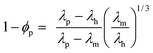DOI:
10.1039/C0JM04243A
(Paper)
J. Mater. Chem., 2011,
21, 4402-4407
Enhanced thermal conductivity over percolation threshold in polyimide blend films containing ZnO nano-pyramidal particles: advantage of vertical double percolation structure†
Received
5th December 2010
, Accepted 18th January 2011
First published on 14th February 2011
Abstract
The thermal conductivity along the out-of-plane direction in polyimide (PI) blend films containing ZnO nano-pyramidal particles (ZnO-NPs) was investigated. PI blend films composed of a sulfur- and a fluorine-containing PI were prepared viaspin-coating and thermal curing of precursor solutions containing ZnO-NPs. Microphase-separated structures with “vertical double percolation (VDP)” morphology were spontaneously formed in the films, in which two phases were separately aligned along the out-of-plane direction, and ZnO-NPs were preferentially precipitated in the fluorine-containing PI phase. The blend film exhibits 410% enhancement of thermal conductivity at 27 vol% of ZnO-NPs, whereas only 90% enhancement was observed in the monophase PI film containing homogeneously dispersed ZnO-NPs. These results indicate that the VDP structure with selective incorporation of ZnO-NPs functions as an effective thermal conductive pathway.
Introduction
Owing to the demands from power-electric and micro-electronic devices, polymer/inorganic hybrid films containing micrometre (µm)-size ceramic particles, such as Al2O3, AlN, and BN, have been widely adopted to enhance their thermal conductivity.1–3 Such hybrid films or sheets exhibiting high thermal conductivity, preferably in the direction perpendicular to the film surface, become increasingly important for electronic packaging and semiconductor chips because their heat dissipation ability limits the reliability, performance, and miniaturization of advanced electronic circuits. In the case of polymer/inorganic hybrids, a thermal conduction mechanism based on the percolation theory has been widely accepted.4 It is well known that a high volume fraction (generally >30 vol.%) of thermally conductive particles over the percolation threshold is necessary to obtain high thermal conductivity of polymer hybrids due to the interfacial contact resistance between the particles and the polymer matrix. A high volume fraction of inorganic components in the polymer matrix, however, causes a number of limitations, in terms of uneven surface, low flexibility, fragility, heavy weight, and poor mechanical performance, as a result of weak polymer/particle interfacial adhesion and agglomeration of inorganic particles. To overcome the limitations of polymer hybrids, many attempts have been made to reduce the interfacial contact area by the use of anisotropic particles with a high aspect ratio such as carbon nanotubes, and to increase the interfacial interactions by chemical modification of particle surfaces for reducing the percolation threshold,5,6 but successful examples are rather limited. In this study, we applied the so-called “double percolation technique” to achieve effective improvement in thermal conductivity. This technique has been used to lower the percolation threshold of electrical conductivity in conductive polymer hybrid materials,7–9 but few applications have been reported for the enhancement of thermal conductivity. For this technique, two-phase immiscible polymer blends, in which at least one phase is continuous, can be used as a polymer matrix, and conducting fillers are selectively and preferentially localized only in the continuous phase.
