89 (br.s, 1H), 5.08 (d, J = 7.0 Hz, 1H), 5.20 (d, J = 2.0 Hz, 1H), 5.98–6.03 (m, 1H), 7.18–7.34 (m, 25H). MS-ESI: 742 [M + H+]. Anal. Calcd for C46H47NO8: C, 74.47; H, 6.39; N, 1.89; Found: C, 74.52; H, 6.35; N, 1.88%
2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-methyl acrylate (20)
Compound 20 was prepared from 18 (84 mg, 0.12 mmol) as described in the preparation of 19, providing 20 (88 mg, 96%) as a colorless oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 3.30 (t, J = 9.0 Hz, 1H), 3.37 (br.s, 1H), 3.70–3.73 (m, 4H), 3.92 (br.s, 1H), 4.26 (dd, J = 6.5, 10.0 Hz, 2H), 4.31–4.37 (m, 2H), 4.52 (d, J = 12.0 Hz, 1H), 4.57–4.67 (m, 4H), 4.73 (d, J = 11.5 Hz, 2H), 5.09 (d, J = 12.0 Hz, 1H), 5.16 (d, J = 12.5 Hz, 1H), 5.36 (br.s, 1H), 6.04 (br.s, 1H), 7.15–7.45 (m, 25H). MS-ESI: 742 [M + H+]. Anal. Calcd for C46H47NO8: C, 74.47; H, 6.39; N, 1.89; Found: C, 74.70; H, 6.56; N, 1.64%
(2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-D-glycero-D- galacto-heptitolyl)methyl propionate (21)
To a solution of 19 (170 mg, 0.23 mmol) in dry CH2Cl2 (1.3 mL) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (8.0 mL) was added 4% solution of NiCl2·6H2O in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (2.3 mL, 0.39 mmol) at 0 °C. After stirring for 20 min, a portion of NaBH4 (35 mg, 0.92 mmol) was added. The reaction mixture was stirred for another five hours at room temperature. The reaction was quenched by HOAc, then the solvent was removed and the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 16
1 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 21 (145 mg, 85%) as a yellow oil. 1H NMR (400 MHz, COMPOUND LINKS
1) to provide 21 (145 mg, 85%) as a yellow oil. 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundd6-DMSO, 70 °C): δ 1.97–2.00 (m, 2H), 2.24–2.27 (m, 2H), 3.52 (s, 3H), 3.63 (br.s, 1H), 3.73–3.82 (m, 2H), 3.91 (br.s, 2H), 4.01 (br.s, 1H), 4.36 (d, J = 12.0 Hz, 2H), 4.42 (d, J = 12.4 Hz, 1H), 4.47 (d, J = 12.0 Hz, 1H), 4.57 (d, J = 12.4 Hz, 1H), 4.61 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 2H), 4.69 (d, J = 8.3 Hz, 1H), 4.86 (d, J = 12.8 Hz, 1H), 5.01 (d, J = 12.4 Hz, 1H), 7.20–7.35 (m, 25H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundd6-DMSO): δ 18.99, 30.19, 51.17, 53.85, 54.44, 66.48, 67.70, 71.67, 71.88, 71.95, 73.42, 74.29, 76.20, 79.68, 127.28, 127.36, 127.43, 127.69, 128.02, 128.17, 128.24, 129.12, 129.45, 134.54, 136.68, 138.26, 138.48, 138.87, 154.96, 157.00, 173.03. MS-ESI: 766 [M + Na+]. Anal. Calcd for C46H49NO8: C, 74.27; H, 6.64; N, 1.88; Found: C, 74.53; H, 6.87; N, 1.79%
2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-methyl propionate (22)
Compound 22 was prepared from 20 (125 mg, 0.17 mmol) as described in the preparation of 21, providing 22 (100 mg, 80%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundd6-DMSO, 70 °C): δ 1.55–1.63 (m, 1H), 2.12–2.20 (m, 1H), 2.23–2.34 (m, 2H), 3.50–3.53 (m, 4H), 3.57–3.63 (m, 1H), 3.89 (dd, J = 3.0, 10.0 Hz, 1H), 3.94 (t, J = 2.5 Hz, 1H), 4.02 (dd, J = 6.5, 10.0 Hz, 1H), 4.44 (s, 2H), 4.55 (s, 3H), 4.58 (d, J = 12.0 Hz, 1H), 4.63 (d, J = 12.0 Hz, 1H), 4.64 (d, J = 11.5 Hz, 1H), 4.68 (d, J = 12.0 Hz, 1H), 4.73 (br.s, 1H), 5.07 (ABq, J = 12.5 Hz, 2H), 7.21–7.34 (m, 25H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO): δ 23.60, 23.95, 30.12, 51.13, 52.34,54.05, 54.49, 66.76, 69.27, 70.96, 71.29, 71.51, 71.76, 71.93, 73.62, 73.86, 75.02, 127.23, 127.33, 127.36, 127.42, 127.53, 127.76, 128.09, 128.13, 128.15, 128.21, 128.32, 136.68, 137.97, 138.32, 138.56, 138.85, 156.06, 173.05. MS-ESI: 766 [M + Na+]. Anal. Calcd for C46H49NO8: C, 74.27; H, 6.64; N, 1.88; Found: C, 74.02; H, 6.64; N, 1.86%
2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-propanol (23)
To a solution of 21 (46 mg, 0.06 mmol) in dry THF (3.5 mL) was added LiAlH4 (4.70 mg, 0.12 mmol) at −15 °C. The reaction mixture was stirred overnight at room temperature. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundEthyl acetate (1.0 mL) was added dropwise to decompose the excess LiAlH4, followed by addition of 2 N COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundNaOH (1.5 mL). The suspension was filtered through a bed of Celite. The filtrate was extracted with EtOAc (3 × 30 mL) and washed with saturated NaHCO3 aqueous solution (10 mL) and brine (10 mL). The organic phases were combined, dried (Na2SO4), filtered, and concentrated. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 23 (38 mg, 86%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 23 (38 mg, 86%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundd6-DMSO, 70 °C): δ 1.29–1.37 (m, 1H), 1.38–1.46 (m, 1H), 1.72–1.75 (m, 2H), 3.38 (dd, J = 6.0, 11.5 Hz, 2H), 3.60 (br.s, 1H), 3.72 (dd, J = 3.0, 8.5Hz, 1H), 3.80 (dd, J = 5.0, 8.5 Hz, 1H), 3.92–3.96 (m, 2H), 4.02 (br.s, 1H), 4.12 (t, J = 5.0 Hz, 1H), 4.36 (d, J = 12.0 Hz, 1H), 4.42 (d, J = 12.0 Hz, 1H), 4.47 (d, J = 12.0 Hz, 2H), 4.58 (s, 2H), 4.64–4.71 (m, 3H), 4.87 (d, J = 13.0 Hz, 1H), 5.01 (d, J = 12.5 Hz, 1H), 7.20–7.33 (m, 25H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO): δ 19.82, 29.10, 54.15, 60.28, 65.82, 66.64, 67.89, 71.72, 71.88, 71.96, 73.46, 74.41, 76.41, 79.79, 127.29, 127.33, 127.47, 127.76, 128.01, 128.12, 128.17, 128.21, 128.25, 136.91, 138.18, 138.58, 138.89, 156.85. MS-ESI: 738 [M + Na+]. Anal. Calcd for C45H49NO7: C, 75.50; H, 6.90; N, 1.96; Found: C, 75.46; H, 6.93; N, 1.85%
2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-propanol (24)
Compound 24 was prepared from 22 (54 mg, 0.07 mmol) as described in the preparation of 23, providing 24 (25 mg, 79% based on the recovery of starting material) as a yellow oil. The material 22 (21 mg) was recovered. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundd6-DMSO, 60 °C): δ 1.24–1.33 (m, 1H), 1.39–1.41 (m, 2H), 1.84–1.91 (m, 1H), 3.34 (br.s, 2H), 3.50 (dd, J = 5.5, 9.5 Hz, 1H), 3.61 (t, J = 8.5 Hz, 1H), 3.86 (dd, J = 3.0, 10.0 Hz, 1H), 3.94–3.95 (m, 1H), 3.99 (dd, J = 6.5, 10.5 Hz, 1H), 4.14–4.16 (m, 1H), 4.41 (d, J = 12.0 Hz, 1H), 4.46 (d, J = 12.5 Hz, 1H), 4.52–4.70 (m, 8H), 5.08 (ABq, J = 13.0 Hz, 2H), 7.25–7.33 (m, 25H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO): δ 25.35, 29.51, 53.08, 53.44, 54.60, 60.63, 66.69, 69.24, 70.95, 71.22, 71.49, 71.90, 73.54, 73.88, 75.12, 75.23, 127.22, 127.26, 127.33, 127.45, 127.56, 127.77, 127.95, 128.10, 128.13, 128.22, 128.34, 136.76, 138.02, 138.32, 138.66, 138.82, 155.80. MS-ESI: 738 [M + Na+]. Anal. Calcd for C45H49NO7: C, 75.50; H, 6.90; N, 1.96; Found: C, 75.50; H, 6.97; N, 1.83%
1,5-Dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-propanol (6)
A mixture of 23 (80 mg, 0.11 mmol) and 10% Pd/C (10.0 mg) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (2.0 mL) and 1.25 M HCl solution was stirred for 72 h under H2 atmosphere. The solid was removed by filtration through Celite and the filtrate was concentrated. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (CH2Cl2/MeOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and C-18 reversed-phase column chromatography (COMPOUND LINKS
3) and C-18 reversed-phase column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O as eluent), giving 6 (23 mg, 94%) as a white solid in the form of hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.66–1.79 (m, 3H), 1.96–2.09 (m, 1H), 3.57–3.59 (m, 1H), 3.63–3.72 (m, 3H), 3.90 (dd, J = 2.5, 8.5Hz, 1H), 3.93 (d, J = 6.5 Hz, 2H), 4.14 (dd, J = 4.5, 8.0 Hz, 1H), 4.21(s, 1H). 13C NMR (75 MHz, D2O): δ 24.21, 31.04, 56.98, 58.08, 60.87, 63.73, 68.57, 69.70, 71.65. HRMS (ESI, positive) Calcd for [(M + H) +] C9H20NO5 222.1336; Found: 222.1331
1,5-Dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-propanol (8)
To a solution of 24 (21 mg, 0.03 mmol) in dry CH2Cl2 (3.0 mL) was added BCl3 (1 M solution in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhexane, 0.45 mL, 0.45 mmol). The reaction mixture was stirred for 5 h at 0 °C, and then the solids formed were dissolved by addition of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (1.0 mL) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (1.0 mL). The solvent was removed and the residue was purified by flash chromatography on C18-reversed phase COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O as eluent), thus providing 8 (6.2 mg, 82%) as a yellow solid in the form of hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.55–1.62 (m, 1H), 1.64–1.79 (m, 2H), 1.81–1.92 (m, 1H), 3.37–3.41 (m, 1H), 3.51 (dd, J = 5.5, 9.5 Hz, 1H), 3.59–3.68 (m, 2H), 3.85 (dd, J = 6.5, 13.0 Hz, 1H), 3.96 (dd, J = 2.5, 12.5 Hz, 1H), 4.04 (dd, J = 2.5, 11.0 Hz, 1H), 4.07–4.10 (m, 2H). 13C NMR (125 MHz, D2O): δ 24.89, 27.82, 55.19, 56.79, 58.99, 61.74, 63.90, 67.88, 69.72. HRMS (ESI, positive) Calcd for [(M + H)+] C9H20NO5 222.1336; Found: 222.1333.
