Themed issue: Chemical transformations of nanoparticles
Dmitri V.
Talapin
a and
Yadong
Yin
b
aUniversity of Chicago, USA
bUniversity of California, Riverside, USA
 Dmitri V. Talapin Dmitri V. Talapin |
 Yadong Yin Yadong Yin |
For a long time, chemists studied systems where composition and structure could be defined at the atomic level. Such determinism is natural in molecular compounds and in macroscopic bulk crystalline solids. As molecules became larger and larger, it was more and more difficult to keep this level of control. For the first time, chemists experienced this problem in polymers. Indeed, description of macromolecular substances required statistical approaches dealing with the average molecular weight and polydispersity. This transition from fully deterministic to statistical descriptions of chemical species was an important paradigm shift that required accepting new chemistry rules and inventing new characterization techniques. It definitely paid off. It is difficult to overestimate the importance of polymers for modern society…
The invention of nanomaterials introduced another broad class of objects that did not fit into traditional chemistry framework but show tremendous potential in many fields, from medical diagnostics to catalysis and optoelectronics. Properly engineered nanomaterials can serve in light-emitting devices and fluorescent bio-tags; they can be used to serve as a low-cost alternative to bulk semiconductors in large area solar cells. Their success on all these arenas will depend on development of efficient and robust synthetic methodology for tailoring physical and chemical properties.
This themed issue focuses on chemical transformation that involve nanoparticles containing hundreds to thousands of atoms in the inorganic core. These chemically synthesized nanoparticles are typically capped with a shell of surface ligands (Fig. 1). The chemical transformations can involve both the core and the shell, resulting in two different research directions. In the former case, one can speak about truly unprecedented chemistries developed to modify the nanoparticle cores. For clarity, we can describe these transformations by making analogy to isomerization, addition, substitution, elimination and rearrangement reactions well known in molecular chemistry (Table 1). However, instead of a process that involves one or several chemical bonds, chemical transformations at nanoparticle level involve hundreds to thousands of chemical bonds. Should we then view these processes in direct analogy to the solid-state reactions? Not really. In many cases, these transformations do not obey the rules observed for bulk solids. For example, the Schaak group synthesized Au–Rh, Au–Pt, Pt–Rh, and Pd–Rh alloy nanoparticles which could not be prepared in the bulk form because of the immiscibility of these metals (DOI: 10.1039/c0jm03913f). Such changes in the miscibility at the nanoscale open up exciting opportunities, e.g., for novel heterogeneous catalysts.
 | ||
| Fig. 1 A colloidal nanoparticle combining an inorganic solid core with a shell of surface ligands. | ||
| Transformation |
|---|
| Isomerization |