Polyimides (PIs) have been widely used in electric and electronic applications such as interlayer dielectric films and substrates for flexible printed circuit boards because of their high thermal stability, good mechanical properties, and favorable electrical properties. Very recently, we investigated the dependence of thermal conductivity for PI films on molecular structure, chain orientation, and molecular packing.10 PIs having rigid molecular structures with lower degrees of molecular orientation of the main chains along the in-plane direction exhibited high thermal conductivity in the direction perpendicular to the film surface. However, the thermal conductivity of PIs is still as low as those of commonly used engineering plastics. The present authors previously reported that immiscible PI blend films containing zero-valent silver nano-particles (Ag-NPs) significantly enhanced out-of-plane thermal diffusivity.11 Interestingly, a “vertical double percolation (VDP) structure” was formed in the films, in which two phases were separately aligned along the out-of-plane direction, and the Ag-NPs were selectively incorporated only in one phase. In this paper, effective enhancement of thermal conductivity of PI blend films, which form the VDP structure, is demonstrated by incorporating zinc oxide nano-pyramidal particles (ZnO-NPs). The percolation threshold of the PI blend films was significantly lower than those of monophase PI films containing homogeneously dispersed ZnO-NPs. In ZnO-NP/PI blend hybrid films, an average diameter of ZnO-NPs (500 nm) larger than that of Ag-NPs (7 nm) should be advantageous, not only for suppressing phonon scattering at polymer/particle interfaces due to a decrease of net interfacial area, but also for spontaneous formation of the VDP structure due to the weaker interactions at the polymer/particle interfaces.11
Experimental
Materials
The procedure for preparing ZnO-NP/PI blend hybrid films is shown in Fig. 1(a). An immiscible PI blend system composed of two polymer components was prepared, i.e., SD as a sulfur-containing PI, and TF as a fluorine-containing PI (Fig. 1(b)). The precursor solutions, poly(amic acid) (PAA), of SD and TF were synthesized from 3,3′,4,4′-biphenyl tetracarboxylic dianhydride (BPDA) and 4,4′-thiodianiline (SDA) dissolved in N,N-dimethylacetamide (DMAc, anhydrous, 99.8%) for SD, and BPDA and 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl (TFDB) dissolved in DMAc for TF in the conventional manner.11 The solid content in the PAA solutions was fixed at 15 wt% for SD and 17 wt% for TF. ZnO nano-pyramidal particles (ZnO-NPs) were synthesized by refluxing zinc acetate dihydrate (purchased from Kanto Chemical Co., Inc.) in an oleylamine solution (purchased from Aldrich) at 240 °C for 40 min. The precipitates were separated by centrifuge, washed with ethanol, and then dried under vacuum at room temperature. The height and the base length of the ZnO-NPs were ca. 500 nm (Fig. 1(c)). The formation of crystalline ZnO was confirmed by wide-angle X-ray diffraction (ESI†).
 |
| | Fig. 1 (a) Preparation procedures for ZnO-NP/PI blend hybrid films. (b) Polymer components for preparing PI blends: BPDA-SDA (SD) and BPDA-TFDB (TF). (c) SEM images of zinc oxide nano-pyramidal particles (ZnO-NPs). | |
ZnO-NP/blend PAA solutions were prepared by blending each PAA solution of SD and TF with ZnO-NPs, followed by stirring for 8 h. The solution was spin-coated onto a 4 inch silicon wafer, followed by soft-baking at 70 °C for 1 h. After peeling from the substrate, the ZnO-NP/blend PAA films were reset onto a 4 inch silicon wafer covered with aluminium foil, and the films were thermally cured by a one-step thermal imidization procedure; the final curing condition was 350 °C for 1.5 h. The heating rate was 4.6 °C min−1 from 70 °C to 350 °C. All curing procedures were conducted under nitrogen flow (2 l min−1). The molar ratios of SD, TF, and ZnO-NPs were set at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–1000, respectively (see Table 1). 1000 mol% of ZnO-NPs correspond to 27.1 vol% of ZnO-NPs in PI blend film (blend-PI). Monophase (only TF) PI films (homo-PIs) containing ZnO-NPs were also prepared as a reference. After spin-coating, drying, and thermal curing, microphase-separated morphologies were observed by an optical microscope and a scanning electron microscope (SEM) on the surfaces of the resultant blend-PI films, whereas the surfaces of the homo-PI films were very flat and homogeneous without phase separation.
0–1000, respectively (see Table 1). 1000 mol% of ZnO-NPs correspond to 27.1 vol% of ZnO-NPs in PI blend film (blend-PI). Monophase (only TF) PI films (homo-PIs) containing ZnO-NPs were also prepared as a reference. After spin-coating, drying, and thermal curing, microphase-separated morphologies were observed by an optical microscope and a scanning electron microscope (SEM) on the surfaces of the resultant blend-PI films, whereas the surfaces of the homo-PI films were very flat and homogeneous without phase separation.