1,5-Dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-propionic acid (7)
To a solution of 21 (55 mg, 0.07 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (2.0 mL) was added 1M COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundNaOH aqueous solution (0.28 mL, 0.28 mmol). After stirring for five hours at 60 °C, a portion of cation exchange resin was added until the pH value remained between 5 and 6. The solvent was removed and the residue was purified by simple column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide the crude product (51 mg). The crude product was treated under the conditions as described in the preparation of 6, yielding 7 (16 mg, 80%, two steps) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.81–1.92 (m, 1H), 1.99–2.03 (m, 1H), 2.33–2.43 (m, 2H), 3.43–3.47 (m, 2H), 3.79–3.84 (m, 3H), 4.05–4.08 (m, 1H), 4.13 (d, J = 3.0 Hz, 1H). 13C NMR (125 MHz, D2O): δ 21.15, 35.92, 55.06, 56.12, 60.07, 67.78, 68.40, 70.20, 182.93. HRMS (ESI, positive) Calcd for [(M + H)+] C9H18NO6 236.1129; Found: 236.1120.
1) to provide the crude product (51 mg). The crude product was treated under the conditions as described in the preparation of 6, yielding 7 (16 mg, 80%, two steps) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.81–1.92 (m, 1H), 1.99–2.03 (m, 1H), 2.33–2.43 (m, 2H), 3.43–3.47 (m, 2H), 3.79–3.84 (m, 3H), 4.05–4.08 (m, 1H), 4.13 (d, J = 3.0 Hz, 1H). 13C NMR (125 MHz, D2O): δ 21.15, 35.92, 55.06, 56.12, 60.07, 67.78, 68.40, 70.20, 182.93. HRMS (ESI, positive) Calcd for [(M + H)+] C9H18NO6 236.1129; Found: 236.1120.
1,5-Dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-propionic acid (9)
Compound 9 was prepared from 22 (90 mg, 0.12 mmol) as described in the preparation of 7, providing 9 (27 mg, 83%) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.98 (dd, J = 7.5, 14.5 Hz, 2H), 2.34–2.45 (m, 2H), 3.37–3.41 (m, 1H), 3.48 (t, J = 7.0 Hz, 1H), 3.88 (dd, J = 6.5, 13.0 Hz, 1H), 4.00 (d, J = 3.0 Hz, 1H), 4.03 (dd, J = 3.0, 9.0 Hz, 1H), 4.06 (d, J = 3.5 Hz, 1H), 4.13 (t, J = 3.0 Hz, 1H). 13C NMR (125 MHz, D2O): δ 25.24, 34.35, 55.32, 56.74, 59.61, 64.43, 68.86, 70.07, 182.43. HRMS (ESI, positive) Calcd for [(M + H)+] C9H18NO6 236.1129; Found: 236.1122.
3,4,6-Tri-O-benzyl-N-benzyloxycarbonyl-1,2,5-trideoxy-1,5-imino-D-galacto-1-ene-heptitol-1-aldehyde (25)
To a solution of 17 (35 mg, 0.05 mmol) in dry THF (3.0 mL) was added a solution of NaH (1.0 mg, 0.03 mmol) in THF (1.0 mL). The reaction mixture was stirred for 3 h at 30 °C. The reaction was quenched by NH4Cl aqueous solution and the solvent was removed under reduced pressure. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 25 (24 mg, 83%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 25 (24 mg, 83%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 3.83–3.86 (m, 2H), 3.87 (t, J = 4.5 Hz, 1H), 4.02 (t, J = 4.5 Hz, 1H), 4.44 (d, J = 12.5 Hz, 1H), 4.56(d, J = 12.0 Hz, 1H), 4.62 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 1H), 4.72 (d, J = 12.0 Hz, 1H), 4.81–4.85 (m, 1H), 5.18 (d, J = 12.0 Hz, 1H), 5.21 (d, J = 12.5 Hz, 1H), 6.03 (d, J = 4.0 Hz, 1H), 7.25–7.34 (m, 20H), 9.33 (s, 1H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 54.50, 65.68, 68.54, 69.29, 71.90, 72.84, 73.00, 77.12, 120.01, 127.51, 127.59, 127.64, 127.80, 127.89, 128.16, 128.29, 128.41, 128.45, 128.59, 135.31, 136.69, 137.54, 137.91, 138.33, 154.10, 186.75. HRMS (ESI, positive) Calcd for [(M + Na)+] C36H35NO6Na 600.2357; Found: 600.2356.
3,4,6-Tri-O-benzyl-N-benzyloxycarbonyl-1,2,5-trideoxy-1,5-imino-D-galacto-1-ene-heptitol-1-methanol (26)
To a solution of 25 (46 mg, 0.08 mmol) in freshly dry COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (4.0 mL) was added a solution of NaBH4 (4.6 mg, 0.12 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (0.8 mL) at 0 °C. The reaction mixture was stirred for 2 h at 0 °C. The solvent was removed under reduced pressure and the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 7
1 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 26 (34 mg, 74%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 26 (34 mg, 74%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 3.78 (t, J = 4.5 Hz, 1H), 3.83 (dd, J = 2.5, 11.0 Hz, 1H), 3.89–3.95 (m, 2H), 4.17 (d, J = 5.5 Hz, 2H), 4.40 (d, J = 12.0 Hz, 1H), 4.50 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 3H), 4.73 (d, J = 12.0 Hz, 1H), 4.92–4.96 (m, 1H), 5.15 (d, J = 12.5 Hz, 1H), 5.18 (d, J = 12.5 Hz, 1H), 5.30 (d, J = 4.5 Hz, 1H), 7.22–7.35 (m, 20H). 13C NMR (75 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 54.70, 64.41, 66.10, 68.07, 68.39, 71.24, 72.58, 72.76, 76.57, 112.65, 127.44, 127.60, 127.88, 128.23, 128.32, 128.44, 128.54, 135.71, 137.63, 138.22, 138.57, 154.43. MS-ESI: 580 [M + H+]. Anal. Calcd for C36H37NO6: C, 74.59; H, 6.43; N, 2.42; Found: C, 74.67; H, 6.61; N, 2.35%
3,4,6-Tri-O-benzyl-1,2,5-trideoxy-1,5-imino-D-galacto-1-ene-heptitol-N-cyclic carbamate (27)
Compound 27 was prepared from 25 (21 mg, 0.04 mmol) as described in the preparation of 23, providing 27 (12 mg, 70%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 3.85 (br.s, 1H), 4.00 (dd, J = 3.5, 11.0 Hz, 1H), 4.06 (t, J = 4.5 Hz, 1H), 4.11–4.14 (m, 1H), 4.45 (d, J = 12.0 Hz, 1H), 4.55 (br.s, 1H), 4.62 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 1H), 4.67 (d, J = 12.0 Hz, 1H), 4.69–4.82 (m, 5H), 7.24–7.37 (m, 15H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 51.31, 65.84, 68.41, 71.47, 72.18, 72.68, 75.49, 92.10, 127.40, 127.58, 127.71, 127.94, 128.28, 128.35, 128.52, 136.05, 137.52, 138.37, 138.50, 155.64. HRMS (ESI, positive) Calcd for [(M + Na)+] C29H29NO5Na 494.1938; Found: 494.1934.
1,2,5-Trideoxy-1,5-imino-L-glycero-D-galacto-heptitol-1-methanol (10)
A mixture of 26 (24 mg, 0.04 mmol) and 10% Pd/C (5.0 mg) in THF (2.0 mL), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O (1.0 mL) and 1.0 M HCl aqueous solution (cat.) was stirred for 72 h under H2 atmosphere. The solid was removed by filtration through Celite and the filtrate was concentrated. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (CH2Cl2/MeOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and C–18 reversed-phase column chromatography (COMPOUND LINKS
3) and C–18 reversed-phase column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O as eluent), giving 10 (7.7 mg, 80%) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.86–1.97 (m, 2H), 3.37–3.42 (m, 2H), 3.72 (dd, J = 6.5, 12.5 Hz, 1H), 3.83 (dd, J = 3.5, 12.5 Hz, 1H), 3.87–3.91 (m, 2H), 3.93–3.97 (m, 1H), 4.10 (s, 1H). 13C NMR (75 MHz, D2O): δ 27.44, 57.41, 59.81, 60.54, 61.78, 66.30, 68.18. HRMS (ESI, positive) Calcd for [(M + H)+] C7H16NO4 178.1074; Found: 178.1079.
