Synthesis of quasi-oriented α-MoO3 nanobelts and nanoplatelets on TiO2 coated glass
Murat E.
Kurtoglu
ab,
Travis
Longenbach
a and
Yury
Gogotsi
*a
aA.J. Drexel Nanotechnology Institute and Department of Materials Science and Engineering, Drexel University, Philadelphia, PA 19104, USA. E-mail: gogotsi@drexel.edu
bGurallar ArtCraft Glassware, Ataturk Bulvari 5.km, Kutahya, Turkey
First published on 12th April 2011
Abstract
We report on a new method to produce quasi-oriented α-MoO3 nanobelts on TiO2 coated glass substrates. Using a simple yet efficient wet-chemical method with subsequent calcination, various morphologies of α-MoO3 including oriented nanobelts, rods and platelets were produced. The crystal morphology can be controlled by changing the substrate and/or the process parameters. Some applications of the resulting structures were demonstrated by coating them with TiO2 and carbon. The photocatalytic activity of the TiO2 coated α-MoO3 nanobelts was several times higher than that of the smooth TiO2 coatings prepared on soda-lime glass. A very thin layer (20–30 nm) of carbon coated on nanobelts by sputtering resulted in a highly light absorbing film with an average reflectivity of 4%, while fluorosilane coated nanobelts showed superhydrophobic properties.
Introduction
One-(1-D) and two-dimensional (2-D) nanostructures are receiving a lot of attention due to their unique electronic, chemical, optical and mechanical properties that differ from thin films or bulk materials.1–3 In particular, high surface area metal oxide nanostructures offer significant potential in several application areas such as gas sensors, piezoelectric devices, and catalysis.4 While nanotubes and nanowires have been very extensively studied,5 nanobelts received less attention until recently.6 In particular, α-MoO3 nanobelts have been utilized in several applications including field emission devices,7 gas sensors,8 as positive templates,9 Li-ion battery electrodes because of their layered structure,10,11 as well as the synthesis of MoS2 nanotubes12 and MoO2 nanorods.13There are two common phases of molybdenum trioxide (MoO3); a thermodynamically stable orthorhombic phase (α-MoO3) and a monoclinic phase (β-MoO3). α-MoO3 has a layered atomic structure with layers stacked along [010] by van der Waals bonding and each layer is composed of two sub-layers forming corner-sharing octahedra along [001] and [100]. It can form a variety of morphologies due to this unusual crystal structure that favors anisotropic growth. Consequently, a rich variety of nanostructures including nanorods,14,15nanowires,16nanotubes17 and nanobelts7 have been reported for α-MoO3.
Several methods have been used for the synthesis of α-MoO3 nanobelts. Li et al. reported field emission properties of α-MoO3 nanobelts which were prepared by heating a piece of molybdenum sheet at high temperatures in a chamber and evaporating the molybdenum trioxide onto a silicon single crystal.7 Several other authors have published hydrothermal processes to produce α-MoO3 nanobelts.18–21 Lou et al. reported fork-like α-MoO3 structures by capping the (001) planes with TiO2 in a hydrothermal process.22 However, hydrothermal processes are not applicable to the synthesis of coatings on a large surface area. Recently Xie et al. suggested a method in which α-MoO3 nanobelts were formed by placing a molybdenum sheet on a glass substrate and heating this system to 480 °C on a heater plate for 2 days.23 While this is the simplest process reported thus far for the production of α-MoO3 nanobelts, which can be applied to relatively large areas, it is still a very slow process with a prolonged, energy-inefficient annealing step. Here, we report the synthesis of quasi-oriented α-MoO3 nanobelts on TiO2 coated glass substrates of any dimension using a very simple wet chemical approach followed by annealing at a temperature as low as 400 °C with a short dwell time (ca. 30 min).