Table 1 Compositions and film thicknesses of ZnO-NP/PI hybrid films
| Sample |
Molar ratios in hybrid PIs |
ZnO content |
Film thickness |
| SD |
TF
|
ZnO
|
vol% |
wt% |
µm
|
|
Blend-0 |
50 |
50 |
0 |
0.0 |
0.0 |
25.9 |
|
Blend-100 |
50 |
50 |
100 |
3.6 |
12.6 |
25.5 |
|
Blend-200 |
50 |
50 |
200 |
6.9 |
22.5 |
26.8 |
|
Blend-300 |
50 |
50 |
300 |
10.1 |
30.3 |
29.2 |
|
Blend-400 |
50 |
50 |
400 |
13.0 |
36.7 |
30.5 |
|
Blend-500 |
50 |
50 |
500 |
15.7 |
42.0 |
30.4 |
|
Blend-600 |
50 |
50 |
600 |
18.3 |
46.5 |
29.6 |
|
Blend-700 |
50 |
50 |
700 |
20.7 |
50.3 |
31.9 |
|
Blend-800 |
50 |
50 |
800 |
23.0 |
53.7 |
33.4 |
|
Blend-900 |
50 |
50 |
900 |
25.1 |
56.6 |
26.1 |
|
Blend-1000 |
50 |
50 |
1000 |
27.1 |
59.1 |
31.1 |
|
Homo-0
|
0 |
100 |
0 |
0.0 |
0.0 |
22.0 |
|
Homo-100
|
0 |
100 |
100 |
3.5 |
11.7 |
24.4 |
|
Homo-200
|
0 |
100 |
200 |
6.7 |
21.0 |
24.3 |
|
Homo-300
|
0 |
100 |
300 |
9.7 |
28.4 |
26.8 |
|
Homo-400
|
0 |
100 |
400 |
12.5 |
34.6 |
27.0 |
|
Homo-500
|
0 |
100 |
500 |
15.2 |
39.9 |
25.6 |
|
Homo-600
|
0 |
100 |
600 |
17.7 |
44.3 |
23.7 |
|
Homo-700
|
0 |
100 |
700 |
20.0 |
48.1 |
27.9 |
|
Homo-800
|
0 |
100 |
800 |
22.2 |
51.5 |
28.5 |
|
Homo-900
|
0 |
100 |
900 |
24.3 |
54.4 |
30.0 |
|
Homo-1000
|
0 |
100 |
1000 |
26.3 |
57.0 |
30.4 |
Measurements
Surface images of the ZnO-NP/PI blend hybrid films were taken with an Olympus SZX12 microscope. Cross-sectional images of the films were observed using an FE-SEM (S-4500, Hitachi) with a backscattering electron mode. X-Ray diffraction patterns were obtained by Cu-Kα radiation using a Rigaku Miniflex diffractometer (wavelength 0.154 nm). For measuring the IR absorption spectrum of each domain in the microphase-separated structures in PI blend films, a Micro FT-IR ATR (IRT-3000, Jasco International Co., Ltd.) was used. The FT-IR spectrum of each domain was separately measured with a spot size of 20 µm.
The thermal diffusivities along the out-of-plane direction (D⊥) of PI hybrid films were measured at room temperature with an AC temperature wave analyzer (ai-Phase mobile 1, ai-Phase Co. Ltd.), which is based on the thermal wave analysis (TWA) method.12,13 In the TWA technique, an AC temperature wave generated from the heater in direct contact with one side of the film passes through the film in the thickness (out-of-plane) direction. The phase delay of the temperature wave can be detected by a sensor placed on the other side of the film. When the angular frequency of the temperature wave and the film thickness are known, the D⊥ value is readily estimated from the phase delay. The thicknesses of the PI films were measured by a thickness meter equipped with the TWA analyzer. Each film was measured three times, which guarantees error ranges of ±5% in D⊥ and ±3% in thickness, and the average value was adopted as the experimental value. The specific heats of PI films were measured with a Shimadzu DSC-60 analyzer with a heating rate of 5 °C min−1 under nitrogen.14 The densities of PI films were measured by pycnometeric method using distilled water as the medium.