1-Tosyl-2,3,4,6-tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol (29)
To a solution of 28 (40 mg, 0.07 mmol) in CH2Cl2 (3.0 mL) was added TsCl (21 mg, 0.11 mmol), Et3N (20 μL, 0.14 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMAP (cat.). The reaction mixture was stirred for 12 h at room temperature. The solvent was removed and the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 29 (46 mg, 90%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 29 (46 mg, 90%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 2.40 (s, 3H), 3.22 (br.s, 1H), 3.41–3.47 (m, 2H), 3.54 (dd, J = 2.5, 7.0 Hz, 1H), 3.69 (br.s, 1H), 3.77 (br.s, 1H), 3.88 (t, J = 3.5 Hz, 1H), 4.03–4.10 (m, 2H), 4.40 (d, J = 12.0 Hz, 1H), 4.45 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.53 (d, J = 12.0 Hz, 1H), 4.56 (d, J = 11.0 Hz, 1H), 4.63 (d, J = 12.0 Hz, 2H), 7.15–7.17 (m, 2H), 7.24–7.36 (m, 20H), 7.75 (d, J = 8.0 Hz, 2H). 13C NMR (75 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 21.61, 50.38, 53.22, 68.06, 68.43, 72.80, 73.00, 73.14, 73.20, 74.65, 75.68, 127.44, 127.54, 127.64, 127.83, 127.97, 128.35, 129.03, 129.83, 132.68, 137.82, 138.22, 138.38, 138.44, 144.77. HRMS (ESI, positive) Calcd for [(M + H)+] C42H46NO7S 708.2989; Found: 708.2987.
2,3,4,6-Tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-acetonitrile (30)
To a solution of 29 (48 mg, 0.07 mmol) in dry COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtoluene (2.0 mL) was added a solution of TBACN (91 mg, 0.35 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtoluene (2.0 mL). The reaction mixture was stirred for 8 h at 50 °C. The solvent was removed and the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 30 (24 mg, 63%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 30 (24 mg, 63%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.85 (br.s, 1H), 2.43 (dd, J = 9.0, 17.0Hz, 1H), 2.61 (dd, J = 6.0, 17.0 Hz, 1H), 3.18 (br.s, 1H), 3.44–3.48 (m, 1H), 3.52 (dd, J = 5.5, 9.5 Hz, 1H), 3.60 (dd, J = 2.5, 7.5 Hz, 1H), 3.72–3.73 (m, 1H), 3.83 (br.s, 1H), 3.95 (t, J = 3.5 Hz, 1H), 4.50 (s, 2H), 4.55 (d, J = 11.5 Hz, 1H), 4.56 (d, J = 11.5 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 11.5 Hz, 1H), 4.71 (d, J = 12.0 Hz, 2H), 7.25–7.37 (m, 20H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 18.00, 49.55, 53.29, 67.94, 73.06, 73.29, 73.36, 74.62, 76.48, 77.00, 77.26, 118.47, 127.49, 127.62, 127.68, 127.72, 127.92, 128.00, 128.09, 128.31, 128.37, 128.41, 128.48, 137.74, 138.12, 138.33, 138.38. MS-ESI: 585 [M + Na+]. Anal. Calcd for C36H38N2O4: C, 76.84; H, 6.81; N, 4.98; Found: C, 76.85; H, 6.84; N, 4.95%
1,5-Dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-acetonitrile (11)
Compound 11 was prepared from 30 (24 mg, 0.04 mmol) as described in the preparation of 8, providing 11 (7.8 mg, 78%) as a yellow semi-solid in the form of hydrochloride salt. 1H NMR (300 MHz, D2O): δ 2.89 (dd, J = 6.0, 17.4 Hz, 1H), 3.05 (dd, J = 7.2, 17.4 Hz, 1H), 3.40–3.55 (m, 1H), 3.72–3.80 (m, 3H), 3.99–4.06 (m, 3H). 13C NMR (75 MHz, D2O): δ 17.63, 52.89, 58.99, 60.26, 67.87, 69.02, 71.28, 119.86. HRMS (ESI, positive) Calcd for [(M + H)+] C8H15N2O4 203.1026; Found: 203.1032.
2,3,4,6-Tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-bicyclo[4.1.0] aziridine (31)
To a solution of 29 (29 mg, 0.04 mmol) in dry COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtoluene (3.0 mL) was added DBU (61 μL, 0.40 mmol). The reaction mixture was stirred for 8 h at 80 °C. The solvent was removed and the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 31 (18 mg, 82%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to provide 31 (18 mg, 82%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.54 (d, J = 4.0 Hz, 1H), 2.09 (d, J = 6.0 Hz, 1H), 2.50–2.53 (m, 1H), 2.63–2.66 (m, 1H), 3.33 (dd, J = 1.5, 8.5 Hz, 1H), 3.68 (dd, J = 5.5, 9.0 Hz, 1H), 3.72 (t, J = 8.5 Hz, 1H), 4.00 (t, J = 1.5 Hz, 1H), 4.44 (d, J = 11.5 Hz, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.49–4.51 (m, 1H), 4.59 (d, J = 12.0 Hz, 1H), 4.68 (s, 2H), 4.74 (d, J = 11.5 Hz, 1H), 4.78 (d, J = 11.5 Hz, 1H), 4.89 (d, J = 11.5 Hz, 1H), 7.23–7.37 (m, 20H). 13C NMR (125 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 34.88, 35.78, 64.45, 70.92, 72.29, 72.52, 73.38, 74.17, 74.71, 76.93, 81.44, 127.36, 127.48, 127.57, 127.67, 127.76, 127.96, 128.14, 128.33, 138.07, 138.54, 138.70, 138.88. HRMS (ESI, positive) Calcd for [(M + H)+] C36H39N2O4 536.2795; Found:536.2809.
1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-chloromethane (12)
Compound 12 was prepared from 31 (35 mg, 0.07 mmol) as described in the preparation of 8, providing 12 (14 mg, 93%) as a yellow solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 3.63–3.66 (m, 1H), 3.87 (dd, J = 3.5, 8.5Hz, 1H), 3.94–4.04 (m, 4H), 4.11 (dd, J = 4.0, 12.0 Hz, 1H), 4.22–4.25 (m, 2H). 13C NMR (75 MHz, D2O): δ 39.03, 56.31, 56.53, 58.66, 66.43, 67.20, 69.69. HRMS (ESI, positive) Calcd for [(M + H)+] C7H15NO4Cl 212.0684; Found: 212.0686.
2,3,4,6-Tetra-O-benzyl-N-hexyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-chloromethane (34)
To a solution of 32 (30 mg, 0.05 mmol) in CH2Cl2 (3.0 mL) was added MsCl (7.3 μL, 0.10 mmol), Et3N (13 μL, 0.10 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMAP (cat.). The reaction mixture was stirred for 12 h at room temperature. The solvent was removed and the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 30
1 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 34 (29 mg, 94%) as a colorless oil. 1H NMR (500 MHz, C6D5N): δ 0.82 (t, J = 6.5 Hz, 3H), 1.12–1.62 (m, 8H), 2.79–2.91 (m, 2H), 3.52 (br.s, 1H), 3.67–3.70 (m, 1H), 3.95–4.11 (m, 4H), 4.39 (s, 2H), 4.57 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.73 (d, J = 12.0 Hz, 2H), 4.80 (d, J = 12.0 Hz, 1H), 4.85 (d, J = 12.0 Hz, 2H), 4.92 (s, 1H), 5.03 (br.s, 1H), 7.18–7.55 (m, 20H). 13C NMR (75 MHz, COMPOUND LINKS
1) to provide 34 (29 mg, 94%) as a colorless oil. 1H NMR (500 MHz, C6D5N): δ 0.82 (t, J = 6.5 Hz, 3H), 1.12–1.62 (m, 8H), 2.79–2.91 (m, 2H), 3.52 (br.s, 1H), 3.67–3.70 (m, 1H), 3.95–4.11 (m, 4H), 4.39 (s, 2H), 4.57 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.73 (d, J = 12.0 Hz, 2H), 4.80 (d, J = 12.0 Hz, 1H), 4.85 (d, J = 12.0 Hz, 2H), 4.92 (s, 1H), 5.03 (br.s, 1H), 7.18–7.55 (m, 20H). 13C NMR (75 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.13, 22.70, 26.83, 29.74, 31.83, 41.68, 50.27, 57.95, 68.21, 72.70, 73.14, 73.26, 74.60, 75.42, 77.19, 78.18, 127.36, 127.50, 127.63, 127.72, 128.20, 128.30, 138.47, 138.71, 139.09. MS-ESI: 656 [M + H+], 658 [M + 2 + H+]. Anal. Calcd for C41H50ClNO4: C, 75.03; H, 7.68; N, 2.13; Found: C, 75.13; H, 7.66; N, 2.07%
2,3,4,6-Tetra-O-benzyl-N-nonyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-chloromethane (35)
Compound 35 was prepared from 33 (38 mg, 0.06 mmol) as described in the preparation of 34, providing 35 (35 mg, 89%) as a colorless oil. 1H NMR (300 MHz, C6D5N): δ 0.74 (t, J = 6.9 Hz, 3H), 1.12–1.35 (m, 14H), 2.68–2.84 (m, 2H), 3.42 (br.s, 1H), 3.54–3.65 (m, 1H), 3.85–4.00 (m, 4H), 4.29 (br.s, 2H), 4.46 (d, J = 11.7 Hz, 1H), 4.52 (d, J = 11.7 Hz, 1H), 4.61–4.77 (m, 5H), 4.82 (s, 1H), 4.91 (br.s, 1H), 7.17–7.44 (m, 20H). 13C NMR (75 MHz, C6D5N): δ 13.98, 22.63, 27.07, 29.31, 29.66, 29.69, 30.24, 31.80, 42.19, 50.03, 56.96, 60.94, 68.52, 70.94, 72.53, 72.90, 73.16, 75.71, 80.03, 127.60, 127.83, 128.41, 128.46, 139.09, 139.30, 139.62. HRMS (ESI, positive) Calcd for [(M + H)+] C44H57ClNO4 698.3971; Found: 698.3979.
