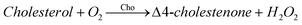Fully integrated lab-on-a-disc for simultaneous analysis of biochemistry and immunoassay from whole blood†‡
Beom Seok
Lee
a,
Yang Ui
Lee
a,
Han-Sang
Kim
a,
Tae-Hyeong
Kim
b,
Jiwoon
Park
b,
Jeong-Gun
Lee
a,
Jintae
Kim
a,
Hanshin
Kim
a,
Wee Gyo
Lee
c and
Yoon-Kyoung
Cho
*b
aSamsung Electronics Co. Ltd., 416, Maetan-3Dong, Yeongtong-Gu, Suwon-City, Gyeonggi-Do 443-746, Korea
bUlsan National Institute of Science and Technology (UNIST), Banyeon-ri 100, Ulsan, 689-798, Korea. E-mail: ykcho@unist.ac.kr; Fax: +82-52-217-2509; Tel: +82-52-217-2511
cAjou University School of Medicine, San 5,Woncheon-Dong, Yeongtong-Gu, Suwon-City, 442-721, Korea. E-mail: ykcho@unist.ac.kr
First published on 1st November 2010
Abstract
We report a fully integrated device that can perform both multiple biochemical analysis and sandwich type immunoassay simultaneously on a disc. The whole blood is applied directly to the disposable “lab-on-a-disc” containing different kinds of freeze-dried reagents for the blood chemistry analysis as well as reagents required for the immunoassay. The concentrations of different kinds of analytes are reported within 22 min by simply inserting a disc to a portable device. Using the innovative laser irradiated ferrowax microvalves together with the centrifugal microfluidics, the total process of plasma separation, metering, mixing, incubation, washing, and detection is fully automated. The analyzer is equipped with an optical detection module to measure absorbances at 10 different wavelengths to accommodate the various kinds of reaction protocols. Compared to the conventional blood analysis done in clinical laboratories, it is advantageous for point-of-care applications because it requires a smaller amount of blood (350 μL vs. 3 mL), takes less time (22 min vs. several days), does not require specially trained operators or expensive instruments to run biochemical analysis and immunoassay separately.
1. Introduction
“Lab-on-a-chip” have garnered a great deal of attention because of the possibility of miniaturization and automation for point-of-care diagnostics.1,2 However, examples of fully integrated systems including a sample preparation step, especially with the capabilities of handling real samples such as whole blood, have been rare.3–5 It is mainly because of the complexity and the cost issues in the fluidic control of multiple reagents.“Lab-on-a-disc” can be a simple alternative because it requires only a single motor to control multiple fluidic transports.6–23 A range of biological assays such as immunoassays,14–17cell lysis and homogenization,13,18,19DNA analysis,20–22 and sorting of bioparticles24 have been demonstrated on a centrifugal microfluidic platform. In order to control the fluidic passage, the majority of the centrifugal microfluidic platforms so far utilized passive types of valves, either hydrophobic valve; hydrophobic surface prevents further liquid flow, or capillary valve, liquid stoppped by a capillary pressure barrier at junctions where the channel diameter suddenly expands.25
However, the fully integrated systems for point-of-care diagnostics that are capable of handling raw samples such as whole blood has also been rare even in centrifugal microfluidc platforms.14,17,23,25,26 It is partially because of the practical limitations of the passive type of valves. For example, the disc needs to be sequentially operated from low to high spin speed, not the reverse, even though the blood separation step is usually the first step and requires the highest spin speed. Moreover, the fine tuning of the spin speed as well as the local surface properties of the microchannels were required in order to have robust control of the valves. As a result, only a limited number of diagnostic tests that do not require complex fluidic design have been developed on a disc platform and launched to the market.27–29
We have developed a unique phase change based active type of valve, laser irradiated ferrowax microvalve (LIFM), that is based on the phase transition of ferrowax, paraffin wax embedded with 10 nm sized iron oxide nanoparticles.30 Both Normally Opened LIFM (NO-LIFM) and Normally Closed LIFM (NC-LIFM) were possible. It has been demonstrated that the optical control of multiple microvalves is fast, robust, simple to operate, and requires minimal chip space and thus is well suited for fully integrated lab-on-a-chip applications.
Using the innovative LIFM together with the pathogen specific magnetic particles, we reported a fully integrated pathogen specific DNA extraction from whole blood on a lab-on-a-disc.13 We also have demonstrated that our novel centrifugal microfluidic design enables a full integration of sandwich type immunoassay starting from whole blood within 30 min.14
In this report, we report a fully integrated portable device that can perfom not only immunoassay but also multiple kinds of biochemical analysis simultaneously on a disc starting from whole blood. Testing the constituents of blood serum or plasma provides important evidence for diagnosis and treatment of illness. Concentrations of the most important components such as enzymes, lipids, electrolytes, and proteins are measured by biochemical analysis. On the other hand, the targets that have low concentrations, e.g. below 10−6 mol L−1, such as hormones, cancer markers, and disease related markers are usually tested by enzyme-linked immuno-sorbent assay (ELISA) techniques.31
Nowadays, most blood tests are done in clinical laboratories using large automated analyzers operated by highly trained staff. Although high throughput analysis is possible, it requires transport of patient's blood from a sample collection site to a clinical laboratory. Therefore, rapid acquisition of each patient's data is not easy.