Results and discussion
Characterization of ZnO-NP/PI blend hybrid films
The morphology of phase-separated structures, which were spontaneously formed in the blend-PIs through spin-coating and thermal curing, was investigated as follows. Fig. 2(a) shows a cross-sectional SEM image of the blend-PI obtained by the backscattering electron mode, in which the molar ratios of SD, TF, and ZnO-NPs were 50, 50, and 300, respectively. For convenience, the notation ‘Blend-300’ will be used for denoting a blend ratio (see Table 1). In the SEM image, the brighter regions correspond to ZnO-rich phases, and the darker regions correspond to ZnO-poor phases because a region which contains many atoms with higher atomic numbers is more brightly observed in backscattering electron images.15 ZnO-NPs were clearly observed in a magnified image of a ZnO-NP-rich phase (Fig. 2(b)).
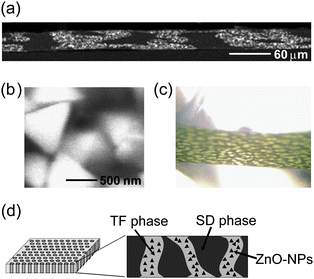 |
| | Fig. 2 Characterization of microphase-separated blend films of Blend-300. (a) Cross-sectional SEM image observed by backscattering electron mode. (b) Magnified SEM image of ZnO-rich phase in (a). (c) Far-field image by optical microscope (50×). (d) Schematic representation of an ideal “vertical double percolation (VDP)” structure. | |
Fig. 2(c) shows a microscopic image of the surface of Blend-300. The surface morphology was composed of a continuous ZnO-NP-rich phase (darker region) and a ZnO-NP-poor phase (brighter region) surrounded by the ZnO-NP-rich phase. A combination of these images obviously indicates the formation of microphase-separated structures, in which two phases are separately aligned and penetrate the films in the out-of-plane direction. The average domain size was approximately 50 µm. This type of phase separation has been designated as a “vertical double percolation (VDP) structure”. By controlling the viscosities of PAA solutions of SD and TF, and the spinning rate in the spin-coating process, the preparation procedure was optimized to generate a VDP structure in the blend-PIs. Micro-FT-IR ATR spectroscopy was used for characterizing the chemical composition of ZnO-NP-rich and ZnO-NP-poor phases.16 In Fig. 3, the strong absorption bands around 1050–1200 cm−1, which are assignable to the C–F band in the TF component,17,18 reveal that ZnO-NP-rich phases are mainly composed of TF, while ZnO-NP-poor phases are mainly composed of SD. Since ZnO-NPs were preferentially precipitated in the TF-rich phase, the VDP structure schematically shown in Fig. 2(d) can be proposed. It is highly expected that the TF-rich phases with larger amounts of ZnO-NPs, which are continuous in the vertical direction, function as effective pathways for thermal conduction in the blend-PI films.