Experimental
To produce α-MoO3, molybdenum pentachloride (MoCl5) (Alfa-Aesar, 99.6%) was dissolved in N,N-dimethyl formamide (DMF) (Sigma-Aldrich, anhydrous, 99.8%), which yielded a green transparent solution. 150 μl of this solution was dropped onto a TiO2 coated (ca. 70 nm) glass slide (2 × 2 cm) using a micropipette. TiO2 coating of the glass slides was done via a sol–gel method as reported previously.24 Briefly, 120 g ethyl alcohol (200-proof, Electron Microscopy Sciences) and 15 g Ti-(O-i-C3H7)4 (Alfa-Aesar, 98%) were mixed and subsequently stirred with a magnetic stirrer for 30 min. Then, a mixture of 0.96 ml deionized water (18 MΩ cm), 0.52 g HCl (Fisher Science, 38%), 1 g poly (ethylene glycol) (Sigma-Aldrich, M.W. = 2000) and 6.3 g ethyl alcohol was added drop-wise to the main solution. The solution was stirred for 2 h. Then, 9.6 g acetylacetone (Sigma-Aldrich, 98%) and 5 ml of deionized water were added to the prepared sol and stirred for an additional hour. The film was prepared by dip-coating (MTI Dip Coater HL-01). For this purpose, a glass microscope slide (Erie Scientific) was dipped into the prepared solution and withdrawn at a constant speed of 100 mm min−1 followed by calcination at 400 °C for 30 min. After the deposition of the MoCl5-DMF mixture on TiO2 coated glass slides, the mixture turned from green to brown with increasing wait time and humidity, which is an indication of hydrolysis. Coated samples were placed into a furnace, which was preheated to 500 °C, within 10 min from the deposition of the MoCl5-DMF mixture and calcined for 30 min. The same procedures were repeated on a plain glass microscope slide and TiO2 coated quartz slides to evaluate the substrate effect. Samples were characterized by scanning electron microscopy (Zeiss Supra 50VP), transmission electron microscopy (JEOL JEM 2100), UV-Vis spectrophotometry (Thermo Scientific Evolution 600), and X-ray diffraction analysis (Siemens D500).Results and discussion
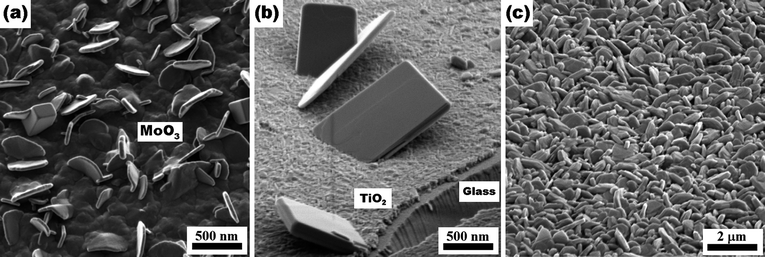
SEM images show that the substrates were covered with belt-like anisotropic crystals with their long end directed upwards, as shown in Fig. 1a-1b. Obtained nanobelts were typically 6 to 8 μm in length and less than 0.4–0.5 μm in width. The typical thickness of the nanobelts was approximately 50 nm. XRD analysis (Fig. 1d) was in agreement with the previously reported α-MoO3 patterns, strong anisotropy was evident from the relative intensities of the peaks, particularly the (021) peak, which is similar to the reported XRD patterns of the aligned MoO3 structures.7,25 As seen from the TEM image and the respective SAED pattern (Fig. 1c), the nanobelts were single crystalline and had a principal growth direction along <001> and the sideways growth along <100>. | ||
| Fig. 1 (a) Low- and (b) high-magnification SEM images of oriented MoO3 nanobelts on soda-lime glass substrates (calcined at 500 °C). (c) TEM image and single crystal SAED pattern (inset). (d) XRD spectrum of the nanobelts (■: Na2MoO4). | ||
Our experiments have shown that the obtained morphology is strongly dependent on the type of substrate, the solvent used for dissolving MoCl5, and the heating rate. When plain soda-lime glass was used as a substrate, rod-like structures with their growth direction parallel to the substrate were obtained (Fig. 2a). XRD analysis (Fig. 2b) of the structures shows that these crystals were mainly composed of α-MoO3 with (0k0) planes aligned parallel to the surface. Some sodium molybdate formation (Na2MoO4) was also evident from the diffraction pattern. Thus, this approach allows the synthesis of adherent α-MoO3 coatings on glass. We assume that sodium diffusion was responsible for the change in the α-MoO3 growth habit.
 | ||
| Fig. 2 SEM images and XRD patterns of α-MoO3 on TiO2 coated (a,b) plain soda-lime glass and (c,d) quartz slides. Both samples were calcined at 500 °C. (■: Na2MoO4). | ||
When TiO2 coated quartz substrates were used, nanoplatelets (Fig. 2c) with their (0k0) planes perpendicular to the substrate were observed. The nanoplatelets were more robust to handling (scratching, peeling off etc.) compared with nanobelts due to a smaller aspect ratio and the intersecting plates forming a rigid structure. Therefore, despite their smaller aspect ratio and lower surface area, they may be more suitable for many practical applications as a coating when durability and wear resistance is required.