Moreover, two different kinds of assay protocols often require separate instruments resulting in complicated flow of patient samples. Therefore, even in large-scale automated analyzers, integrated systems, e.g. Cobas 6000, have been developed to reduce the laboratory workload by combining two separate modules, e.g. originally independent analyzers for biochemical analysis and immunoassay. Despite the advantages, there can be maintenance issues as well, e.g. the system cannot be used even if one of the modules has a problem.
To meet the need for rapid analysis in point-of-care testing, emergency rooms, and small hospital settings, we have developed a robust, low-cost, easy to use, and versatile blood analyzer that is capable of doing both biochemical analysis as well as immunoassay. In our analyzer, whole blood is applied directly to a disposable plastic disc containing various kinds of reagents required to run both biochemistry analysis and immunoassay. As the instrument spins the disc, the total reaction process, e.g. plasma separation, metering, mixing, washing, and detection, is fully automated. The analyzer is equipped with an optical detection module to measure the absorbance at 10 different wavelengths to accommodate the various kinds of reaction protocols.
In this report, a disc preloaded with reagents for lipid test panel composed of six different kind of biochemical analytes, total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL), triglycerides (TRIG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glucose (GLU) and CK-MB (muscle and brain fraction of creatine kinase), was demonstrated as a model system.
The list of target analytes in the biochemical analysis part is the same as those from the commercially available system.32 The measurement of the serum lipids and lipoproteins can provide useful information to characterize the risk of developing cardiovascular diseases (CVD). In addition, low-density lipoprotein cholesterol (LDL), very low-density lipoprotein cholesterol (VLDL), and a total cholesterol/high-density lipoprotein cholesterol ratio (CHOL/HDL) could be also calculated from the measurements of CHOL, HDL, and TRIG.32
For the demonstration of ELISA, CK-MB was used. Normally the concentration of CK-MB is low. However, if heart muscle becomes injured as in a heart attack, the enzymes will leak out of the damaged cells causing their levels to rise in the blood stream. Thus testing for an increase of the CK-MB enzyme can diagnose or confirm a recent heart attack.33
The concentration of all of the above mentioned target analytes could be obtained within 22 min with one simple manual step of loading 350 μL of whole blood to the disc. The blood analyzer automatically runs both biochemical analysis and immunoassay simultaneously. To the best of our knowledge, it is the first portable point-of-care type device that can perform both immunoassay as well as biochemical analysis simultaneously. It is worthwhile to note that it is not a module type but a single motor system which is versatile enough to accommodate two different reaction principles.
2. Materials and methods
2.1 Biochemical analysis
Most commercially available reagents for multiple blood chemistry analysis are for the automated analyzers in clinical laboratories. In those high throughput style automated systems, the ratio of plasma to reagents or type of dilution buffers can be easily adjusted by automatic liquid handling system. Therefore, each assay has been developed to use different plasma to reagent ratios to achieve the best performance.In the previously reported blood analyzers for point-of-care testing, 2–12 tests could be performed on a rotor.34,35 The fluidic control is done by using passive type valves such as capillary or siphon valves, which limit the number of reaction steps that could be integrated on a disc. Therefore, only one dilution ratio of plasma to reagents was used for each analyte.34,35 Instead the composition of reagent and optical path length of each assay cuvette had to be specially optimized to accommodate various assay conditions on a rotor.34,35
Table 1 shows the diverse reaction protocols of each analyte of the biochemical analysis test panel. The disc design shown in Fig. 1 was optimized to accommodate these diverse reaction conditions. For example, the disc is designed to use two different dilution ratios of plasma to reagents to have better performance in limit of detection and dynamic range. It can accomodate 10 analytes that require 1 μL of plasma sample per 100 μL of reagent in cuvettes connected to the dilution chamber A. In addition, 8 analytes that need 5 μL of sample per 100 μL of reagent are tested in cuvettes connected to the dilution chamber B. In this report, we have only evaluated total 6 items related to the CVD. Four items such as CHOL, GLU, HDL, TRIG uses 1 μL of plasma sample per 100 μL of reagent. The other two target analytes such as ALT and AST use 5 μL of sample per 100 μL of reagent.