N-Hexyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-chloromethane (13)
A mixture of 34 (49 mg, 0.07 mmol) and 10% Pd/C (10.0 mg) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH (4.0 mL) and 4.0 M HCl aqueous solution (cat.) was stirred for 72 h under H2 atmosphere. The solid was removed by filtration through Celite and the filtrate was concentrated. The residue was purified by C-18 reversed-phase column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O as eluent), giving 13 (20 mg, 78%) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 0.88 (t, J = 7.0 Hz, 3H), 1.27–1.42 (m, 6H), 1.82 (br.s, 2H), 3.49–3.52 (m, 2H), 3.83–4.17 (m, 7H), 4.32 (br.s, 2H). 13C NMR (100 MHz, D2O): δ 15.97, 24.46, 27.66, 27.90, 33.15, 37.74, 40.84, 54.11, 59.27, 64.52, 68.31, 71.55. HRMS (ESI, positive) Calcd for [(M + H)+] C13H27ClNO4 296.1623; Found: 296.1624.
N-Nonyl-1,5-dideoxy-1,5-imino-D-glycero-D-galacto-heptitol-1-chloromethane (14)
Compound 14 was prepared from 35 (24 mg, 0.03 mmol) as described in the preparation of 13, providing 14 (10 mg, 77%) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, CD3OD): δ 0.89 (t, J = 7.0 Hz, 3H), 1.23–1.48 (m, 14H), 2.71–2.78 (m, 2H), 2.89 (s, 1H), 3.31–3.35 (m, 1H), 3.51–3.52 (m, 1H), 3.71 (dd, J = 3.0, 11.0 Hz, 1H), 3.80–3.84 (m, 3H), 3.99–4.06 (m, 2H). 13C NMR (75 MHz, CD3OD): δ 14.44, 23.73, 28.08, 30.31, 30.42, 30.66, 30.70, 33.06, 41.20, 50.89, 60.76, 61.99, 69.36, 71.52, 73.04. HRMS (ESI, positive) Calcd for [(M + H)+] C16H33ClNO4 338.2093; Found: 338.2097.
2,3,4,6-Tetra-O-benzyl-N-nonyl-1,5-dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-chloromethane (37)
Compound 37 was prepared from 36 (20 mg, 0.03 mmol) as described in the preparation of 34, providing 37 (16 mg, 80%) as a colorless oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 0.88 (t, J = 7.0 Hz, 3H), 1.09–1.40 (m, 14H), 2.63–2.69 (m, 1H), 2.79–2.86 (m, 1H), 3.07 (td, J = 3.5, 9.0 Hz, 1H), 3.15–3.19 (m, 1H), 3.54–3.58 (m, 2H), 3.64 (dd, J = 3.5, 10.5 Hz, 1H), 3.66–3.74 (m, 2H), 3.78 (dd, J = 3.0, 9.0 Hz, 1H), 3.90 (dd, J = 2.5, 5.0 Hz, 1H), 4.31 (d, J = 12.0 Hz, 1H), 4.35 (d, J = 12.0 Hz, 1H), 4.38 (d, J = 12.0 Hz, 1H), 4.43 (d, J = 12.5 Hz, 1H), 4.47 (d, J = 2.5 Hz, 2H), 4.51 (d, J = 12.0 Hz, 1H), 4.53 (d, J = 12.5 Hz, 1H), 7.21–7.32 (m, 20H). 13C NMR (75 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.12, 22.67, 23.92, 27.21, 29.25, 29.53, 29.66, 31.89, 43.10, 49.52, 58.64, 59.30, 67.73, 71.59, 72.23, 72.38, 73.28, 74.33, 74.40, 77.18, 127.55, 127.77, 128.04, 128.18, 128.27, 128.51, 138.13, 138.39, 138.57. MS-ESI: 698 [M + H+]. Anal. Calcd for C44H56ClNO4: C, 75.67; H, 8.08; N, 2.01; Found: C, 75.94; H, 8.35; N,1.85%
N-Nonyl-1,5-dideoxy-1,5-imino-D-glycero-L-galacto-heptitol-1-chloromethane (15)
Compound 15 was prepared from 37 (25 mg, 0.04 mmol) as described in the preparation of 13, providing 15 (10 mg, 75%) as a white solid in the form of hydrochloride salt. 1H NMR (500 MHz, D2O): δ 0.85–0.87 (m, 3H), 1.28–1.41 (m, 12H), 1.75–1.76 (m, 2H), 3.24–3.34 (m, 1H), 3.41–3.50 (m, 2H), 3.91–4.10 (m, 5H), 4.13 (t, J = 4.0 Hz, 1H), 4.20–4.22 (m, 1H), 4.41–4.42 (m, 1H). 13C NMR (100 MHz, D2O): δ 13.36, 19.98, 21.98, 25.34, 28.14, 28.25, 28.39, 31.06, 38.63, 48.28, 54.38, 59.17, 60.86, 62.52, 67.26, 68.37. HRMS (ESI, positive) Calcd for [(M + H)+] C16H33ClNO4 338.2093; Found: 338.2097.
2,3,4,6-Tetra-O-benzyl-N-benzyloxycarbonyl-1,5-dideoxy-1,5-imino-L-glycero-L-galacto-heptitol-1-acid (40)
To a solution of 38 (71 mg, 0.10 mmol) in CH2Cl2 (4.0 mL) was added BAIB (68 mg, 0.20 mmol) and TEMPO (16.5 mg, 0.10 mmol). The reaction mixture was stirred for 30 h at 40 °C. The solvent was removed under reduced pressure, the residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 15
1 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide yellow crude product. The crude product was dissolved in COMPOUND LINKS
1) to provide yellow crude product. The crude product was dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetonitrile (1.5 mL), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium dihydrogen phosphate in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (1.5 mL, 100 mg mL−1), 30% H2O2 (0.38 mL) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium chlorite in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (3.0 mL, 130 mg mL−1) were added to the mixture. After stirring for 36 h at room temperature, the aqueous solution was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate. The combined organic layers were dried (Na2SO4) and evaporated in vacuo. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (petroleum ether/EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 40 (54 mg, 75%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
1) to yield 40 (54 mg, 75%) as a yellow oil. 1H NMR (500 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 3.44 (s, 1H), 3.69–3.74 (m, 2H), 4.00 (s, 1H), 4.37–4.51 (m, 8H), 4.59 (d, J = 11.5 Hz, 1H), 4.70–4.78 (m, 2H), 4.98–5.08 (m, 2H), 7.22–7.30 (m, 25H). HRMS (ESI, positive) Calcd for [(M + H)+] C43H44NO8 702.3061; Found: 702.3082.
1,5-Dideoxy-1,5-imino-L-glycero-L-galacto-heptitol-1-formic acid (16)
A mixture of 40 (27 mg, 0.04 mmol) and 10% Pd/C (8.0 mg) in THF (2.0 mL), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O (1.0 mL) and HOAc (0.4 mL) was stirred for 72 h under H2 atmosphere. The solid was removed by filtration through Celite and the filtrate was concentrated. The residue was purified by column chromatography on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel (CH2Cl2/MeOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and C-18 reversed-phase column chromatography (COMPOUND LINKS
3) and C-18 reversed-phase column chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O as eluent), giving 16 (8.0 mg, 100%) as a white solid. 1H NMR (500 MHz, D2O): δ 3.69 (br.s, 2H), 3.86 (dd, J = 7.0, 12.5Hz, 1H), 3.95–3.98 (m, 2H), 4.07 (dd, J = 2.5, 8.5 Hz, 1H), 4.40 (br.s, 1H). 13C NMR (75 MHz, D2O): δ 57.30, 61.53, 62.12, 67.94, 72.26, 72.50. HRMS (ESI, positive) Calcd for [(M + H)+] C7H14NO6 208.0816; Found: 208.0819.
Cell proliferation assay
Male BALB/c mouse splenocytes (8 × 105cells per well), retreated with Con A alone (2.5 μg mL−1 concentration) or along with 30 μM concentration of synthetic iminosugar compounds, were incubated at 37 °C for 48 h under 5% CO2 in a RPMI-1640 medium containing 10% fetal bovine serum (FBS). The proliferation of the mouse splenocytes was assayed using the CCK-8 reduction method. CCK-8 (20 μL) was added to each well and the plates were incubated for 2 h at 37 °C. Optical density was measured using a Microplate Reader at 450 nm. All data were presented as mean ± SEM. The cytotoxicity was assayed by the CCK-8 method using the cells without the Con A-induced proliferation.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China.
References
-
(a)
A. E. Stütz, Iminosugars as Glycosidase Inhibitors, Wiley-VCH: Weinheim, 1999 Search PubMed;
(b) N. Asano, R. J. Nash, R. J. Molyneux and G. W. J. Fleet, Tetrahedron: Asymmetry, 2000, 11, 1645–1680 CrossRef CAS.
-
(a) V. H. Lillelund, H. H. Jensen, X. Liang and M. Bols, Chem. Rev., 2002, 102, 515–554 CrossRef CAS;
(b)
P. Compain and O. R. Martin, Iminosugars: from synthesis to therapeutic applications, John Wiley & Sons Ltd, 2007, pp. 1–4 Search PubMed.
- T. Nishikawa and N. Ishida, J. Antibiot., 1965, 18, 132–133 CAS.
