Acidic ZSM-5 zeolite-coated long period fiber grating for optical sensing of ammonia†
Xiling
Tang
a,
Justin
Provenzano
a,
Zhi
Xu
a,
Junhang
Dong
*a,
Hongbiao
Duan
b and
Hai
Xiao
b
aDepartment of Chemical and Materials Engineering, University of Cincinnati, Cincinnati, OH 45221, USA. E-mail: Junhang.dong@uc.edu; Fax: (+513) 556-3473; Tel: (+513) 556-3992
bDepartment of Electrical and Computer Engineering, Missouri University of Science and Technology, Rolla, MO 65409, USA
First published on 22nd October 2010
Abstract
A ZSM-5 zeolite thin film with Si/Al ratio of about 23 has been grown on a long period fiber grating (LPFG) for optical gas sensing by monitoring its resonance wavelength (λR) shift caused by molecular sorption into the zeolite cavity. The sensing selectivity, sensitivity and speed of response of the zeolite-coated LPFG (Z-LPFG) are determined by the adsorption equilibria and transport properties of the analyte molecules in the zeolite pores. The ZSM-5 zeolite was modified through ammonium ion exchange and subsequent calcination to form acidic ZSM-5 (H-ZSM-5). The surface acidified Z-LPFG (HZ-LPFG) achieved dramatically improved sensitivity and selectivity for detecting ammonia gas. Also, increasing operation temperature improves sensing selectivity for the strongly adsorbing ammonia over weakly adsorbing gases and enhances response speed but compromises detection sensitivity due to reduced amount of adsorption for ammonia.
Introduction
Zeolites are aluminosilicate crystals composed of TO4 (T = Si4+, Al3+) tetrahedral primary units which are interconnected by oxygen atoms to form ordered micropores defined by O–T–O rings. Conventionally, zeolites have been used as gas adsorbents, membranes, and shape-selective catalysts for molecular separations and catalytic reactions. In recent years, research has revealed that zeolites also possess a number of sorbate-dependent optical properties, such as UV–vis–IR absorption spectra, fluorescence, refractive index, birefringence, and Raman spectra.1–5 With the enormous surface-to-mass ratio for analyte concentration and excellent thermal and chemical stabilities, zeolites are well suited for sensing chemicals through monitoring their optical properties which vary upon loading of analyte species. However, the realization of zeolite-based optical chemical probes has been hindered by the challenges in integrating zeolites with optical devices and the lack of in depth understanding on the sorbate-dependent optical behaviors.Recently, we successfully integrated zeolites with fiber optic devices by growing highly siliceous MFI-type zeolite thin films on the straight-cut fiber endface and the surface of long period fiber grating (LPFG).6,7 These zeolite-fiber optical devices were demonstrated for sensing chemicals based on the change of zeolite optical refractive index (nz) caused by adsorption of analyte molecules from the environment.8,9 The LPFG is an index module created by inscribing periodic index perturbations into the core of an optical fiber using laser irradiation. As lights travel through the LPFG, some particular wavelengths, which is termed as resonant wavelength (λR), are coupled from lossless core mode to high-loss cladding modes, resulting in a series of attenuation bands in the transmission spectrum.10–13 When the LPFG is coated with a thin film, its λR shifts when the refractive index of the overcoat (nov) changes. Thus optical chemical sensors can be constructed by coating thin films of chemically sensitive photonic materials on the LPFG.6,14–16 The relationship between λR and nov is described by17
 | (1) |
The refractive indices of the microporous zeolite crystals (nz) are smaller than that of the dense silica cladding, ncl. The nz increases with increasing the load of sorbate molecules in the zeolitic cavities.5,8,20 Therefore, the zeolite coated LPFG (Z-LPFG) can measure the concentration of adsorbing gases by monitoring the shift of λR in the normal cladding mode. In our previous studies, the λR of the pure-silica MFI zeolite coated LPFG shifted ∼1.45 nm and ∼ 0.7 nm when contacting 5.5 ppmv isopropanol vapor and 0.22 ppmv toluene vapor, respectively.6 The magnitude of these responses in λR promises high detection sensitivity because wavelength displacement as small as 0.001 nm can be measured by commercial optical spectrum analyzers or tunable lasers.