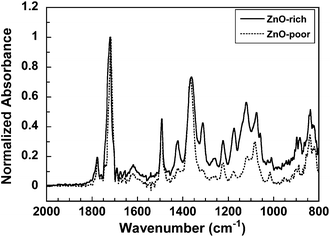 |
| | Fig. 3 Micro-FT-IR ATR spectra of the ZnO-rich phases and ZnO-poor phases in Blend-300 film. Peak intensities are normalized at 1724 cm−1. | |
Thermal conductivity of ZnO-NP/PI blend hybrid films
To evaluate the thermal conductivity of PI hybrid films, the out-of-plane thermal diffusivity (D⊥) was measured by temperature wave analysis (TWA) at room temperature. TWA can estimate the thermal diffusivity of films from frequency-dependent phase shifts of temperature waves.12,13 Experimental thermal conductivity (λ) was evaluated based on the following relation:where ρ is the density, and Cp is the specific heat at constant pressure. In this study, ρ and Cp were estimated using the following equations:19| | | ρh = ρp·ϕp + ρm·(1 − ϕp) | (2) |
| | | Cph = Cpp·ϕp + Cpm·(1 − ϕp), | (3) |
where ϕp is the volume fraction of the dispersed particles. The subscripts, “h”, “p”, and “m”, represent hybrid films, dispersed particles (ZnO-NPs), and polyimide matrix (PIs), respectively. The ρ and Cp values of hybrid films (ρh and Cph) were calculated from the literature data of ZnO (ρp = 5.68 g cm−3, and Cpp = 0.50 J g−1 K−1)20 and the experimental data of PIs (ρm = 1.46 g cm−3 and Cpm = 1.11 J g−1 K−1 for the pristine SD/TF blend, see Table 2).
Table 2
ZnO-NP content ϕp, thermal conductivity λ, density ρ, specific heat Cp and thermal diffusivity D⊥ of ZnO-NP/PI hybrid films
| Sample |
ϕ
p (vol%) |
λ/W m−1 K−1 |
ρ/g cm−3 |
C
p/J g−1 K−1 |
D
⊥/10−8 m2s−1 |
|
Blend-0 |
0.0 |
0.30 |
1.46 |
1.11 |
18.5 |
|
Blend-100 |
3.6 |
0.31 |
1.61 |
1.03 |
18.6 |
|
Blend-200 |
6.9 |
0.35 |
1.75 |
0.97 |
20.8 |
|
Blend-300 |
10.1 |
0.45 |
1.88 |
0.92 |
26.0 |
|
Blend-400 |
13.0 |
0.51 |
2.01 |
0.89 |
29.0 |
|
Blend-500 |
15.7 |
0.57 |
2.12 |
0.85 |
31.4 |
|
Blend-600 |
18.3 |
0.71 |
2.23 |
0.83 |
38.6 |
|
Blend-700 |
20.7 |
0.91 |
2.33 |
0.80 |
48.5 |
|
Blend-800 |
23.0 |
1.17 |
2.43 |
0.78 |
61.4 |
|
Blend-900 |
25.1 |
1.26 |
2.52 |
0.76 |
65.2 |
|
Blend-1000 |
27.1 |
1.54 |
2.60 |
0.75 |
78.8 |
|
Homo-0
|
0.0 |
0.26 |
1.53 |
1.13 |
15.3 |
|
Homo-100
|
3.5 |
0.26 |
1.67 |
1.05 |
14.6 |
|
Homo-200
|
6.7 |
0.29 |
1.81 |
0.99 |
16.0 |
|
Homo-300
|
9.7 |
0.30 |
1.93 |
0.95 |
16.1 |
|
Homo-400
|
12.5 |
0.30 |
2.05 |
0.91 |
16.4 |
|
Homo-500
|
15.2 |
0.32 |
2.16 |
0.88 |
17.0 |
|
Homo-600
|
17.7 |
0.36 |
2.26 |
0.85 |
18.6 |
|
Homo-700
|
20.0 |
0.41 |
2.36 |
0.82 |
21.0 |
|
Homo-800
|
22.2 |
0.45 |
2.45 |
0.80 |
22.9 |
|
Homo-900
|
24.3 |
0.47 |
2.54 |
0.79 |
23.3 |
|
Homo-1000
|
26.3 |
0.49 |
2.62 |
0.77 |
24.4 |
First, the relationship between the D⊥ values and the phase-separation morphology of blend-PI films was investigated, for which a series of blend-PIs and homo-PIs with different molar ratios of ZnO-NPs were prepared: Blend-0–1000 and Homo-0–1000. Fig. 4(a) shows the experimental D⊥ values of the blend-PIs and homo-PIs. In the case of homo-PIs, the ZnO-NPs are homogeneously dispersed in the PI matrix (G, H, and I in Fig. 4(b) (Homo-300, 800, and 1000)), and their D⊥ values were gradually increased as the ZnO content increased. The D⊥ of Homo-1000 was larger than that of Homo-0 by 59%. In contrast, the blend-PIs having VDP structures exhibited significantly larger D⊥ values compared to the homo-PIs (A, B, and C in Fig. 4(b) (Blend-300, 800, and 1000)). The D⊥ of Blend-1000 was larger than that of Blend-0 by 326%. The significant enhancement of D⊥ values (18.5–78.8 × 10−8 m2s−1) obtained for the blend-PIs having VDP structures is explainable by the following two factors. (1) According to the material design strategy of this study, the