-
(a) N. Asano, Curr. Top. Med. Chem., 2003, 3, 471–484 CrossRef CAS;
(b) A. A. Watson, G. W. J. Fleet, N. Asano, R. J. Molyneux and R. J. Nash, Phytochemistry, 2001, 56, 265–295 CrossRef CAS.
- Y. Nishimura, T. Satoh, T. Kudo, S. Kondo and T. Takeuchi, Bioorg. Med. Chem., 1996, 4, 91–96 CrossRef CAS.
-
(a) J. M. H. van Den Elsen, D. A. Kuntz and D. R. Rose, EMBO J., 2001, 20, 3008–3017 CrossRef CAS;
(b) T. M. Wrodnigg, A. J. Steiner and B. J. Ueberbacher, Anti-Cancer Agents Med. Chem., 2008, 8, 77–85 CrossRef CAS.
-
(a) L. J. Scott and C. M. Spencer, Drugs, 2000, 59, 521–549 CrossRef CAS;
(b) K. Yasuda, H. Kizu, T. Yamashita, Y. Kameda, A. Kato, R. J. Nash, G. W. J. Fleet, R. J. Molyneux and N. Asano, J. Nat. Prod., 2002, 65, 198–202 CrossRef CAS.
-
(a) L. Somsák, V. Nagy, Z. Hadady, T. Docsa and P. Gergely, Curr. Pharm. Des., 2003, 9, 1177–1189 CrossRef;
(b) A. Kato, N. Kato, E. Kano, I. Adachi, K. Ikeda, L. Yu, T. Okamoto, Y. Banda, H. Ouchi, H. Takahata and N. Asano, J. Med. Chem., 2005, 48, 2036–2044 CrossRef CAS.
- D. Durantel, N. Branza-Nichita, S. Carrouée-Durantel, T. D. Butters, R. A. Dwek and N. Zitzmann, J. Virol., 2001, 75, 8987–8998 CrossRef CAS.
-
(a) K. T. Weber, D. Hammache, J. Fantini and B. Ganem, Bioorg. Med. Chem. Lett., 2000, 10, 1011–1014 CrossRef CAS;
(b) L. A. Augustin, J. Fantini and D. R. Mootoo, Bioorg. Med. Chem., 2006, 14, 1182–1188 CrossRef CAS.
-
(a) T. M. Block, X. Lu, F. M. Platt, G. R. Foster, W. H. Gerlich, B. S. Blumberg and R. A. Dwek, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 2235–2239 CrossRef CAS;
(b) A. Mehta, B. Conyers, D. L. J. Tyrrell, K.-A. Walters, G. A. Tipples, R. A. Dwek and T. M. Block, Antimicrob. Agents Chemother., 2002, 46, 4004–4008 CrossRef CAS.
-
(a) D. Pavlović, D. C. A. Neville, O. Argaud, B. Blumberg, R. A. Dwek, W. B. Fischer and N. Zitzmann, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6104–6108 CrossRef;
(b) A. S. Metha, B. Gu, B. Conyers, S. Ouzounov, L. Wang, R. M. Moriarty, R. A. Dwek and T. M. Block, Antimicrob. Agents Chemother., 2004, 48, 2085–2090 CrossRef;
(c) E. Steinmann, T. Whitfield, S. Kallis, R. A. Dwek, N. Zitzmann, T. Pietschmann and R. Bartenschlager, Hepatology, 2007, 46, 330–338 CrossRef CAS.
-
(a) J.-Q. Fan, S. Ishii, N. Asano and Y. Suzuki, Nat. Med., 1999, 5, 112–115 CrossRef CAS;
(b) N. Asano, S. Ishii, H. Kizu, K. Ikeda, K. Yasuda, A. Kato, O. R. Martin and J.-Q. Fan, Eur. J. Biochem., 2000, 267, 4179–4186 CrossRef CAS.
-
(a) T. D. Butters, R. A. Dwek and F. M. Platt, Chem. Rev., 2000, 100, 4683–4696 CrossRef CAS;
(b) Z. Yu, A. R. Sawkar, L. J. Whalen, C.-H. Wong and J. W. Kelly, J. Med. Chem., 2007, 50, 94–100 CrossRef CAS.
- G.-N. Wang, G. Reinkensmeier, S.-W. Zhang, J. Zhou, L.-R. Zhang, L.-H. Zhang, T. D. Butters and X.-S. Ye, J. Med. Chem., 2009, 52, 3146–3149 CrossRef CAS.
- E. R. Benjamin, J. J. Flanagan, A. Schilling, H. H. Chang, L. Agarwal, E. Katz, X. Wu, C. Pine, B. Wustman, R. J. Desnick, D. J. Lockhart and K. J. Valenzano, J. Inherited Metab. Dis., 2009, 32, 424–440 CrossRef CAS.
-
(a) K. Afarinkia and A. Bahar, Tetrahedron: Asymmetry, 2005, 16, 1239–1287 CrossRef CAS;
(b) B. G. Winchester, Tetrahedron: Asymmetry, 2009, 20, 645–651 CrossRef CAS;
(c) P. Compain, V. Chagnault and O. R. Martin, Tetrahedron: Asymmetry, 2009, 20, 672–711 CrossRef CAS;
(d) G. Horne, F. X. Wilson, J. Tinsley, D. H. Williams and R. Storer, Drug Discovery Today, 2011, 16, 107–118 CrossRef CAS.
- X.-S. Ye, F. Sun, M. Liu, Q. Li, Y. Wang, L.-H. Zhang and X.-L. Zhang, J. Med. Chem., 2005, 48, 3688–3691 CrossRef CAS.
- J. Zhou, Y. Zhang, X. Zhou, J. Zhou, L.-H. Zhang, X.-S. Ye and X.-L. Zhang, Bioorg. Med. Chem., 2008, 16, 1605–1612 CrossRef CAS.
- G.-L. Zhang, C. Chen, Y. Xiong, L.-H. Zhang, J. Ye and X.-S. Ye, Carbohydr. Res., 2010, 345, 780–786 CrossRef CAS.
- U. Lehmann, S. Awasthi and T. Minehan, Org. Lett., 2003, 5, 2405–2408 CrossRef CAS.
-
(a) H. R. Rosen, Gastroenterology, 2008, 134, 1789–1801 CrossRef CAS;
(b) P. F. Halloran, N. Engl. J. Med., 2004, 351, 2715–2729 CrossRef CAS.
-
(a) L. Zhang, F. Sun, Y. Li, X. Sun, X. Liu, Y. Huang, L.-H. Zhang, X.-S. Ye and J. Xiao, ChemMedChem, 2007, 2, 1594 CrossRef CAS;
(b) L. Zhang, F. Sun, Q. Wang, J. Zhou, L.-H. Zhang, X.-L. Zhang and X.-S. Ye, ChemMedChem, 2009, 4, 756–760 CrossRef CAS.
- T. M. Werkhoven, R. van Nispen and J. Lugtenburg, Eur. J. Org. Chem., 1999, 2909–2914 CrossRef CAS.
- A. Dondoni, D. Perrone and M. T. Semola, J. Org. Chem., 1995, 60, 7927–7933 CrossRef CAS.
- V. Liautard, V. Desvergnes, K. Itoh, H.-W. Liu and O. R. Martin, J. Org. Chem., 2008, 73, 3103–3115 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOMe, THF; b) NiCl2·6H2O, NaBH4, CH2Cl2, MeOH, 0 °C; c) LiAlH4, THF; d) Pd/C, H2, MeOH, 1.25 M HCl; e) BCl3, CH2Cl2, 0 °C.
CHCOOMe, THF; b) NiCl2·6H2O, NaBH4, CH2Cl2, MeOH, 0 °C; c) LiAlH4, THF; d) Pd/C, H2, MeOH, 1.25 M HCl; e) BCl3, CH2Cl2, 0 °C.