It has been also observed that the response time of the Z-LPFG in chemical sensing coincides with the equilibrating time for the molecular adsorption,7,21 which is controlled by the sorption and desorption kinetics as well as the molecular diffusivity in the zeolite pores. Because the adsorption-induced change of zeolite refractive index cannot reveal the identity of adsorbed molecules, the sensing selectivity/specificity of the Z-LPFG relies on the gas adsorption selectivity in the zeolite film. In this research we synthesize a ZSM-5 zeolite film on the LPFG and investigate the effects of zeolite surface acidification and operating temperature on its performance in ammonia gas sensing.
Experimental
Zeolite film synthesis
The LPFG was obtained using point-by-point CO2-laser irradiation on the Corning fused silica SMF-28 single mode fiber (refractive index 1.4682).11 The grating section was 5-cm long with a 520-μm grating period (Λ). The ZSM-5 zeolite thin film was grown on the 125 μm-diameter LPFG fiber surface by in situ hydrothermal crystallization in a precursor solution with a nominal Si/Al atomic ratio of 15. The synthesis solution was a clear solution derived by mixing 11.3 ml of tetrapropylammonium hydroxide (TPAOH, 1M, Sigma-Aldrich), 19.2 ml of tetraethyl orthosilicate (TEOS, 98%, Acros), 60 ml of DI water and 0.458 g of NaAlO2 (Sigma-Aldrich). TPAOH is the structure directing agent (SDA). The apparatus for zeolite film synthesis on the LPFG has been described in a previous publication.9 The hydrothermal synthesis was conducted at 180 °C under autogeneous pressure for 4 h. After the hydrothermal reaction, the zeolite-coated LPFG was rinsed thoroughly with DI water and then dried and calcined in air at 500 °C for 2 h to remove the SDA from the zeolite channels. The entire process of zeolite film growth was monitored online by continuously scanning the LPFG transmission spectrum† to ensure that the optical function of the zeolite-coated LPFG is preserved and the zeolite film is thick enough to avoid thickness influence.Zeolite modification and characterization
The resultant ZSM-5 film on the fiber surface was examined by field emission scanning electron microscopy (FESEM) and the actual elemental composition of the zeolite thin film was determined by energy dispersive spectroscopy (EDS). Adsorption isotherms of NH3, CO2, and CH4 were measured by a Micromeritics ASAP 2020 unit for the ZSM-5 zeolite particles collected from the residual liquid in the synthesis vessel. Both the zeolite-coated LPFG and ZSM-5 particles were treated in a 0.1 M NH4Cl solution at 80 °C for 60 min after SDA removal to exchange the extraframework Na+ ions with NH4+. The ion exchanged ZSM-5 film and particles were then dried and calcined in air at 350 °C to convert the [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(NH+4)] to [
Si–O−(NH+4)] to [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(H+)] acidic sites in the zeolite structure.
Si–O−(H+)] acidic sites in the zeolite structure.
Optical gas sensing
The transmission spectrum and λR of the Z-LPFG were measured by an apparatus same as that reported in previous works.6 The zeolite-coated LPFG segment of the fiber was mounted in a 4-mm-ID stainless steel tube (total chamber volume ∼9.6 cm3) which was placed in a temperature-programmable tubular furnace. The transmission spectrum covers a near IR wavelength range of 1510–1640 nm. The source light was provided by a tunable laser equipped with a laser power detector (Agilent 8164A) and a computer data acquisition system. In operation, one end of the Z-LPFG connects to the tunable laser (light source) and the other end (output) connects to the laser power meter. The output light intensity was measured point-by-point by the power meter to obtain the transmission spectrum and the λR value. A wavelength increment of 1 nm and a dwelling time of 1 s were used in the gas sensing experiments. In all measurements, the total flow rate of the sample gas was kept at a small value of 10 cm3 (STP)/min for maintaining temperature stability and avoiding mechanical disturbance to the suspended fiber sensor.In the gas sensing test, N2 was used as the carrier gas and the calibrating baseline of the sensor's optical response was measured in pure N2 in a temperature range of 25–350 °C. The baseline λR,N2 is given as a function of temperature, (λR,N2)T = λ0R,N2 + ζT, because the λR of a LPFG is known to exhibit excellent linear dependence on temperature. The ζ values were typically around 0.11 nm/° C with slight variations among different Z-LPFGs. Thus, the gas sensing signal at a specific temperature T, which is the shift of λR in response to switching from N2 to an analyte gas “i” (ΔλR,i)T, is given by:
| (ΔλR,i)T = (λR,i)T − (λR,N2)T | (2) |
The ΔλR of the Z-LPFG was measured for N2-carried single gases including NH3 and CO2 to evaluate the sensing selectivity for NH3 over CO2. Other gases such as H2, CH4, CO, H2S and H2O were also tested as they are common interfering molecules in potential applications like biomass-derived syngas and environments of food storage and animal farms. The ideal sensing selectivity of NH3 against a gas i, αNH3/i, is defined as
 | (3) |
 | (4) |
Results and discussion
Morphology and chemistry of the zeolite film
The zeolite film had a typical morphology of ZSM-5 films with low Si/Al ratios in which the individual particles do not have smooth surface and well-defined orientation planes. Although the outer surface of the ZSM-5 films is microscopically rough, a 3–4 μm thick dense layer is clearly formed at the fiber surface as shown by the SEM images in Fig. 1. This film thickness ensures that the cladding modes are confined in the cladding so that the influence of film thickness variation on λR is negligible for different sensors. | ||
| Fig. 1 SEM images of fractured cross-section of a ZSM-5 zeolite coated LPFG fiber (Insert: high magnification showing zeolite/fiber interface). | ||
The EDS test confirmed that both the zeolite film and particles collected from residual liquid had similar average Si/Al ratios of ∼22.8 (±1.5) and a Na+/Al3+ atomic ratios of ∼0.74 (±0.1). The Na+/Al3+ atomic ratio in the zeolite decreased to ∼0.1 after the NH4+ ion exchange and firing. The MFI zeolite unit cell composition is Mm+x/m[Si96−xAlxO192] where Mm+ is the extraframework cation compensators, which are Na+ and H+ in the current ZSM-5 zeolites. Thus, in each unit cell, the as-synthesized ZSM-5 contained ∼3.0 [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(Na+)] sites and ∼1.0 [
Si–O−(Na+)] sites and ∼1.0 [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(H+)] site while the modified HZSM-5 contained ∼0.4 [
Si–O−(H+)] site while the modified HZSM-5 contained ∼0.4 [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(Na+)] sites and ∼3.6 [
Si–O−(Na+)] sites and ∼3.6 [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(H+)] sites. The modified ZSM-5 film on LPFG and ZSM-5 zeolite particles had greatly increased numbers of acidic sites and hence are denoted hereafter as HZ-LPFG and HZSM-5, respectively.
Si–O−(H+)] sites. The modified ZSM-5 film on LPFG and ZSM-5 zeolite particles had greatly increased numbers of acidic sites and hence are denoted hereafter as HZ-LPFG and HZSM-5, respectively.
Z-LPFG and HZ-LPFG responses to NH3 and CO2
The ΔλR of the zeolite-coated LPFG was tested before and after the modification for their optical responses to NH3 and CO2 at room temperature (23 °C) and atmospheric total pressure. Fig. 2 (a) presents ΔλR,NH3 andΔλR,CO2 as a function of the gas concentration in N2 and Fig. 2 (b) presents the NH3/CO2 sensing selectivity, αNH3/CO2, and zeolite gas adsorption selectivity, βNH3/CO2. The refractive index of the porous MFI zeolite crystal is about 1.34 (<ncl ≈ 1.46) in the non-adsorbing N2 gas and increases with increasing the amount of gas adsorption.8 Thus, blue shifts of λR, i.e.ΔλR < 0, occur when the zeolite film adsorbs NH3 and CO2. | ||
| Fig. 2 Effect of zeolite acidification on (a) ΔλR in response to NH3 and CO2 and (b) NH3/CO2 sensing selectivity at 23 °C and adsorption selectivity at 35 °C under a total pressure of po = 101.3 kPa. | ||
At same volume concentration, ΔλR,NH3 of the Z-LPFG was greater than ΔλR,CO2 because the adsorbance of NH3 is larger than that of CO2. In Fig. 2 (b), the sensing selectivity αNH3/CO2 followed the trend of the adsorption selectivity βNH3/CO2, which decreased with increasing gas concentration. Both αNH3/CO2 and βNH3/CO2 increase sharply as the gas concentrations approach the lower end. At low concentration, the NH3 adsorption is dominated by strong chemisorption, [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(H+)]+NH3 → [
Si–O−(H+)]+NH3 → [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O−(NH+4)], and the NH3 adsorbance is much greater than that of CO2 which is physically adsorbed in the zeolites. The HZSM-5 zeolite particles and HZ-LPFG have larger populations of acidic sites and therefore exhibit higher βNH3/CO2 and αNH3/CO2 than the unmodified ZSM-5 particles and Z-LPFG. The βNH3/CO2 and αNH3/CO2 decrease with increasing NH3 and CO2 gas concentrations because of the increases in the less selective physisorption with increasing adsorbate partial pressure.