|
| Addition I |

|
| Addition II |

|
| Substitution |
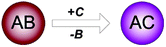
|
| Elimination |

|
| Rearrangement |

|
In addition to unexpected chemical compositions, nanoscale structural engineering can enhance the materials performance by precise control over the surface structure. It is known that high-index facets or platinum group metals can exhibit higher catalytic activity than the low-index facets. Shi-Gang Sun and co-workers studied the structure and stability of high-index facets at the surface of Pt nanocrystals (DOI: 10.1039/c1jm10125k) and found that nanocrystals enclosed by high-index {310}, {311}, and {331} facets possess higher stability than the nanocrystals with low-index {100} and {110} facets. This work represents an important class of chemical transformations which do not change chemical composition but result in distinctively different shapes (Fig. 2). Following the analogy with traditional chemistry, we may consider them as “isomerization reactions”. In the same vein, Liz-Marzán observed “isomerization” of star-shape Au nanoparticles into spherical particles caused by the Ostwald ripening (DOI: 10.1039/c1jm10603a).
 | ||
| Fig. 2 Different shapes allow controlling the surface structure of nanoparticle catalysts (S.-G. Sun, DOI: 10.1039/c1jm10125k). | ||
The important class of nanoparticle transformations is the “addition reaction” where a nanoparticle reacts with some molecular species to form another nanoparticle of a different chemical phase (we will call this process “Addition I”). The second type of addition reaction (“Addition II”) results in the nanoparticle heterostructures with dumbbell or core–shell morphology. The readers can find examples of both reactions in this themed issue. Thus, De and co-workers studied the transformation of Pd nanocrystals into PdH0.7 nanoparticles in the presence of NaBH4 (DOI: 10.1039/c1jm10207a). Urban et al. demonstrated reversible adsorption of CO2 at the surface of MgO nanocrystals revealing interesting opportunities for development of nanoparticles for capturing CO2 (DOI: 10.1039/c1jm11784j). Another interesting type of addition reaction was demonstrated by the Robinson group who observed the phase transformation of Co nanoparticles first into Co2P and then into CoP phase (DOI: 10.1039/c1jm10337g). The phase transformation was accompanied by the shape transformation from spherical nanoparticles to hollow nanostructures via the nano-Kirkendall effect.
The addition reactions of type II are widely used for synthesis of nano-heterostructures with core–shell and dumbbell morphologies. In this case, the molecular reactant does not induce a chemical transformation of original nanoparticles but serves as the precursor for the nucleation and growth of a new inorganic phase connected to the seed particle. The examples of such transformations can be found in the papers by Puntes et al. (DOI: 10.1039/c1jm10313j) and Ying et al. (DOI: 10.1039/c1jm10349k) who explored the preparation of metallic nano-heterostructures.
A typical example of a substitution reaction in nanoparticles is the cation exchange in II–VI semiconductor nanocrystals. Green et al. studied this process using CdSe and CdTe nanoparticles and nanorods as starting materials and treating them with different metal ions, e.g., Hg2+ which partially replaced Cd2+ ions in the nanocrystal lattice (DOI: 10.1039/c1jm10248f).
Two works from this themed issue demonstrate chemical transformations that can be classified as the elimination reactions. Both An et al. (DOI: 10.1039/c1jm10244c) and Sun et al. (DOI: 10.1039/c1jm10475f) studied the partial reduction of AgCl nanoparticles resulting in the hybrid AgCl:Ag nanoparticles exhibiting high photocatalytic activity and durability under sunlight illumination.
Finally, the work of de Mello Donega represents a striking example of a chemical transformation where PbSe/CdSe core–shell nanoparticles rearrange into nanostructures with a dumbbell morphology upon annealing under vacuum (Fig. 3). The structural reconstruction of the PbSe/CdSe nano-heterostructures has also a pronounced effect on their optical properties, affecting both the absorption and emission transitions (DOI: 10.1039/c0jm04458j).
 | ||
| Fig. 3 Rearrangement of PbSe/CdSe core–shell nanoparticles into a nanoparticle dimer (de Mello Donega, DOI: 10.1039/c0jm04458j). | ||
Returning to Fig. 1, we want to emphasize the importance of surface ligands which control many properties of nanomaterials, such as solubility and compatibility with the surrounding medium. In many cases, the original surface ligands used in nanoparticle synthesis have to be replaced with new ligands that provide desired chemical or physical properties. For example, Hyeon et al. describes the transformation of hydrophobic iron oxide nanoparticles to hydrophilic ones by dextran coating. The hydrophilization of the nanoparticle surface allowed them to be utilized as a highly efficient MRI contrast agent (DOI: 10.1039/c1jm10432b).
In addition to solubility and biocompatibility, surface ligands can also add novel functionalities. For example, S. Sun et al. designed surface ligands containing a fluorescent ruthenium complex (Fig. 4). The grafting of this complex to the surface of magnetic Fe3O4 nanoparticles produced a rather unique material combining magnetic and luminescent functionalities (DOI: 10.1039/c0jm03119d). The authors propose using these multifunctional nanoparticles as dual probes for biological imaging applications.
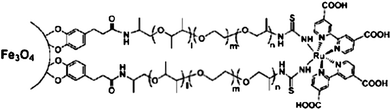 | ||
| Fig. 4 The conjugate of magnetic Fe3O4 nanoparticles and fluorescent Ru(dcbpy)2(NCS)2 complex (S. Sun, DOI: 10.1039/c0jm03119d). | ||
The above examples mostly focus on the structures and properties of individual nanoparticles. At the same time, in recent years the nanoparticles have been widely explored as the building blocks for macroscopic functional materials. This emerging class of nanocrystal solids attracts growing interest for the inexpensive fabrication of solar cells and photodetectors, for solid-state lighting and other technologies. The surface ligands separate individual particles in the nanocrystal solids and often negatively affect electrical transport in these materials. Several publications from this themed issue deal with the design of electronically transparent surface ligands for nanocrystal solids. For example, the Wei and Reiss groups have managed to dramatically improve the conductivity in the films of PbSe and CdSe quantum dots by treating them with small molecule linkers such as 1,2-ethanedithiol and butylamine (DOI: 10.1039/c0jm04417b and DOI: 10.1039/c1jm10538h). In an elegant study, Weiss et al. used diarylethylene surface ligands to cross-link the films of CdSe quantum dots and observed switchable photoconductivity dependent on sample illumination history (DOI: 10.1039/c0jm04397d). Finally, Milliron et al. report a very interesting and innovative work on the use of polyoxometallates as surface coatings for indium-tin oxide nanoparticles (DOI: 10.1039/c1jm10514k). This work opens up interesting directions for the development of complex oxide-based composite materials.
Before concluding this editorial, we would like to point the readers' attention to several problems that still need to be addressed. Future developments of synthetic methodology should not only pursue the goal of making more and more sophisticated nanostructures, but should also perfect the run-to-run reproducibility of nanoparticle syntheses. The other extremely important problem, relevant to nanoscience in general, is standardization of nanomaterials. The properties of nanomaterials synthesized by different research groups often vary because of the lack of commonly accepted standards. The research community has to agree upon the list of experimental conditions and characterization techniques, sufficient for quantitative reproducibility of properties for every important type of nanomaterials. Only in that case can results from different researchers be directly compared with each other. This problem is not unique to nanomaterials. Other fields, including semiconductors and polymers, have faced very similar challenges in their early days. Development of the protocols accepted by research and industrial communities helped to unify the efforts of different research groups and greatly facilitated the transfer of these materials from research labs to industry.
We want to thank all the authors who contributed to this themed issue. Their contributions provided excellent examples of the chemical transformations that involve inorganic nanoparticles. We also want to thank the editorial staff of Journal of Materials Chemistry for their help with organizing this issue. We hope that many readers will find this themed issue interesting, useful, and will inspire further developments in this exciting area of research.
| This journal is © The Royal Society of Chemistry 2011 |