ZnO-NPs were preferentially concentrated in the TF phases of blend-PIs, while the SD phases functioned as ZnO-NP-excluding domains. Fig. 4(c) shows magnified SEM images of the ZnO-NP-rich phases in (C) Blend-1000 (above) and (I) Homo-1000 (below). In Blend-1000, the ZnO-NPs are more concentrated in the TF phases compared to Homo-1000 with the same content of ZnO-NPs. (2) The formation of the VDP structure leads to densification of the ZnO-NPs with continuity of high concentration and connectivity along the out-of-plane direction. For reference, films of blend-PIs with deformed VDP structures were prepared by varying the rotation rates in the spin-coating process (D, E, and F in Fig. 4(b)). In these cases, the D⊥ values are larger than those of homo-PIs, though they are smaller than those of blend-PIs having VDP structures. These phenomena clearly demonstrate that the VDP structures formed in the blend-PIs function as effective thermally conductive pathways. Moreover, it should also be noted that the blend-PIs containing no ZnO-NPs (Blend-0) exhibited larger D⊥ values than those of homo-PIs (Homo-0). In solid polymer films, thermal energy is more efficiently transferred through covalent bonds in the direction of the main chains than in the direction perpendicular to the main chains because polymer chains weakly interact with each other via van der Waals and dipole–dipole interactions. In general, PI films exhibit smaller D⊥ values than those in the film plane because the spin-coating process induces preferential in-plane orientation of the main chains in the film plane.10 Thus, our results suggest that the in-plane orientation was partly disrupted in the PI blend films, which should enhance thermal diffusion along the out-of-plane direction.
 |
| | Fig. 4 (a) Thermal diffusivity (D⊥) of hybrid films with different molar ratios of ZnO-NPs for (●) PI blend films with VDP structures, (▲) PI blend films with deformed VDP structure, and (■) Homo-PIs. (b) Cross-sectional SEM images observed by backscattering electron mode. (A–C) PI blends with “VDP” structures, (D–F) PI blends with deformed VDP structures, and (G–I) Homo-PI. The symbols (A–I) in (a) correspond to the SEM images of (A–I) in (b), respectively. (c) Magnified SEM images of ZnO-rich phase in (C) Blend-1000 (above) and (I) Homo-1000 (below). | |
Fig. 5(a) shows the experimental thermal conductivities (λ) of the hybrid films estimated using eqn (1)–(3) and the calculated thermal conductivities based on Bruggeman's theory.21 In the case of homo-PIs, their λ values gradually increased by increasing the ZnO content. For Homo-1000 (26.3 vol% of ZnO content) film, the λ value was increased by 90% compared to that of Homo-0. In contrast, blend-PIs having VDP structures exhibited significantly larger λ values compared to the homo-PIs. For Blend-1000 (27.1% of ZnO content), the λ value was increased by 410% than that of Blend-0, and it exhibited the largest λ value (1.54 W m−1 K−1) in this study. To elucidate the effect of the VDP structure formed in PI blend films, ideal models for blend-PIs with VDP structures and homo-PIs with homogeneously dispersed ZnO-NPs were constructed. In the former, the TF phase forms mutually separated cylindrical structures containing homogeneously dispersed ZnO particles, and the SD phase forms residual domains without ZnO particles. In the latter, ZnO particles are homogeneously dispersed in the homo-PI (TF) phase (see Fig. 5(b)). Then, the thermal conductivities of blend-PIs (λh-blend) and homo-PIs (λh-homo) were calculated using the following eqn (4) and (5), respectively:22