In order to better understand the growth process, we prepared a set of samples on glass slides and calcined them by heating at the rate of 50 °C min−1 to 350 °C and 400 °C. Samples were taken out of the furnace at each temperature and quenched in air. At 350 °C, formation of plate-like structures was evident on the surface (Fig. 3a), whereas nanobelts were formed at 400 °C (Fig. 3b).
 | ||
| Fig. 3 MoO3 structures obtained after quenching at (a) 350 °C and (b) 400 °C. (c) SEM image of the film prepared by using 2-propanol as the solvent. | ||
Based on the observations above, it can be concluded that anisotropic growth of α-MoO3 nanobelts with their (0k0) planes perpendicular to the substrate plane were favored on TiO2. The low lattice mismatch between the (010) plane of α-MoO3 (a = 3.963 Å, b = 3.696 Å, c = 13.856 Å) and the (001) plane of anatase TiO2 (a = b = 3.785 Å, c = 9.513 Å) (Fig. 4a) favors the preferential nucleation of the (010) planes of α-MoO3 to the (001) planes of TiO2. However, growth along [010] occurs through the creation of van der Waals bonds, which limits the growth rate compared to other principal growth directions, in which the growth occurs through the creation of ionic bonds (Fig. 4b). Accordingly, planar growth rates along the principal axes of α-MoO3 decrease in the following order: (001) > (100) ≫ (010).26 Since there is an excess supply of the α-MoO3 precursor due to the fast heating rates, growth proceeds along the fastest direction, i.e. [001]. When the heating rate was decreased to 5 °C min−1, nanoplatelets were formed instead of nanobelts. The TiO2 (anatase) films deposited on glass are polycrystalline and there is a mixture of faces exposed in random directions. Typically, an anatase crystal has a tetragonal prism structure consisting of eight (011) and two (001) faces (Fig. 4c).27 (001) planes of TiO2 laying parallel to the surface cannot nucleate nanobelts since (a) growth will be restricted by the compressive stress induced on the substrate due to fast heating and (b) the α-MoO3 supply is provided in the direction perpendicular to the film plane. The growth starts from the TiO2 film surface and proceeds outwards since heterogeneous nucleation is typically faster than homogeneous nucleation. Thus, those (001) planes of TiO2, which are oriented at an angle to the substrate, act as preferred nucleation and growth sites for the formation of α-MoO3 nanobelts. A schematic of the proposed mechanism is shown in Fig. 4d-e.
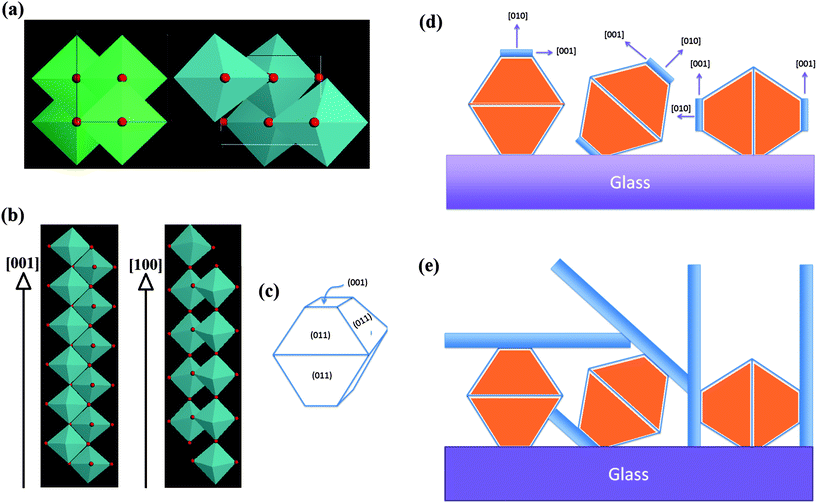 | ||
| Fig. 4 (a) (001) Anatase plane (left) and (010) MoO3 plane. (b) Principal growth directions of MoO3. (c) A typical anatase crystal. (d) nucleation of (010) MoO3 on (001) faces of polycrystalline anatase and (e) growth of nanobelts. | ||
It is important to note that the rate of nucleation and growth should be carefully controlled to sustain the desired growth. For example, when samples were heated with a speed of 5 °C min−1, nanobelts were not observed. This can be explained by the high nucleation density due to the slow evaporation of DMF (b.p. = 153 °C), during which nuclei are created, but growth is limited as the temperature is well below the crystallization temperature. The solvent used to dissolve MoCl5 was also one of the determining parameters on the obtained structures, as suggested by several authors.28,29 When 2-propanol was used as a solvent in place of DMF, hydrolysis occurred quickly, as evident from the dark blue color of the deposited solution only a few minutes after coating. Dimethyl