| Analytes | Type of assay | Wavelength/nm | Sample volume/μLa | Normal range (Unit) | Principle b | Medically related disorders |
|---|---|---|---|---|---|---|
| a Sample volume per 100 μL of reagents. b IFCC noPLP: International Federation of Clinical Chemistry. Without pyridoxal phosphate, COD-POD: Cholesteroloxidase-Peroxidase, LPL: Lipoprotein lipase, GPO: α-Glycerol phosphate oxidase, GK: Glycerol kinase. | ||||||
| ALT | Kinetic | 340 | 5 | 0–47 (IU/L) | IFCC noPLP | Liver diseases, including viral hepatitis and cirrhosis |
| AST | Kinetic | 340 | 5 | 0–38 (IU/L) | IFCC noPLP | Liver disease including hepatitis and viral jaundice |
| GLU | End Point | 340 | 1 | 73–118 (mg/dL) | Hexokinase | Carbohydrate metabolism disorders, including adult and juvenile diabetes mellitus and hypoglycaemia |
| CHOL | End Point | 550 | 1 | 120–240 (mg/dL) | COD-POD | Cardiovascular diseases (CVD) risk |
| HDL | End Point | 570 | 1 | >40 (mg/dL) | Direct enzymatic | Cardiovascular diseases (CVD) risk |
| TRIG | End Point | 550 | 1 | <200 (mg/dL) | LPL, GPO GK | Metabolic disorders and overall risk |
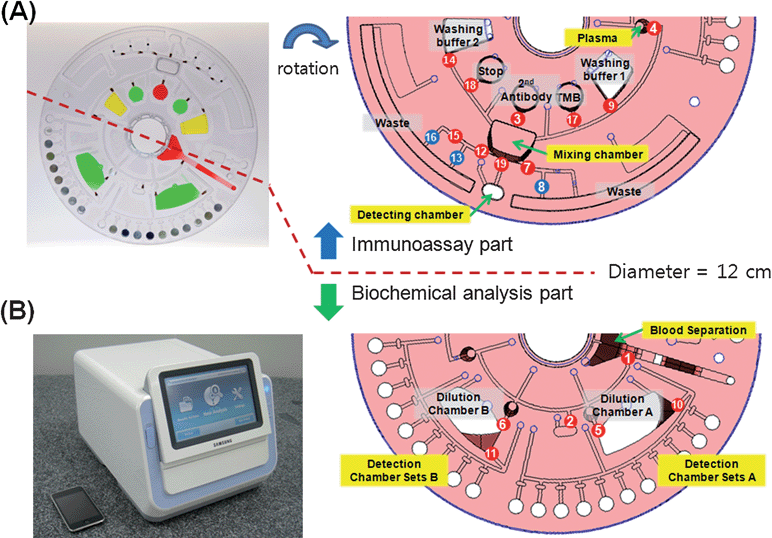 | ||
| Fig. 1 (A) Photograph of a disc. Detection wells on the clinical chemistry side are preloaded with lyophilized reagents. Other chambers for liquid type reagent are loaded with food dye solution for demonstration. In the right hand side, the disc design shows the detailed microfluidic layout. The number indicates the order of the LIFM operation. The top half of the disc for the immunoassay part is rotated for easier demonstration. The blue circles with numbers are (NO)-LIFM. The other half of the disc for the clinical chemistry analysis part is shown in the bottom. (B) A photo image of the Samsung Blood Analyzer (25(W) × 35(D) × 25(H) cm). | ||
In addition, two items such as ALT and AST are based on kinetic measurements; the analyte concentration is proportional to the slope of the absorbance change. Four items such as GLU, CHOL, HDL, and TRIG use end-point measurements; the saturated absorbance is proportional to the analyte concentration. Each analyte needs detection in a different wavelength. For example, ALT, AST, and GLU require measuring absorbances at 340 nm while CHOL and TRIG use absorbance measured at 550 nm.
Most blood chemistry reagents developed for large-sized automated analyzers are in liquid form. Reagents should be stored at −4 °C and have relatively short shelf life, from several weeks to a month after the reagents are reconstituted. In order to have a longer shelf life, we used lyophilized reagent. It is known that the shelf life of the lyophilized reagents is typically longer than one year if kept in a moisture free environment.
The linearity and imprecision tests were performed by using normal and abnormal standard serum samples (Randox, Ireland). For the correlation tests, the proposed method using the “lab-on-a-disc” on the Samsung Blood Analyzer was compared to a conventional laboratory method on Toshiba TBA 200FR Chemistry Analyzer using 213 clinical (serum) samples in Aju Hospital in Korea.
2.2 Immunoassay
As shown in Fig. 1, the other half of the disc was used for the bead-based immunoassay. The basic principle of the immunoassay is similar to the previous report except that larger silica beads are used instead of polystyrene particles.14 The beads are preloaded to the mixing chamber before the bonding step and the larger sized silica beads are much easier to handle than micron-sized polystyrene particles. Moreover, the silica beads have higher mass and therefore efficient mixing was easily achievable in a centrifugal microfluidic platform. Furthermore, the covalent bonding of antibodies was easier on silica surfaces than polystyrene particles.The silica balls (Sigma Aldrich) with a diameter of 1 mm were modified with 3-aminopropyl triethoxy silane (APTES), reacted with glutaraldehyde, and then incubated with CK-MB capture antibody (30-C40A, Fitzerald). The detailed experimental condition for the bead-based immunossay can be found elsewhere.14 An advantage of using bead-based immunoassay is that the disc can be easily modified to test other kinds of biomarkers, e.g. cardiac troponin (cTn) or C-reactive protein (CRP), simply by using different kinds of beads modified with corresponding biomarkers.
2.3 Fabrication of the disc
The diameter of the plastic disposable disc is 12 cm and the assay cuvettes A and B are 105 μL and 101 μL, respectively. Both have an optical path length of 6.2 mm. The top and bottom layer of the disc are made of molded Cyclic Olefin Copolymer (COC) for optical clarity. The microfluidic channels used to transfer liquids between reservoirs are formed on the bottom layer. The width of the channel is 1 mm and the depth of the channel is 100 μm. The top plate has holes for sample injection or air vent. The two pieces are bonded by using a UV curing glue after loading the valve material. The detailed procedure of the fabrication and the operation protocols are described elsewhere.13,302.4 Instrumentation
The operator simply pushes the open button on the instrument and places the disc in the loader after inserting whole blood (≥ 350 μL) to the blood application site of the disc. The loader is closed by pushing the close button and the instrument automatically runs the preloaded spin program.The concentrations of the target analytes measured by biochemical analysis and the immunoassay are reported within 22 min. The instrument automatically separates plasma from the whole blood, meters plasma samples with predetermined volumes, removes any extra amount of plasma, mixes with the dilution buffer with predetermined dilution ratios for blood chemistry, distributes diluted plasma to each of 18 assay cuvettes, and measures the concentration of each analyte using a built-in spectrophotometer in one side of the disc. Simultaneously, the half of the disc is used for immunoassay. Metered plasma is incubated with the glass balls coated with antibody and the detection probe, and washed with washing buffers, then incubated with tetramethyl benzidine (TMB) solution, mixed with stopping solution and finally the absorbance is measured with the same spectrophotometer of the system.