| | | λh-blend = ϕTF·λ(TF + ZnO) + (1 − ϕTF)·λSD | (4) |
where
ϕTF is the volume ratio of the TF phase to the SD phase (fixed as
ϕTF = 0.5 in this study). The thermal conductivity of TF phases containing ZnO particles (
λTF + ZnO) was estimated based on Bruggeman's theory,
21 which was proposed to estimate the thermal conductivity of hybrid materials:
| | 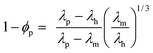 | (6) |
 |
| | Fig. 5 (a) Thermal conductivity of hybrid films: (●) PI blends and (■) Homo-PIs. The lines are the λ values calculated for an ideal PI blend model (rigid line) and a Homo-PI model (broken line) based on the Bruggeman theory. (b) Schematic representations for ideal model structures for PI blends with VDP structures (left) and for homo-PIs with homogeneously dispersed ZnO-NPs (right). | |
The thermal conductivity of ZnO was taken from the literature (λp = 54 W m−1 K−1).20 The solid and broken lines in Fig. 5(a) represent the calculated λ values for blend-PIs and homo-PIs, respectively. Note that the experimental λ values of the blend-PIs are fairly close to those of the calculated values (λh-blend). These facts indicate that, although the VDP structures generated in the PI blend films do not possess ideally cylindrical shapes along the out-of-plane direction, the structures effectively function as ideal thermal conductive pathways. Moreover, the resulting PI blend films containing ZnO-NPs kept the inherent thermal stability of PI films (i.e., the degradation temperatures at 5% weight loss were higher than 500 °C), and the films were still flexible and bendable with a 27.1 vol% of ZnO-NPs (in Blend-1000).
Conclusions
The thermal conductivities (λ) of blend-PI films containing ZnO-NPs were systematically investigated. The blend films composed of sulfur- (SD) and fluorine- (TF) containing PIs exhibited distinct microphase-separated structures with a vertical double percolation (VDP) morphology when they were prepared under appropriate conditions, in which two immiscible phases are separately aligned along the out-of-plane direction. Blend films with selective incorporation of ZnO-NPs in TF-domains exhibited significantly larger λ values than those of monophase homo-PI films containing homogeneously dispersed ZnO-NPs. The blend-PI system thus obtained possesses two advantages over conventional homo-PI systems. (1) A larger λ value is achievable with smaller ZnO content in blend-PI films compared to homo-PI films due to the reduced percolation threshold. This is highly advantageous in practical applications, e.g., better flexibility, light weight, and higher electric insulation properties. (2) The λ values of pristine blend-PI films (without ZnO-NPs) are larger than those of homo-PI films, which impart larger λ values to blend-PI films when ZnO-NPs are incorporated. We believe that the novel material design concept for the PI blend system with VDP structures is a promising way to enhance the thermal conductivity of polymer dielectric materials for future electric and electronic applications.
Acknowledgements
The authors sincerely thank Jun Koki at the Center for Advanced Materials Analysis, Tokyo Institute of Technology for valuable advice and support on the FE-SEM measurement. This work was partly supported by NEDO/METI project “Superhybrid Materials R & D”.
References
- G. Droval, J. F. Feller, P. Salagnac and P. Glouannec, Polym. Adv. Technol., 2006, 17, 732 CrossRef CAS.
- Y. Xu, D. D. L. Chung and C. Mroz, Composites, Part A, 2001, 32, 1749 CrossRef.
- H. He, R. Fu, Y. Hun, Y. Shen and X. Song, J. Mater. Sci., 2007, 42, 6769 CrossRef.