sulfoxide gave similar results to DMF while methanol produced results similar to 2-propanol. In the case of 2-propanol, the resulting structure is a film composed of fine crystals of α-MoO3, indicative of a high nucleation rate (Fig. 3c). On the other hand, it was quite surprising to observe nanoplatelets on TiO2 coated quartz (Fig. 2c) rather than nanobelts, similar to the ones obtained on TiO2 coated soda-lime glass. There are only two differences between TiO2 coated quartz and glass substrates. The first is the high alkali content of soda-lime glass. In particular, sodium can diffuse easily through TiO2 films30,31 and may react with MoCl5 to form NaCl and/or Na2MoO4, although this amount should be lower compared to an uncoated surface. The second is the thermal expansion of the substrate. Quartz has one of the lowest coefficients of thermal expansion (CTE) (ca. 0.5 × 10−6°C−1)32 while soda lime glass has a very high CTE (ca. 7.6 × 10−6°C−1)33. Thus, coatings on a glass substrate tend to experience a lower compressive stress than the ones on quartz. However, we believe that the alkali content is more important, as evidenced by the decrease in nanobelt density with increasing TiO2 coating thickness (not shown). Increasing the TiO2 thickness from ca. 80 nm to 300 nm significantly decreased the nanobelt density per unit of surface area; a thicker titania film led to a decrease in sodium content. It is important to note that no major morphological differences were detected with the TiO2 films on glass and quartz, which might have led to different MoO3 structures. Although the exact mechanism is still under investigation, some sodium diffusion through the TiO2 film appears to be necessary to obtain highly anisotropic α-MoO3 structures. It is possible that the NaCl crystals may be acting as capping agents to ensure an anisotropic growth of MoO3 structures, as suggested recently by these authors.34
One-dimensional nanostructures attract great attention in solar cells and photocatalytic applications for light trapping as well as in electrochemical applications35 due to their high surface area and surface roughness. α-MoO3 nanobelts grown on an inexpensive glass substrate by such an easy method can be utilized as templates to produce active structures for solar cells and photocatalysis. To demonstrate the light trapping ability, obtained α-MoO3 nanobelts were sputter coated with carbon. As can be seen from the total reflection spectra (Fig. 5), the reflection of carbon-coated nanobelts was less than 4%, compared to 20% for carbon-coated glass. Nanoplatelets on quartz samples also produced low reflection values (less than 7%).
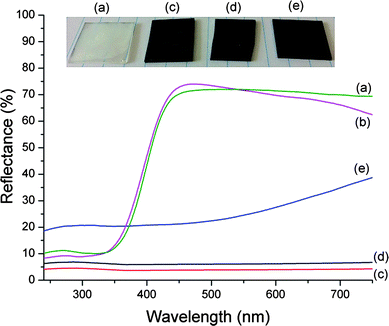 | ||
| Fig. 5 Total reflection spectra and photographs of (a) MoO3 nanobelts, (b) MoO3 nanoplatelets, (c) carbon-coated MoO3 nanobelts, (d) carbon-coated MoO3 nanoplatelets, and (e) carbon-coated glass. | ||
For photocatalytic applications, α-MoO3 nanobelts were first coated by SiO2 using dip coating and then again dip coated with TiO2. The resultant film was much more active compared to the film coated on plain glass, possibly due to its much higher surface area (Fig. 6). Approximately 10 times more dye was decomposed within the first 2 h by the TiO2-coated nanobelts compared to the conventionally prepared24TiO2 film on glass. Although we used a dip-coating process, a gas phase deposition method should be more suitable as it is difficult to prepare a uniform coating on these structures by liquid phase deposition methods.