The optic module is composed of 10 photodiodes (S1133) with wavelengths of 340, 405, 450, 500, 550, 570, 600, 630, 660, and 700 nm, and 10 light emitting diodes (LED) with corresponding wavelengths. For absorbance detection, each detection cuvette on the disc is aligned with the optical module. In order to avoid any vibration effect, current has been optimized and the signal from the photodiode is amplified and converted to digital out by a 24 bit ADC. The signals, corresponding to the target cuvettes, are measured while the disc is rotating.
The performance of the built-in spectrophotometer was evaluated using the absorbance of standard material, NIST (National Institute of Standards and Technology-traceable absorbance standards STAN-ABS-VIS (Ocean Optics, Dunedin, FL)) at various wavelengths. The data was compared with the values measured by a standard spectrophotometer, Hitachi U-3310 (Hitachi High-Technologies, Minato-ku, Tokyo, JAPAN). The absorbance was linear to ≥ 3 AU.
In order to control the temperature, a heater (30 W of output power) and a digital output type temperature sensor are used. The temperature was controlled to be 37 ± 0.5 °C with P type feedback control.
The instrument has a LCD board and a system control board. The system control board has a microcontroller, that controls the main function of the instrument. It controls the motor speed, laser position, measures and processes the detector signals. The LCD board has a microcontroller that controls graphic user interface functions.
The software is designed so that people with minimal training are able to use it. It prompts the user to insert the disc and to enter a patient's identification number to begin the test. After finishing the analysis, the LCD monitor displays the results.
3. Results and discussion
3.1 Centrifugal microfluidics
As shown in Fig. 1A, the disc is designed to accommodate two different assay protocols, blood chemistry analysis and bead-based immunoassay. The valve numbers shown in Fig. 1 indicate the order of the valve actuation.Table 2 describes the detailed spin program that was used to synchronize both assays. For example, the spin steps such as 1–3, 6–8, 11–13, and 18–24 shown in italics are for both assays. First, the separation and metering of the plasma is a common step for both biochemical analysis and immunoassay. Then, the transfer of plasma and dilution for biochemical analysis were accomplished during the mixing step in immunoassay as shown in spin steps from 6 to 8. In addition, the transfer of diluted serum to the detection chamber and the following mixing step could be achieved during the washing step in the immunoassay part as shown in spin steps from 11 to 13. Furthermore, the signal detection at each time step for bioanalytical chemistry was done during the incubation time for the immunoassay as shown in spin steps from 18 to 24.
| Spin No. | Spin speed (rpm) | Valve No. | Operation | ||
|---|---|---|---|---|---|
| Blood chemistry | Immunoassay | Blood chemistry | Immunoassay | ||
| a Spin steps that are common in both blood chemistry and immunoassay are shown in italic. The final detection was done while the disc was rotating at spin speed lower than 1000 rpm. The total analysis time could vary depending upon the spin speed in each step. In the current work, the total analysis time was 22 min. | |||||
| 1 | 3600 | Separate plasma (Fig. 2A) | |||
| 2 | < 3000 | 1 | Transfer plasma to chamber A, B, C | ||
| 3 | < 3000 | 2 | Remove excess plasma (Fig. 2B) | ||
| 4 | < 3000 | 3 | Transfer 2nd Ab-HRP (Fig. 3A) | ||
| 5 | < 3000 | 4 | Transfer plasma to mixing chamber (Fig. 3B) | ||
| 6 | < 3000 | 5 | Transfer plasma to dilution chamber A | Mix beads, plasma, and detection probe ( Fig. 3C) | |
| 7 | < 3000 | 6 | Transfer plasma to dilution chamber B | ||
| 8 | vibration | Mix plasma with dilution buffer ( Fig. 2C) | |||
| 9 | < 3000 | 7, 8 | Remove residue and close the channel | ||
| 10 | < 3000 | 9 | Transfer washing buffer 1 | ||
| 11 | >3000 | 10 | Distribute diluted plasma A to each detection chamber | Mix beads and washing buffer ( Fig. 3D) | |
| 12 | >3000 | 11 | Distribute diluted plasma B to each detection chamber (Fig. 2D) | ||
| 13 | vibration | Mixing diluted serum with pre-stored reagents | |||
| 14 | < 3000 | 12, 13 | Remove residue and close the channel | ||
| 15 | < 3000 | 14 | Transfer washing buffer 2 | ||
| 16 | vibration | Mix beads and washing buffer | |||
| 17 | < 3000 | 15, 16 | Remove residue and close the channel | ||
| 18 | < 1000 | Detection (1 min) | |||
| 19 | < 3000 | 17 | Transfer TMB | ||
| 20 | vibration | Mix beads with TMB (6 min) | |||
| 21 | < 1000 | Detection (3 min) | |||
| 22 | vibration | ||||
| 23 | < 1000 | Detection (5 min) (Fig. 2E and Fig. 2F) | |||
| 24 | vibration | ||||
| 25 | < 3000 | 18 | Transfer stopping solution (Fig. 3E) | ||
| 26 | vibration | Mix beads with stopping solution | |||
| 27 | < 3000 | 19 | Transfer to detection chamber | ||
| 28 | < 1000 | Detection (Fig. 3F) | |||