![Reagents and conditions: a) DBU, toluene, 80 °C; b) BCl3, CH2Cl2, 0 °C; c) MsCl, Et3N, DMAP; d) Pd/C, H2, MeOH, 4 N HCl. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene.](/image/article/2011/MD/c1md00098e/c1md00098e-s5.gif)

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HOAc = 4
HOAc = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy free radical, BAIB = (diacetoxyiodo)benzene
1. TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy free radical, BAIB = (diacetoxyiodo)benzene


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 13
1 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 19 (170 mg, 92%) as a colorless oil. 1H NMR (500 MHz, CDCl3): δ 3.47 (dd, J = 3.0, 9.0 Hz, 1H), 3.73 (s, 3H), 3.80–3.88 (m, 1H), 4.00–4.07 (m, 3H), 4.19–4.57 (m, 4H), 4.59–4.65 (m, 1H), 4.68 (d, J = 11.5 Hz, 2H), 4.72 (d, J = 12.0 Hz, 2H), 4.78 (d, J = 11.5 Hz, 1H), 4.89 (br.s, 1H), 5.08 (d, J = 7.0 Hz, 1H), 5.20 (d, J = 2.0 Hz, 1H), 5.98–6.03 (m, 1H), 7.18–7.34 (m, 25H). MS-ESI: 742 [M + H+]. Anal. Calcd for C46H47NO8: C, 74.47; H, 6.39; N, 1.89; Found: C, 74.52; H, 6.35; N, 1.88%
1) to provide 19 (170 mg, 92%) as a colorless oil. 1H NMR (500 MHz, CDCl3): δ 3.47 (dd, J = 3.0, 9.0 Hz, 1H), 3.73 (s, 3H), 3.80–3.88 (m, 1H), 4.00–4.07 (m, 3H), 4.19–4.57 (m, 4H), 4.59–4.65 (m, 1H), 4.68 (d, J = 11.5 Hz, 2H), 4.72 (d, J = 12.0 Hz, 2H), 4.78 (d, J = 11.5 Hz, 1H), 4.89 (br.s, 1H), 5.08 (d, J = 7.0 Hz, 1H), 5.20 (d, J = 2.0 Hz, 1H), 5.98–6.03 (m, 1H), 7.18–7.34 (m, 25H). MS-ESI: 742 [M + H+]. Anal. Calcd for C46H47NO8: C, 74.47; H, 6.39; N, 1.89; Found: C, 74.52; H, 6.35; N, 1.88%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 16
1 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 21 (145 mg, 85%) as a yellow oil. 1H NMR (400 MHz, d6-DMSO, 70 °C): δ 1.97–2.00 (m, 2H), 2.24–2.27 (m, 2H), 3.52 (s, 3H), 3.63 (br.s, 1H), 3.73–3.82 (m, 2H), 3.91 (br.s, 2H), 4.01 (br.s, 1H), 4.36 (d, J = 12.0 Hz, 2H), 4.42 (d, J = 12.4 Hz, 1H), 4.47 (d, J = 12.0 Hz, 1H), 4.57 (d, J = 12.4 Hz, 1H), 4.61 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 2H), 4.69 (d, J = 8.3 Hz, 1H), 4.86 (d, J = 12.8 Hz, 1H), 5.01 (d, J = 12.4 Hz, 1H), 7.20–7.35 (m, 25H). 13C NMR (125 MHz, d6-DMSO): δ 18.99, 30.19, 51.17, 53.85, 54.44, 66.48, 67.70, 71.67, 71.88, 71.95, 73.42, 74.29, 76.20, 79.68, 127.28, 127.36, 127.43, 127.69, 128.02, 128.17, 128.24, 129.12, 129.45, 134.54, 136.68, 138.26, 138.48, 138.87, 154.96, 157.00, 173.03. MS-ESI: 766 [M + Na+]. Anal. Calcd for C46H49NO8: C, 74.27; H, 6.64; N, 1.88; Found: C, 74.53; H, 6.87; N, 1.79%
1) to provide 21 (145 mg, 85%) as a yellow oil. 1H NMR (400 MHz, d6-DMSO, 70 °C): δ 1.97–2.00 (m, 2H), 2.24–2.27 (m, 2H), 3.52 (s, 3H), 3.63 (br.s, 1H), 3.73–3.82 (m, 2H), 3.91 (br.s, 2H), 4.01 (br.s, 1H), 4.36 (d, J = 12.0 Hz, 2H), 4.42 (d, J = 12.4 Hz, 1H), 4.47 (d, J = 12.0 Hz, 1H), 4.57 (d, J = 12.4 Hz, 1H), 4.61 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 2H), 4.69 (d, J = 8.3 Hz, 1H), 4.86 (d, J = 12.8 Hz, 1H), 5.01 (d, J = 12.4 Hz, 1H), 7.20–7.35 (m, 25H). 13C NMR (125 MHz, d6-DMSO): δ 18.99, 30.19, 51.17, 53.85, 54.44, 66.48, 67.70, 71.67, 71.88, 71.95, 73.42, 74.29, 76.20, 79.68, 127.28, 127.36, 127.43, 127.69, 128.02, 128.17, 128.24, 129.12, 129.45, 134.54, 136.68, 138.26, 138.48, 138.87, 154.96, 157.00, 173.03. MS-ESI: 766 [M + Na+]. Anal. Calcd for C46H49NO8: C, 74.27; H, 6.64; N, 1.88; Found: C, 74.53; H, 6.87; N, 1.79%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 23 (38 mg, 86%) as a yellow oil. 1H NMR (500 MHz, d6-DMSO, 70 °C): δ 1.29–1.37 (m, 1H), 1.38–1.46 (m, 1H), 1.72–1.75 (m, 2H), 3.38 (dd, J = 6.0, 11.5 Hz, 2H), 3.60 (br.s, 1H), 3.72 (dd, J = 3.0, 8.5Hz, 1H), 3.80 (dd, J = 5.0, 8.5 Hz, 1H), 3.92–3.96 (m, 2H), 4.02 (br.s, 1H), 4.12 (t, J = 5.0 Hz, 1H), 4.36 (d, J = 12.0 Hz, 1H), 4.42 (d, J = 12.0 Hz, 1H), 4.47 (d, J = 12.0 Hz, 2H), 4.58 (s, 2H), 4.64–4.71 (m, 3H), 4.87 (d, J = 13.0 Hz, 1H), 5.01 (d, J = 12.5 Hz, 1H), 7.20–7.33 (m, 25H). 13C NMR (125 MHz, DMSO): δ 19.82, 29.10, 54.15, 60.28, 65.82, 66.64, 67.89, 71.72, 71.88, 71.96, 73.46, 74.41, 76.41, 79.79, 127.29, 127.33, 127.47, 127.76, 128.01, 128.12, 128.17, 128.21, 128.25, 136.91, 138.18, 138.58, 138.89, 156.85. MS-ESI: 738 [M + Na+]. Anal. Calcd for C45H49NO7: C, 75.50; H, 6.90; N, 1.96; Found: C, 75.46; H, 6.93; N, 1.85%
1) to provide 23 (38 mg, 86%) as a yellow oil. 1H NMR (500 MHz, d6-DMSO, 70 °C): δ 1.29–1.37 (m, 1H), 1.38–1.46 (m, 1H), 1.72–1.75 (m, 2H), 3.38 (dd, J = 6.0, 11.5 Hz, 2H), 3.60 (br.s, 1H), 3.72 (dd, J = 3.0, 8.5Hz, 1H), 3.80 (dd, J = 5.0, 8.5 Hz, 1H), 3.92–3.96 (m, 2H), 4.02 (br.s, 1H), 4.12 (t, J = 5.0 Hz, 1H), 4.36 (d, J = 12.0 Hz, 1H), 4.42 (d, J = 12.0 Hz, 1H), 4.47 (d, J = 12.0 Hz, 2H), 4.58 (s, 2H), 4.64–4.71 (m, 3H), 4.87 (d, J = 13.0 Hz, 1H), 5.01 (d, J = 12.5 Hz, 1H), 7.20–7.33 (m, 25H). 13C NMR (125 MHz, DMSO): δ 19.82, 29.10, 54.15, 60.28, 65.82, 66.64, 67.89, 71.72, 71.88, 71.96, 73.46, 74.41, 76.41, 79.79, 127.29, 127.33, 127.47, 127.76, 128.01, 128.12, 128.17, 128.21, 128.25, 136.91, 138.18, 138.58, 138.89, 156.85. MS-ESI: 738 [M + Na+]. Anal. Calcd for C45H49NO7: C, 75.50; H, 6.90; N, 1.96; Found: C, 75.46; H, 6.93; N, 1.85%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and C-18 reversed-phase column chromatography (H2O as eluent), giving 6 (23 mg, 94%) as a white solid in the form of hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.66–1.79 (m, 3H), 1.96–2.09 (m, 1H), 3.57–3.59 (m, 1H), 3.63–3.72 (m, 3H), 3.90 (dd, J = 2.5, 8.5Hz, 1H), 3.93 (d, J = 6.5 Hz, 2H), 4.14 (dd, J = 4.5, 8.0 Hz, 1H), 4.21(s, 1H). 13C NMR (75 MHz, D2O): δ 24.21, 31.04, 56.98, 58.08, 60.87, 63.73, 68.57, 69.70, 71.65. HRMS (ESI, positive) Calcd for [(M + H) +] C9H20NO5 222.1336; Found: 222.1331
3) and C-18 reversed-phase column chromatography (H2O as eluent), giving 6 (23 mg, 94%) as a white solid in the form of hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.66–1.79 (m, 3H), 1.96–2.09 (m, 1H), 3.57–3.59 (m, 1H), 3.63–3.72 (m, 3H), 3.90 (dd, J = 2.5, 8.5Hz, 1H), 3.93 (d, J = 6.5 Hz, 2H), 4.14 (dd, J = 4.5, 8.0 Hz, 1H), 4.21(s, 1H). 13C NMR (75 MHz, D2O): δ 24.21, 31.04, 56.98, 58.08, 60.87, 63.73, 68.57, 69.70, 71.65. HRMS (ESI, positive) Calcd for [(M + H) +] C9H20NO5 222.1336; Found: 222.1331
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide the crude product (51 mg). The crude product was treated under the conditions as described in the preparation of 6, yielding 7 (16 mg, 80%, two steps) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.81–1.92 (m, 1H), 1.99–2.03 (m, 1H), 2.33–2.43 (m, 2H), 3.43–3.47 (m, 2H), 3.79–3.84 (m, 3H), 4.05–4.08 (m, 1H), 4.13 (d, J = 3.0 Hz, 1H). 13C NMR (125 MHz, D2O): δ 21.15, 35.92, 55.06, 56.12, 60.07, 67.78, 68.40, 70.20, 182.93. HRMS (ESI, positive) Calcd for [(M + H)+] C9H18NO6 236.1129; Found: 236.1120.
1) to provide the crude product (51 mg). The crude product was treated under the conditions as described in the preparation of 6, yielding 7 (16 mg, 80%, two steps) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.81–1.92 (m, 1H), 1.99–2.03 (m, 1H), 2.33–2.43 (m, 2H), 3.43–3.47 (m, 2H), 3.79–3.84 (m, 3H), 4.05–4.08 (m, 1H), 4.13 (d, J = 3.0 Hz, 1H). 13C NMR (125 MHz, D2O): δ 21.15, 35.92, 55.06, 56.12, 60.07, 67.78, 68.40, 70.20, 182.93. HRMS (ESI, positive) Calcd for [(M + H)+] C9H18NO6 236.1129; Found: 236.1120.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 25 (24 mg, 83%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.83–3.86 (m, 2H), 3.87 (t, J = 4.5 Hz, 1H), 4.02 (t, J = 4.5 Hz, 1H), 4.44 (d, J = 12.5 Hz, 1H), 4.56(d, J = 12.0 Hz, 1H), 4.62 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 1H), 4.72 (d, J = 12.0 Hz, 1H), 4.81–4.85 (m, 1H), 5.18 (d, J = 12.0 Hz, 1H), 5.21 (d, J = 12.5 Hz, 1H), 6.03 (d, J = 4.0 Hz, 1H), 7.25–7.34 (m, 20H), 9.33 (s, 1H). 13C NMR (125 MHz, CDCl3): δ 54.50, 65.68, 68.54, 69.29, 71.90, 72.84, 73.00, 77.12, 120.01, 127.51, 127.59, 127.64, 127.80, 127.89, 128.16, 128.29, 128.41, 128.45, 128.59, 135.31, 136.69, 137.54, 137.91, 138.33, 154.10, 186.75. HRMS (ESI, positive) Calcd for [(M + Na)+] C36H35NO6Na 600.2357; Found: 600.2356.