 | ||
| Fig. 6 (a,b) SEM images of the TiO2 coated MoO3 nanobelts and (c) a plot showing photocatalytic degradation of methylene blue (under UV light irradiation) by TiO2 coated MoO3 nanobelts, TiO2 film and a glass substrate. | ||
It is known that hydrophobic surfaces exhibit superhydrophobic behaviour when they are patterned, although patterning a surface is not very straightforward in most cases. In order to demonstrate the ease of forming a patterned superhydrophobic surface with this method, contact angle measurements were performed by the Sessile drop method on plain, carbon-coated, and fluorosilane coated α-MoO3 nanobelts. Flurosilane coating was prepared by soaking nanobelt coated glass in a 10 mM octadeyl-fluorosilane-hexane solution for 30 min followed by drying at 200 °C for 10 min. The plain nanobelt film was superhydrophilic with a contact angle of zero, possibly due to the hydration of the nanobelts, which also resulted in the nanobelt film peeling off the surface, most probably due to stresses associated with the intercalation. After coating with carbon or fluorosilane, the films became stable and did not show any sign of peeling or delamination after soaking in water for at least a day. The carbon coated film was hydrophobic with a contact angle of 105° (Fig. 7a), whereas fluorosilane coated film attained a superhydrophobic state with a contact angle of 154° (Fig. 7b).
 | ||
| Fig. 7 Contact angle of (a) carbon coated and (b) flurosilane coated MoO3 nanobelts, as determined by the Sessile drop method. | ||
Conclusions
In summary, we have demonstrated a simple yet effective method to produce large aspect ratio α-MoO3 structures on TiO2 coated substrates. Depending on the substrate used, it was possible to obtain nanobelts or plate-like structures in which the morphology of the films depends on the composition of the glass substrate, annealing temperature and the heating rate. Such coatings can be utilized in several applications, such as solar cells, photo-catalysis and superhydrophobic surfaces.Acknowledgements
This work was supported by Gurallar ArtCraft Glassware. Authors are thankful to the Centralized Research Facility of Drexel University for use of SEM, TEM, and XRD; Dr Craig Johnson for assistance with TEM and Dr Volker Presser for the contact angle measurements.Notes and references
-
J. Spanier, in Nanomaterials Handbook, ed. Y. Gogotsi, CRC Press, Boca Raton, 2006, p. p. 283 Search PubMed
.
- Z. W. Pan, Z. R. Dai and Z. L. Wang, Science, 2001, 291, 1947–1949 CrossRef CAS
.
- Z. L. Wang, Annu. Rev. Phys. Chem., 2004, 55, 159–196 CrossRef CAS
.
- A. Kolmakov and M. Moskovits, Annu. Rev. Mater. Res., 2004, 34, 151–180 CrossRef CAS
.
-
Y. Gogotsi, ed., Nanotubes and nanofibers, Taylor & Francis, Boca Raton, 2006 Search PubMed
.
- G. N. Yushin, Z. G. Cambaz, Y. Gogotsi, K. L. Vyshnyakova and L. N. Pereselentseva, J. Am. Ceram. Soc., 2008, 91, 83–87 CAS
.
- Y. B. Li, Y. Bando, D. Golberg and K. Kurashima, Appl. Phys. Lett., 2002, 81, 5048–5050 CrossRef CAS
.
- E. Comini, L. Yubao, Y. Brando and G. Sberveglieri, Chem. Phys. Lett., 2005, 407, 368–371 CrossRef CAS
.
- H. G. Yang and H. C. Zeng, Chem. Mater., 2003, 15, 3113–3120 CrossRef CAS
.
- L. Mai, B. Hu, W. Chen, Y. Qi, C. Lao, R. Yang, Y. Dai and Z. Wang, Adv. Mater., 2007, 19, 3712–3716 CrossRef CAS
.