The detailed spin program is explained with the examples of the photo images after each spin step as shown in Fig. 2 and Fig. 3. Images of the rotating disc were obtained in real time by using an instrument equipped with a strobe light (Hanra precision ENG, Korea) and CCD camera (IK-TF5, Toshiba, Japan). The detailed description about imaging the spinning disc is given elsewhere13,30
 | ||
| Fig. 2 CCD images of the biochemical analysis part. (A) Whole blood (350 μL) is loaded on a disc and plasma is separated by spinning. (B) Valve #1 is open and plasma is transferred to each metering chamber. Valve #2 is open and the excess amount of plasma is removed. (C) The metered plasma is transferred to the mixing chamber and diluted with pre-determined dilution ratio. (D) Valve #10 and #11 are open and the diluted plasma is transferred to the detection cuvettes. The lyophilized reagent preloaded on each detection cuvette is melted by spinning the disc in a mixing mode. (E) For the end-point detection, the absorbance values measured after 5 min are used to calculate the concentration of the target analytes such as GLU, CHOL, HDL, and TRIG. (F) The rate of absorbance change is used to calculate the concentration of the target analytes such as ALT and AST. | ||
 | ||
| Fig. 3 CCD images of the immunoassay part. (A) The HRP labelled secondary antibody is transferred to the mixing chamber by opening valve #3. (B) The metered plasma is transferred to the mixing chamber by opening valve #4. (C) Image obtained during the incubation step. The disc is rotated clockwise and counter-clockwise for efficient mixing. (D) Image after opening valve #9 to transfer the washing buffer. (E) Image after opening valve #17 and #18 to transfer the TMB solution and stopping solution, respectively. (D) Image after opening valve #19 to transfer the solution to the detection chamber. | ||
As shown in Fig. 2A, the plasma is separated after spinning the disc at 3600 rpm for 3 min. By opening valve #1, the plasma is transferred to the metering chambers. The volume of the metering chambers 1, 2, and 3 are 11 μL, 45 μL, 50 μL respectively. After filling each metering chamber, valve #2 is opened and the excess amount of plasma is transferred to the excess plasma chamber as shown in Fig. 2B.
Next, 150 μL of the secondary antibody (70-XG47, Fitzgerald) modified with Horseradish peroxidase (HRP) and the metered plasma (50 μL) is transferred to the mixing chamber in the immunoassay side by opening the valve #3 and #4 as shown in Fig. 3A and Fig. 3B.
Then, each metered plasma of 11 μL and 45 μL is transferred to the plasma dilution chamber A and B in the blood chemistry side, respectively, by repeating the sequential illumination of the laser light on the valves #5 and # 6.
Next, the metered plasma is diluted with preloaded buffer with two different dilution ratios on the half of the disc for the biochemical analysis. Fig. 2C shows the image after spin step 8.
Efficient mixing is achieved by repeating the rotation of the disc clockwise and counter-clock wise as shown in Fig. 3C. At the same time, the target analytes are captured on the silica balls. After incubation for 9 min, the plasma residue was removed by operating valves #7 and #8. Finally, the washing buffer was released by operating valve #9.
From spin step from 11 to 13, the diluted plasma sequentially fills the detection cuvettes preloaded with lyophilized reagents by opening valves #10 and #11 as shown in Fig. 2D. In order to avoid cross-contamination and promote the efficient distribution of diluted serum, the thickness of the main distribution channel is thicker, 1 mm, than the capillay channels above the assay cuvettes, 0.03 mm. Therefore, the flow resistance to assay cuvettes is larger than to the main distribution channels. This way, the liquid could be filled to each assay cuvette without air bubbles in the absence of air vent holes.
This step is partially shared by the immunoassay part for washing the beads as shown in Fig. 3D and removing the residue by sequentially operating the valves from #12 to #16. During this step, the lyophilized reagents are dissolved in diluted serum and the absorbances are measured by a built-in spectrophotomer after 1 min, 3 min, and 5 min as shown Fig. 2E and Fig. 2F. The absorbance values are compared to the prestored calibration data and the concentrations of the analytes are determined.
During this detection process on the biochemical analysis side, the valve #17 is opened and the substrate, tetramethyl benzidine (TMB) in dimethyl sulfoxide (DMSO) is transferred to the mixing chamber and incubated for 6 min. Finally, as shown in Fig. 3E, the stopping solution, 1.6 N H2SO4, was transferred to the mixing chamber and then transferred to the detection chamber by opening the valve #19 (Fig. 3F).
In the previous report, one immunoassay took about 30 min.14 However, in this report, we could accomplish both biochemical analysis and an immunoassay simultaneously within 22 min, not only by using higher spin speed in each spin step but also sharing and synchronizing the spining steps using the spin program shown in Table 2.