1) to provide 25 (24 mg, 83%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.83–3.86 (m, 2H), 3.87 (t, J = 4.5 Hz, 1H), 4.02 (t, J = 4.5 Hz, 1H), 4.44 (d, J = 12.5 Hz, 1H), 4.56(d, J = 12.0 Hz, 1H), 4.62 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 12.0 Hz, 1H), 4.65 (d, J = 12.0 Hz, 1H), 4.72 (d, J = 12.0 Hz, 1H), 4.81–4.85 (m, 1H), 5.18 (d, J = 12.0 Hz, 1H), 5.21 (d, J = 12.5 Hz, 1H), 6.03 (d, J = 4.0 Hz, 1H), 7.25–7.34 (m, 20H), 9.33 (s, 1H). 13C NMR (125 MHz, CDCl3): δ 54.50, 65.68, 68.54, 69.29, 71.90, 72.84, 73.00, 77.12, 120.01, 127.51, 127.59, 127.64, 127.80, 127.89, 128.16, 128.29, 128.41, 128.45, 128.59, 135.31, 136.69, 137.54, 137.91, 138.33, 154.10, 186.75. HRMS (ESI, positive) Calcd for [(M + Na)+] C36H35NO6Na 600.2357; Found: 600.2356.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 7
1 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 26 (34 mg, 74%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.78 (t, J = 4.5 Hz, 1H), 3.83 (dd, J = 2.5, 11.0 Hz, 1H), 3.89–3.95 (m, 2H), 4.17 (d, J = 5.5 Hz, 2H), 4.40 (d, J = 12.0 Hz, 1H), 4.50 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 3H), 4.73 (d, J = 12.0 Hz, 1H), 4.92–4.96 (m, 1H), 5.15 (d, J = 12.5 Hz, 1H), 5.18 (d, J = 12.5 Hz, 1H), 5.30 (d, J = 4.5 Hz, 1H), 7.22–7.35 (m, 20H). 13C NMR (75 MHz, CDCl3): δ 54.70, 64.41, 66.10, 68.07, 68.39, 71.24, 72.58, 72.76, 76.57, 112.65, 127.44, 127.60, 127.88, 128.23, 128.32, 128.44, 128.54, 135.71, 137.63, 138.22, 138.57, 154.43. MS-ESI: 580 [M + H+]. Anal. Calcd for C36H37NO6: C, 74.59; H, 6.43; N, 2.42; Found: C, 74.67; H, 6.61; N, 2.35%
1) to provide 26 (34 mg, 74%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.78 (t, J = 4.5 Hz, 1H), 3.83 (dd, J = 2.5, 11.0 Hz, 1H), 3.89–3.95 (m, 2H), 4.17 (d, J = 5.5 Hz, 2H), 4.40 (d, J = 12.0 Hz, 1H), 4.50 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 3H), 4.73 (d, J = 12.0 Hz, 1H), 4.92–4.96 (m, 1H), 5.15 (d, J = 12.5 Hz, 1H), 5.18 (d, J = 12.5 Hz, 1H), 5.30 (d, J = 4.5 Hz, 1H), 7.22–7.35 (m, 20H). 13C NMR (75 MHz, CDCl3): δ 54.70, 64.41, 66.10, 68.07, 68.39, 71.24, 72.58, 72.76, 76.57, 112.65, 127.44, 127.60, 127.88, 128.23, 128.32, 128.44, 128.54, 135.71, 137.63, 138.22, 138.57, 154.43. MS-ESI: 580 [M + H+]. Anal. Calcd for C36H37NO6: C, 74.59; H, 6.43; N, 2.42; Found: C, 74.67; H, 6.61; N, 2.35%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and C–18 reversed-phase column chromatography (H2O as eluent), giving 10 (7.7 mg, 80%) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.86–1.97 (m, 2H), 3.37–3.42 (m, 2H), 3.72 (dd, J = 6.5, 12.5 Hz, 1H), 3.83 (dd, J = 3.5, 12.5 Hz, 1H), 3.87–3.91 (m, 2H), 3.93–3.97 (m, 1H), 4.10 (s, 1H). 13C NMR (75 MHz, D2O): δ 27.44, 57.41, 59.81, 60.54, 61.78, 66.30, 68.18. HRMS (ESI, positive) Calcd for [(M + H)+] C7H16NO4 178.1074; Found: 178.1079.
3) and C–18 reversed-phase column chromatography (H2O as eluent), giving 10 (7.7 mg, 80%) as a white solid in the form of a hydrochloride salt. 1H NMR (500 MHz, D2O): δ 1.86–1.97 (m, 2H), 3.37–3.42 (m, 2H), 3.72 (dd, J = 6.5, 12.5 Hz, 1H), 3.83 (dd, J = 3.5, 12.5 Hz, 1H), 3.87–3.91 (m, 2H), 3.93–3.97 (m, 1H), 4.10 (s, 1H). 13C NMR (75 MHz, D2O): δ 27.44, 57.41, 59.81, 60.54, 61.78, 66.30, 68.18. HRMS (ESI, positive) Calcd for [(M + H)+] C7H16NO4 178.1074; Found: 178.1079.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 29 (46 mg, 90%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 2.40 (s, 3H), 3.22 (br.s, 1H), 3.41–3.47 (m, 2H), 3.54 (dd, J = 2.5, 7.0 Hz, 1H), 3.69 (br.s, 1H), 3.77 (br.s, 1H), 3.88 (t, J = 3.5 Hz, 1H), 4.03–4.10 (m, 2H), 4.40 (d, J = 12.0 Hz, 1H), 4.45 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.53 (d, J = 12.0 Hz, 1H), 4.56 (d, J = 11.0 Hz, 1H), 4.63 (d, J = 12.0 Hz, 2H), 7.15–7.17 (m, 2H), 7.24–7.36 (m, 20H), 7.75 (d, J = 8.0 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 21.61, 50.38, 53.22, 68.06, 68.43, 72.80, 73.00, 73.14, 73.20, 74.65, 75.68, 127.44, 127.54, 127.64, 127.83, 127.97, 128.35, 129.03, 129.83, 132.68, 137.82, 138.22, 138.38, 138.44, 144.77. HRMS (ESI, positive) Calcd for [(M + H)+] C42H46NO7S 708.2989; Found: 708.2987.
1) to provide 29 (46 mg, 90%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 2.40 (s, 3H), 3.22 (br.s, 1H), 3.41–3.47 (m, 2H), 3.54 (dd, J = 2.5, 7.0 Hz, 1H), 3.69 (br.s, 1H), 3.77 (br.s, 1H), 3.88 (t, J = 3.5 Hz, 1H), 4.03–4.10 (m, 2H), 4.40 (d, J = 12.0 Hz, 1H), 4.45 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.53 (d, J = 12.0 Hz, 1H), 4.56 (d, J = 11.0 Hz, 1H), 4.63 (d, J = 12.0 Hz, 2H), 7.15–7.17 (m, 2H), 7.24–7.36 (m, 20H), 7.75 (d, J = 8.0 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 21.61, 50.38, 53.22, 68.06, 68.43, 72.80, 73.00, 73.14, 73.20, 74.65, 75.68, 127.44, 127.54, 127.64, 127.83, 127.97, 128.35, 129.03, 129.83, 132.68, 137.82, 138.22, 138.38, 138.44, 144.77. HRMS (ESI, positive) Calcd for [(M + H)+] C42H46NO7S 708.2989; Found: 708.2987.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 30 (24 mg, 63%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 1.85 (br.s, 1H), 2.43 (dd, J = 9.0, 17.0Hz, 1H), 2.61 (dd, J = 6.0, 17.0 Hz, 1H), 3.18 (br.s, 1H), 3.44–3.48 (m, 1H), 3.52 (dd, J = 5.5, 9.5 Hz, 1H), 3.60 (dd, J = 2.5, 7.5 Hz, 1H), 3.72–3.73 (m, 1H), 3.83 (br.s, 1H), 3.95 (t, J = 3.5 Hz, 1H), 4.50 (s, 2H), 4.55 (d, J = 11.5 Hz, 1H), 4.56 (d, J = 11.5 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 11.5 Hz, 1H), 4.71 (d, J = 12.0 Hz, 2H), 7.25–7.37 (m, 20H). 13C NMR (125 MHz, CDCl3): δ 18.00, 49.55, 53.29, 67.94, 73.06, 73.29, 73.36, 74.62, 76.48, 77.00, 77.26, 118.47, 127.49, 127.62, 127.68, 127.72, 127.92, 128.00, 128.09, 128.31, 128.37, 128.41, 128.48, 137.74, 138.12, 138.33, 138.38. MS-ESI: 585 [M + Na+]. Anal. Calcd for C36H38N2O4: C, 76.84; H, 6.81; N, 4.98; Found: C, 76.85; H, 6.84; N, 4.95%
1) to provide 30 (24 mg, 63%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 1.85 (br.s, 1H), 2.43 (dd, J = 9.0, 17.0Hz, 1H), 2.61 (dd, J = 6.0, 17.0 Hz, 1H), 3.18 (br.s, 1H), 3.44–3.48 (m, 1H), 3.52 (dd, J = 5.5, 9.5 Hz, 1H), 3.60 (dd, J = 2.5, 7.5 Hz, 1H), 3.72–3.73 (m, 1H), 3.83 (br.s, 1H), 3.95 (t, J = 3.5 Hz, 1H), 4.50 (s, 2H), 4.55 (d, J = 11.5 Hz, 1H), 4.56 (d, J = 11.5 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.63 (d, J = 11.5 Hz, 1H), 4.71 (d, J = 12.0 Hz, 2H), 7.25–7.37 (m, 20H). 13C NMR (125 MHz, CDCl3): δ 18.00, 49.55, 53.29, 67.94, 73.06, 73.29, 73.36, 74.62, 76.48, 77.00, 77.26, 118.47, 127.49, 127.62, 127.68, 127.72, 127.92, 128.00, 128.09, 128.31, 128.37, 128.41, 128.48, 137.74, 138.12, 138.33, 138.38. MS-ESI: 585 [M + Na+]. Anal. Calcd for C36H38N2O4: C, 76.84; H, 6.81; N, 4.98; Found: C, 76.85; H, 6.84; N, 4.95%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 31 (18 mg, 82%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 1.54 (d, J = 4.0 Hz, 1H), 2.09 (d, J = 6.0 Hz, 1H), 2.50–2.53 (m, 1H), 2.63–2.66 (m, 1H), 3.33 (dd, J = 1.5, 8.5 Hz, 1H), 3.68 (dd, J = 5.5, 9.0 Hz, 1H), 3.72 (t, J = 8.5 Hz, 1H), 4.00 (t, J = 1.5 Hz, 1H), 4.44 (d, J = 11.5 Hz, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.49–4.51 (m, 1H), 4.59 (d, J = 12.0 Hz, 1H), 4.68 (s, 2H), 4.74 (d, J = 11.5 Hz, 1H), 4.78 (d, J = 11.5 Hz, 1H), 4.89 (d, J = 11.5 Hz, 1H), 7.23–7.37 (m, 20H). 13C NMR (125 MHz, CDCl3): δ 34.88, 35.78, 64.45, 70.92, 72.29, 72.52, 73.38, 74.17, 74.71, 76.93, 81.44, 127.36, 127.48, 127.57, 127.67, 127.76, 127.96, 128.14, 128.33, 138.07, 138.54, 138.70, 138.88. HRMS (ESI, positive) Calcd for [(M + H)+] C36H39N2O4 536.2795; Found:536.2809.