- L. Q. Mai, L. Xu, B. Hu and Y. H. Gu, J. Mater. Res., 2010, 25, 1413–1420 CrossRef CAS
.
- X. L. Li and Y. D. Li, Chem.–Eur. J., 2003, 9, 2726–2731 CrossRef CAS
.
- B. Hu, L. Mai, W. Chen and F. Yang, ACS Nano, 2009, 3, 478–482 CrossRef CAS
.
- G. R. Patzke, A. Michailovski, F. Krumeich, R. Nesper, J.-D. Grunwaldt and A. Baiker, Chem. Mater., 2004, 16, 1126–1134 CrossRef CAS
.
- X. W. Lou and H. C. Zeng, Chem. Mater., 2002, 14, 4781–4789 CrossRef CAS
.
- J. Zhou, N.-S. Xu, S.-Z. Deng, J. Chen, J.-C. She and Z.-L. Wang, Adv. Mater., 2003, 15, 1835–1840 CrossRef CAS
.
- B. C. Satishkumar, A. Govindaraj, M. Nath and C. N. R. Rao, J. Mater. Chem., 2000, 10, 2115–2119 RSC
.
- T. Xia, Q. Li, X. Liu, J. Meng and X. Cao, J. Phys. Chem. B, 2006, 110, 2006–2012 CrossRef CAS
.
- S. Wang, Y. Zhang, X. Ma, W. Wang, X. Li, Z. Zhang and Y. Qian, Solid State Commun., 2005, 136, 283–287 CrossRef CAS
.
- X. K. Hu, Y. T. Qian, Z. T. Song, J. R. Huang, R. Cao and J. Q. Xiao, Chem. Mater., 2008, 20, 1527–1533 CrossRef CAS
.
- L. Fang, Y. Shu, A. Wang and T. Zhang, J. Phys. Chem. C, 2007, 111, 2401–2408 CrossRef CAS
.
- X. W. Lou and H. C. Zeng, J. Am. Chem. Soc., 2003, 125, 2697–2704 CrossRef CAS
.
- Y. L. Xie, F. C. Cheong, Y. W. Zhu, B. Varghese, R. Tamang, A. A. Bettiol and C. H. Sow, J. Phys. Chem. C, 2009, 114, 120–124
.
- M. E. Kurtoglu, T. Longenbach, P. Reddington and Y. Gogotsi, J. Am. Ceram. Soc., 2010 DOI:10.1111/j.1551-2916.2010.04218.x
.
- J. Zhou, S. Z. Deng, N. S. Xu, J. Chen and J. C. She, Appl. Phys. Lett., 2003, 83, 2653–2655 CrossRef CAS
.
- W. G. Chu, L. N. Zhang, H. F. Wang, Z. H. Han, D. Han, Q. Q. Li and S. S. Fan, J. Mater. Res., 2007, 22, 1609–1617 CrossRef CAS
.
- P. M. Oliver, G. W. Watson, E. Toby Kelsey and S. C. Parker, J. Mater. Chem., 1997, 7, 563–568 RSC
.
- M. Epifani, P. Imperatori, L. Mirenghi, M. Schioppa and P. Siciliano, Chem. Mater., 2004, 16, 5495–5501 CrossRef CAS
.
- W. Dong and B. Dunn, J. Mater. Chem., 1998, 8, 665–670 RSC
.
- T. Watanabe, S. Fukayama, M. Miyauchi, A. Fujishima and K. Hashimoto, J. Sol-Gel Sci. Technol., 2000, 19, 71–76 CrossRef CAS
.
- M. E. Kurtoglu, T. Longenbach and Y. Gogotsi, International Journal of Applied Glass Science, 2010 DOI:10.1111/j.2041-1294.2011.00040.x
.
- R. Roy, D. K. Agrawal and H. A. McKinstry, Annu. Rev. Mater. Sci., 1989, 19, 59–81 CrossRef CAS
.
- W. Mi-tang and C. Jin-shu, J. Alloys Compd., 2010, 504, 273–276 CrossRef
.
- X. Yang, H. Tang, R. Zhang, H. Song and K. Cao, Crystal Research and Technology, 2011 DOI:10.1002/crat.201100061
.
- T. Brezesinski, J. Wang, S. H. Tolbert and B. Dunn, Nat. Mater., 2010, 9, 146–151 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2011 |