3.2 Results of blood chemistry
Table 1 describes the principles of the biochemistry assay of each 6 analytes. Here, only two examples of the principles to test ALT and CHOL are given to demonstrate kinetic and end-point reaction, respectivly and others can be found elsewhere.36,37
Alanine aminotransferase (ALT) was measured by an IFCC (International Federation of Clinical Chemistry) recommended method. In this reaction, ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate to form L-glutamate and pyruvate. Lactate dehydrogenase catalyzes the conversion of pyruvate to lactate. Concomitantly, NADH is oxidized to NAD+, as illustrated in the following reaction scheme.
The rate of change of the absorbance at 340 nm is due to the conversion of NADH to NAD+ and it is directly proportional to the amount of ALT present in the sample.
The total cholesterol assay is based on enzymatic reactions (COD-POD method) including cholesterol esterase (ChE), cholesterol oxidase (ChO) and hydrogen peroxidase (POD). Cholesterol esters are hydrolyzed by ChE to produce cholesterol which is then oxidased by ChO to produce Δ4-Cholestenone and an equimolar amount of hydrogen peroxide (H2O2). In the presence of peroxidase, hydrogen peroxide oxidizes p-hydroxybenzenesulfonate and 4-aminoantipyrine to give a quinoneimine dye colored in red. The intensity of the color produced is proportional to the concentration of cholesterol in the sample. The reaction is monitored at 550 nm after 5 min.
The linearity test of the biochemical analysis for each analyte is done by the evaluation method recommended by CLSI/NCCLS EP6-A. The control serum samples (Aju Hospital, Korea) with low and high concentrations were selected based upon the measurements done by a large automated analyzer, Hitachi U-3010. The samples with 5 different concentration was prepared by diluting the sample with the dilution ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 3
0, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 0
3, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and measured twice. For all of the 6 target analytes, the linearity was better than 0.995.
4 and measured twice. For all of the 6 target analytes, the linearity was better than 0.995.
The precision studies were conducted based on CLSI/NCCLS EP5-A2 as shown in Table 3. The imprecision (within-run CV% and total CV%) of 6 analytes have been measured by running 8 lab-on-a-disc per day for 5 days using 2 analyzers. Results for within-run and total precision were determined using two samples, low and high control serum samples (Randox, Ireland). The data shown in Table 3 indicate that the precision of the six assays are as good as the commercially available devices.32
| Item | Unit | Control Serum (Low) | Mean (N = 40)a | Within-run CV (%) | Total CV (%) | Control Serum (High) | Mean (N = 40)a | Within-run CV (%) | Total CV (%) |
|---|---|---|---|---|---|---|---|---|---|
| a Within-run and total imprecision were evaluated in accordance with CLSI/NCCLS (Clinical and Laboratory Standards Institute/National Committee for Clinical Laboratory Standards) EP5-A2 guidelines. Testing was done with control serums of low and high concentration, each 8 discs per day for 5 days, resulting total of 40 data, using two prototype analyzers. The within-run CV % is calculated by the average of the imprecision within a day. The total CV % includes the day by day imprecision. | |||||||||
| ALT | U/L | 37 | 36.95 | 6.3 | 6.0 | 138 | 132.78 | 0.8 | 0.6 |
| AST | U/L | 34 | 30.58 | 1.7 | 2.9 | 156 | 148.78 | 1.7 | 1.8 |
| GLU | mg/dL | 114 | 118.80 | 2.1 | 2.3 | 285 | 284.70 | 0.8 | 0.8 |
| CHOL | mg/dL | 157 | 150.43 | 0.7 | 0.6 | 275 | 277.13 | 0.3 | 0.4 |
| HDL | mg/dL | 56 | 53.55 | 7.4 | 7.2 | 116 | 105.00 | 5.1 | 4.7 |
| TG | mg/dL | 97.4 | 87.23 | 5.8 | 4.9 | 247 | 230.98 | 0.9 | 0.7 |
For the correlation study, 213 serum samples collected at Aju Hospital, Korea are measured by Samsung Blood Analyzer and compared with the data obtained by a conventional machine, Toshiba TBA 200FR Chemistry Analyzer. All the tests were conducted at Aju Hospital, Korea. The results obtained by Samsung Blood Analyzer and a standard analyzer agreed reasonably well as shown in Fig. 4. Representative correlation statistics are shown and the correlation coefficient (R2) is all larger than 0.95.
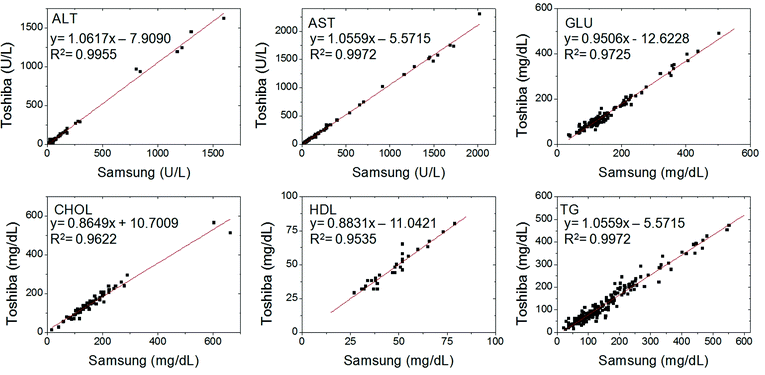 | ||
| Fig. 4 Correlation of biochemical analysis data of 213 clinical serum samples obtained by the lab-on-a-disc using Samsung Blood Analyzer with a conventional method, Toshiba TBA 200FR chemistry analyzer. All showed excellent correlations with the reference values. | ||
Further tests such as limit of detection, effects of physiological interference as well as larger-scale clinical evaluation are currently investigated at three independent clinical laboratories. Nonetheless, the precision as well as correlation study with clinical samples already indicate that the point-of-care type disc-based biochemical assay is in good agreement with the conventional large-sized automated analyzers.