1) to provide 31 (18 mg, 82%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 1.54 (d, J = 4.0 Hz, 1H), 2.09 (d, J = 6.0 Hz, 1H), 2.50–2.53 (m, 1H), 2.63–2.66 (m, 1H), 3.33 (dd, J = 1.5, 8.5 Hz, 1H), 3.68 (dd, J = 5.5, 9.0 Hz, 1H), 3.72 (t, J = 8.5 Hz, 1H), 4.00 (t, J = 1.5 Hz, 1H), 4.44 (d, J = 11.5 Hz, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.49–4.51 (m, 1H), 4.59 (d, J = 12.0 Hz, 1H), 4.68 (s, 2H), 4.74 (d, J = 11.5 Hz, 1H), 4.78 (d, J = 11.5 Hz, 1H), 4.89 (d, J = 11.5 Hz, 1H), 7.23–7.37 (m, 20H). 13C NMR (125 MHz, CDCl3): δ 34.88, 35.78, 64.45, 70.92, 72.29, 72.52, 73.38, 74.17, 74.71, 76.93, 81.44, 127.36, 127.48, 127.57, 127.67, 127.76, 127.96, 128.14, 128.33, 138.07, 138.54, 138.70, 138.88. HRMS (ESI, positive) Calcd for [(M + H)+] C36H39N2O4 536.2795; Found:536.2809.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 30
1 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 34 (29 mg, 94%) as a colorless oil. 1H NMR (500 MHz, C6D5N): δ 0.82 (t, J = 6.5 Hz, 3H), 1.12–1.62 (m, 8H), 2.79–2.91 (m, 2H), 3.52 (br.s, 1H), 3.67–3.70 (m, 1H), 3.95–4.11 (m, 4H), 4.39 (s, 2H), 4.57 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.73 (d, J = 12.0 Hz, 2H), 4.80 (d, J = 12.0 Hz, 1H), 4.85 (d, J = 12.0 Hz, 2H), 4.92 (s, 1H), 5.03 (br.s, 1H), 7.18–7.55 (m, 20H). 13C NMR (75 MHz, CDCl3): δ 14.13, 22.70, 26.83, 29.74, 31.83, 41.68, 50.27, 57.95, 68.21, 72.70, 73.14, 73.26, 74.60, 75.42, 77.19, 78.18, 127.36, 127.50, 127.63, 127.72, 128.20, 128.30, 138.47, 138.71, 139.09. MS-ESI: 656 [M + H+], 658 [M + 2 + H+]. Anal. Calcd for C41H50ClNO4: C, 75.03; H, 7.68; N, 2.13; Found: C, 75.13; H, 7.66; N, 2.07%
1) to provide 34 (29 mg, 94%) as a colorless oil. 1H NMR (500 MHz, C6D5N): δ 0.82 (t, J = 6.5 Hz, 3H), 1.12–1.62 (m, 8H), 2.79–2.91 (m, 2H), 3.52 (br.s, 1H), 3.67–3.70 (m, 1H), 3.95–4.11 (m, 4H), 4.39 (s, 2H), 4.57 (d, J = 12.0 Hz, 1H), 4.61 (d, J = 11.5 Hz, 1H), 4.73 (d, J = 12.0 Hz, 2H), 4.80 (d, J = 12.0 Hz, 1H), 4.85 (d, J = 12.0 Hz, 2H), 4.92 (s, 1H), 5.03 (br.s, 1H), 7.18–7.55 (m, 20H). 13C NMR (75 MHz, CDCl3): δ 14.13, 22.70, 26.83, 29.74, 31.83, 41.68, 50.27, 57.95, 68.21, 72.70, 73.14, 73.26, 74.60, 75.42, 77.19, 78.18, 127.36, 127.50, 127.63, 127.72, 128.20, 128.30, 138.47, 138.71, 139.09. MS-ESI: 656 [M + H+], 658 [M + 2 + H+]. Anal. Calcd for C41H50ClNO4: C, 75.03; H, 7.68; N, 2.13; Found: C, 75.13; H, 7.66; N, 2.07%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 15
1 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide yellow crude product. The crude product was dissolved in acetonitrile (1.5 mL), and sodium dihydrogen phosphate in water (1.5 mL, 100 mg mL−1), 30% H2O2 (0.38 mL) and sodium chlorite in water (3.0 mL, 130 mg mL−1) were added to the mixture. After stirring for 36 h at room temperature, the aqueous solution was extracted with ethyl acetate. The combined organic layers were dried (Na2SO4) and evaporated in vacuo. The residue was purified by column chromatography on silica gel (petroleum ether/EtOAc 3
1) to provide yellow crude product. The crude product was dissolved in acetonitrile (1.5 mL), and sodium dihydrogen phosphate in water (1.5 mL, 100 mg mL−1), 30% H2O2 (0.38 mL) and sodium chlorite in water (3.0 mL, 130 mg mL−1) were added to the mixture. After stirring for 36 h at room temperature, the aqueous solution was extracted with ethyl acetate. The combined organic layers were dried (Na2SO4) and evaporated in vacuo. The residue was purified by column chromatography on silica gel (petroleum ether/EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 40 (54 mg, 75%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.44 (s, 1H), 3.69–3.74 (m, 2H), 4.00 (s, 1H), 4.37–4.51 (m, 8H), 4.59 (d, J = 11.5 Hz, 1H), 4.70–4.78 (m, 2H), 4.98–5.08 (m, 2H), 7.22–7.30 (m, 25H). HRMS (ESI, positive) Calcd for [(M + H)+] C43H44NO8 702.3061; Found: 702.3082.
1) to yield 40 (54 mg, 75%) as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.44 (s, 1H), 3.69–3.74 (m, 2H), 4.00 (s, 1H), 4.37–4.51 (m, 8H), 4.59 (d, J = 11.5 Hz, 1H), 4.70–4.78 (m, 2H), 4.98–5.08 (m, 2H), 7.22–7.30 (m, 25H). HRMS (ESI, positive) Calcd for [(M + H)+] C43H44NO8 702.3061; Found: 702.3082.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and C-18 reversed-phase column chromatography (H2O as eluent), giving 16 (8.0 mg, 100%) as a white solid. 1H NMR (500 MHz, D2O): δ 3.69 (br.s, 2H), 3.86 (dd, J = 7.0, 12.5Hz, 1H), 3.95–3.98 (m, 2H), 4.07 (dd, J = 2.5, 8.5 Hz, 1H), 4.40 (br.s, 1H). 13C NMR (75 MHz, D2O): δ 57.30, 61.53, 62.12, 67.94, 72.26, 72.50. HRMS (ESI, positive) Calcd for [(M + H)+] C7H14NO6 208.0816; Found: 208.0819.
3) and C-18 reversed-phase column chromatography (H2O as eluent), giving 16 (8.0 mg, 100%) as a white solid. 1H NMR (500 MHz, D2O): δ 3.69 (br.s, 2H), 3.86 (dd, J = 7.0, 12.5Hz, 1H), 3.95–3.98 (m, 2H), 4.07 (dd, J = 2.5, 8.5 Hz, 1H), 4.40 (br.s, 1H). 13C NMR (75 MHz, D2O): δ 57.30, 61.53, 62.12, 67.94, 72.26, 72.50. HRMS (ESI, positive) Calcd for [(M + H)+] C7H14NO6 208.0816; Found: 208.0819.