- R. A. Hauser, J. M. Keith, J. A. King and J. L. Holdren, J. Appl. Polym. Sci., 2008, 110, 2914 CrossRef CAS.
- F. H. Gojny, M. H. G. Wichmann, B. Fiedler, I. A. Kinloch, W. Bauhofer, A. H. Windle and K. Schulte, Polymer, 2006, 47, 2036 CrossRef CAS.
- T. Zhou, X. Wang, G. U. Mingyuan and X. Liu, Polymer, 2008, 49, 4666 CrossRef CAS.
- M. Sumita, K. Sakata, Y. Hayakawa, S. Asai, K. Miyasaka and M. Tanemura, Colloid Polym. Sci., 1992, 270, 134 CrossRef CAS.
- T. Matsumura, M. Ochi and K. Nagata, J. Appl. Polym. Sci., 2003, 90, 1980 CrossRef CAS.
- W. Thongruang, R. J. Spontak and C. M. Balik, Polymer, 2002, 43, 3717 CrossRef CAS.
- D. Yorifuji and S. Ando, Macromolecules, 2010, 43, 7583 CrossRef CAS.
- D. Yorifuji and S. Ando, Macromol. Chem. Phys., 2010, 211, 2118 CrossRef CAS.
- T. Hashimoto, J. Morikawa, T. Kurihara and T. Tsuji, Thermochim. Acta, 1997, 304/305, 151 CrossRef.
- J. Morikawa and T. Hashimoto, J. Appl. Phys., 2009, 105, 113506 CrossRef.
- J. Fujino and T. Honda, Netsu Bussei, 2006, 20, 166 CrossRef CAS.
- P. Y. Timbrell, A. Bulinski, S. S. Bamji and J. Densley, IEEE Trans. Electr. Insul., 1990, 25, 730 CrossRef CAS.
- Y. Liu, Z. Yang, Y. Desyaterik, P. L. Gassman, H. Wang and A. Laskin, Anal. Chem., 2008, 80, 633 CrossRef CAS.
- J. Liu, Y. Nakamura, Y. Shibasaki, S. Ando and M. Ueda, Macromolecules, 2007, 40, 4614 CrossRef CAS.
- Y. Terui, S. Matsuda and S. Ando, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 2109 CrossRef CAS.
- K. Murakami, K. Yamada, K. Deguchi, T. Shimizu and S. Ando, J. Photopolym. Sci. Technol., 2010, 23, 501 CrossRef CAS.
- S. Shirasaki, Ceram. Jpn., 1983, 18, 965 Search PubMed.
- D. A. G. Bruggeman, Ann. Phys., 1936, 24, 645 CrossRef.
- S. Agarwal, M. Masud, K. Khan and R. K. Gupta, Polym. Eng. Sci., 2008, 48, 2474 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns of ZnO-NP/PI blend hybrid films. See DOI: 10.1039/c0jm04243a |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–1000, respectively (see Table 1). 1000 mol% of ZnO-NPs correspond to 27.1 vol% of ZnO-NPs in PI blend film (blend-PI). Monophase (only TF) PI films (homo-PIs) containing ZnO-NPs were also prepared as a reference. After spin-coating, drying, and thermal curing, microphase-separated morphologies were observed by an optical microscope and a scanning electron microscope (SEM) on the surfaces of the resultant blend-PI films, whereas the surfaces of the homo-PI films were very flat and homogeneous without phase separation.
0–1000, respectively (see Table 1). 1000 mol% of ZnO-NPs correspond to 27.1 vol% of ZnO-NPs in PI blend film (blend-PI). Monophase (only TF) PI films (homo-PIs) containing ZnO-NPs were also prepared as a reference. After spin-coating, drying, and thermal curing, microphase-separated morphologies were observed by an optical microscope and a scanning electron microscope (SEM) on the surfaces of the resultant blend-PI films, whereas the surfaces of the homo-PI films were very flat and homogeneous without phase separation.