3.3 Results of immunoassay
The calibration curve of CK-MB detection obtained by using the fully automated lab-on-a-disc is shown in Fig. 5A. For the evaluation of the immunoassay, the standard sample (Fitzgerald, 30-AC66, Creatine kinase-MB Isozyme) was used for comparision in a wide range of concentrations. The dynamic range is 0.92–100 ng mL−1, which is comparable with conventional ELISA kits. | ||
| Fig. 5 Experimental results of the immunoassay to measure the concentration of CK-MB using Samsung Blood Analyzer. (A) The calibration curve of CK-MB detection, (B) A correlation study conducted by the proposed lab-on-a-disc and a conventional analyzer, Roche Modular E170. | ||
The conventional definition of Limit of Detection (LoD) is the lowest analyte concentration reliably distinguished from the Limit of Blank (LoB). The LoD is determined by utilising both the measured LoB and test replicates of a sample known to contain a low concentration of analyte.38 Based upon the definition, LoB and LoD was calculated to be 0.70 ng mL−1 and 0.92 ng mL−1, respectively. The normal cut-off range used in conventional analyzer, Roche Modular E170, is below 4.94 ng mL−1.
Fig. 5B shows a correlation study conducted by the proposed lab-on-a-disc using Samsung Blood Analyzer and conventional analyzer, Roche Modular E170. For this study, the same standard sample was used and large-scale clinical evaluation is in preparation.
The performance of the immunoassay done by using the proposed lab-on-a-disc is as good as the conventional methods even though it requires a reduced number of washing steps; twice by using 220 μL of washing buffervs. three times by using 1 mL, and the reaction time is dramatically reduced; 22 min. vs. several hours. Moreover, the concentrations of the clinical test panel composed of relevant biochemical analytes are reported simultaneously to provide more information so that the physicians may make better decisions in a shorter time.
4. Conclusions
We have developed a blood analyzer utilizing the novel ferro-wax valve and centrifual microfuidics technology. It is the first small sized instrument, to the best of our knowledge, that can run both immunoassays and blood chemistry analysis.In this report, we have demonstrated the simultaneous analysis of a blood chemistry panel with 6 analytes including ALT, AST, CHOL, GLU, HDL, and TRIG and immunoassay for CK-MB as a model system. Compared to the conventional blood chemistry analysis done in the clinical laboratories, the “Lab-on-a-Disc” is advantageous for the point-of-care applications because it requires less blood (350 μL vs. 3 mL), takes less time (22 min vs. several days), and does not require a specially trained operator or expensive instruments.
Furthermore, the disc can be easily modified to accomodate different kinds of biological assays e.g. detection for infectious diseases such as Anti-HBV or HBsAg or cancer markers such as CEA (Carcinoembryonic Antigen) or AFP (alpha-fetoprotein) as well as panels with desired combination of biochemical analytes depending upon the application by simply adding different set of reagents and antibody modified beads for biochemical analysis and immunoassay, respectively. For more versatile applications, the disc capable of multiplex immunoassay is currently under development.
Acknowledgements
The authors at UNIST is partially sponsored by WCU (World Class University) program (R32-2008-000-20054-0).Notes and references
- S. K. Sia and L. J. Kricka, Lab Chip, 2008, 8, 1982–1983 RSC.
- F. B. Myers and L. P. Lee, Lab Chip, 2008, 8, 2015–2031 RSC.
- C. J. Easley, J. M. Karlinsey, J. M. Bienvenue, L. A. Legendre, M. G. Roper, S. H. Feldman, M. A. Hughes, E. L. Hewlett, T. J. Merkel, J. P. Ferrance and J. P. Landers, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19272–19277 CrossRef CAS.
- A. Lenshof, A. Ahmad-Tajudin, K. Jrås, A.-M. Swrd-Nilsson, L. Åberg, G. Marko-Varga, J. Malm, H. Lilja and T. Laurell, Anal. Chem., 2009, 81, 6030–6037 CrossRef CAS.
- M. Toner and D. Irimia, Annu. Rev. Biomed. Eng., 2005, 7, 77–103 CrossRef CAS.
- D. C. Duffy, H. L. Gillis, J. Lin, N. F. Sheppard and G. J. Kellogg, Anal. Chem., 1999, 71, 4669–4678 CrossRef CAS.
- G. J. Kellogg, T. E. Arnold, B. L. Carvalho, D. C. Duffy and N. F. Sheppard, in Proc. in MicroTAS, ed. W. O. A. van den Berg, and P. Bergveld, 2000, pp. 239–242 Search PubMed.
- S. Lai, S. Wang, J. Luo, L. J. Lee, S.-T. Yang and M. J. Madou, Anal. Chem., 2004, 76, 1832–1837 CrossRef CAS.
- J. V. Zoval and M. J. Madou, Proc. IEEE, 2004, 92, 140–153 CrossRef CAS.
- M. Grumann, A. Geipel, L. Riegger, R. Zengerle and J. Ducree, Lab Chip, 2005, 5, 560–565 RSC.
- T. Brenner, T. Glatzel, R. Zengerle and J. Ducree, Lab Chip, 2005, 5, 146–150 RSC.
- S. Haeberle, T. Brenner, R. Zengerle and J. Ducree, Lab Chip, 2006, 6, 776–781 RSC.
- Y. K. Cho, J. G. Lee, J. M. Park, B. S. Lee, Y. Lee and C. Ko, Lab Chip, 2007, 7, 565–573 RSC.
- B. S. Lee, J.-N. Lee, J.-M. Park, J.-G. Lee, S. Kim, Y.-K. Cho and C. Ko, Lab Chip, 2009, 9, 1548–1555 RSC.
- L. G. Puckett, E. Dikici, S. Lai, M. Madou, L. G. Bachas and S. Daunert, Anal. Chem., 2004, 76, 7263–7268 CrossRef CAS.
- N. Honda, U. Lindberg, P. Andersson, S. Hoffmann and H. Takei, Clin. Chem., 2005, 51, 1955–1961 CrossRef CAS.
- J. Steigert, M. Grumann, T. Brenner, L. Riegger, J. Harter, R. Zengerle and J. Ducree, Lab Chip, 2006, 6, 1040–1044 RSC.
- J. Kim, S. H. Jang, G. Jia, J. V. Zoval, N. A. D. Silva and M. J. Madou, Lab Chip, 2004, 4, 516–522 RSC.
- J. Siegrist, R. Gorkin, M. Bastien, G. Stewart, R. Peytavi, H. Kido, M. Bergeron and M. Madou, Lab Chip, 2010, 10, 363–371 RSC.
- R. F. Taylor, IVD Technology Magazine, 2008, 39–47 Search PubMed.
- R. Peytavi, F. R. Raymond, D. Gagne, F. J. Picard, G. Jia, J. Zoval, M. Madou, K. Boissinot, M. Boissinot, L. Bissonnette, M. Ouellette and M. G. Bergeron, Clin. Chem., 2005, 51, 1836–1844 CrossRef CAS.
- H. Chen, L. Wang and P. C. H. Li, Lab Chip, 2008, 8, 826–829 RSC.
- R. Gorkin, J. Park, J. Siegrist, M. Amasia, B. S. Lee, J.-M. Park, J. Kim, H. Kim, M. Madou and Y.-K. Cho, Lab Chip, 2010, 10, 1758–1773 RSC.
- R. Martinez-Duarte, R. A. G. Iii, K. Abi-Samra and M. J. Madou, Lab Chip, 2010, 10, 1030–1043 RSC.
- P. Andersson, G. Jesson, G. Kylberg, G. Ekstrand and G. Thorsen, Anal. Chem., 2007, 79, 4022–4030 CrossRef CAS.
- R. M. Rocco, Landmark Papers in Clin. Chem., Elsevier, Amsterdam, 2006 Search PubMed.
- A. I. Piccolo, 3240 Whipple Road, 94587 Union City, CA, USA, http://www.abaxis.com.
- R. L. SpinX Technologies, 1217 Geneva, Switzerland, http://www.spinx-technologies.com.
- C. D. Gyrolab Bioaffy, A. B. Gyros, Dag Hammarskjolds vag 54, SE-751 83 Uppsala, Sweden (formerly Pharmacia Inc., USA), http://www.gyros.com.
- J. M. Park, Y. K. Cho, B. S. Lee, J. G. Lee and C. Ko, Lab Chip, 2007, 7, 557–564 RSC.
- K. Imai, S. Watari, T. Sakazume and S. Mitsuyama, Hitachi Review, 2008, 57, 1–7 Search PubMed.
- Abaxis Inc., USA, (http://www.abaxis.com).
- A. S. Jaffe, L. Babuin and F. S. Apple, J. Am. Coll. Cardiol., 2006, 48, 1–11 CrossRef CAS.
- C. T. Schembri, V. Ostoich, P. J. Lingane, T. L. Burd and S. N. Buhl, Clin. Chem., 1992, 38, 1665–1670 CAS.
- G. T. Schembri, T. L. Burd, A. R. Kopf-Sill, L. R. Shea and R. Braynin, J. Auto. Chem., 1995, 17, 99–104 Search PubMed.
- Z. Noroozi, H. Kido, M. Micic, H. Pan, C. Bartolome, M. Princevac, J. Zoval and M. Madou, Rev. Sci. Instrum., 2009, 80, 075102–075108 CrossRef.
- C. A. Burtis, E. R. Ashwood and N. W. Tietz, Tietz Textbook of Clinical Chemistry, W.B. Saunders Company, 3rd edn, January 15, 1999 Search PubMed.
- D. A. Armbruster and T. Pry, Clin Biochem Rev, 2008, 29, S51 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Movie file and caption. See DOI: 10.1039/c0lc00205d |
| ‡ Published as part of a LOC themed issue dedicated to Korean Research: Guest Editors: Professor Je-Kyun Park and Kahp-Yang Suh. |
| This journal is © The Royal Society of Chemistry 2011 |