Si–O−(NH+4)], and the NH3 adsorbance is much greater than that of CO2 which is physically adsorbed in the zeolites. The HZSM-5 zeolite particles and HZ-LPFG have larger populations of acidic sites and therefore exhibit higher βNH3/CO2 and αNH3/CO2 than the unmodified ZSM-5 particles and Z-LPFG. The βNH3/CO2 and αNH3/CO2 decrease with increasing NH3 and CO2 gas concentrations because of the increases in the less selective physisorption with increasing adsorbate partial pressure.
Effect of temperature on sensor performance
The temperature-dependence of gas adsorption in zeolites varies with the adsorbing strength. Adsorption isotherms were measured for NH3 and CO2 in a temperature range of 35 to 200 °C.†Fig. 3 presents the amounts of adsorption of NH3 and CO2 and adsorption selectivity βNH3/CO2 in ZSM-5 and HZSM-5 zeolites as functions of temperature at a fixed pressure of ∼0.05 bar for both NH3 and CO2. | ||
| Fig. 3 Temperature dependences of amount of adsorption (ads) and βNH3/CO2 for NH3 and CO2 in the ZSM-5 and HZSM-5 zeolites. | ||
Distinct effects of zeolite modification were obtained on the adsorption of NH3 and CO2. At low temperature, physical adsorptions of NH3 is significant in both the ZSM-5 and HZSM-5 zeolites and the total amounts of NH3 adsorption in ZSM-5 and HZSM-5 are almost the same. As temperature increases, the NH3 physisorption diminishes and the NH3 chemisorption remains strong. Hence the HZSM-5 zeolite adsorbs a larger amount of NH3 than the ZSM-5 zeolite at elevated temperature. In contrast, the extraframework alkali metal ions enhance the adsorption of CO2 gas. Thus, replacing the Na+ with H+ in the zeolite reduces the adsorption of the acidic CO2. The relatively weak adsorption of CO2 diminished to nearly zero at 110–150 °C in the HZSM-5 and at ∼200 °C in the ZSM-5 zeolite, respectively. Meanwhile, the chemisorption of NH3 remained significant in both zeolites at 200 °C, namely 13.4 cm3(STP)/g in ZSM-5 and 18.32 cm3(STP)/g in HZSM-5.
Therefore, the modification of zeolite enhances the NH3 chemisorption while reducing the CO2 adsorption that, in combination, dramatically increased the βNH3/CO2 in the HZSM-5, especially at elevated temperatures. As shown in Fig. 3, βNH3/CO2 values are 4.1 in ZSM-5 and 9.1 in HZSM-5 at 35 °C which increased to 9.1 in ZSM-5 and 156.2 in HZSM-5 at 150 °C. The gas adsorption data indicate that the sensing selectivity for NH3 over CO2 can be significantly improved by the surface modification together with proper selection of operation temperature.
Fig. 4 shows the experimental ΔλR for the Z-LPFG and HZ-LPFG in response to 5% CO2 and 5% NH3 measured at atmospheric total pressure as a function of temperature. The Z-LPFG response to CO2 virtually disappeared (i.e.ΔλR,CO2 ≈ 0 nm) at 140 °C where ΔλR,NH3 was −0.35 nm. The HZ-LPFG reached a ΔλR,CO2 of ∼0 nm at 50 °C where its ΔλR,NH3 was as large as −2.9 nm. These demonstrate that the sensing selectivity αNH3/CO2 is significantly enhanced in the HZ-LPFG as compared to the unmodified Z-LPFG. The absolute deviation of ΔλR measurement was within ±0.05 nm based on three times of independent measurements. The HZ-LPFG optical response to the 5% NH3 was found to disappear, i.e.ΔλR,NH3 approaching zero, at around 300 °C. It should be noted that ΔλR of CO2 reaches zero at a temperature well below the temperature where CO2 adsorption becomes negligible because the baseline λR was measured in N2 but not in vacuum.
 | ||
| Fig. 4 The Z-LPFG and HZ-LPFG responses to 5vol% CO2 and 5vol% NH3 carried in N2 as a function of temperature. | ||
Sensor response to common small molecules
We also measured the adsorption isotherms of CH4 in the ZSM-5 and HZSM-5 zeolites† with the results for PCH4/Po at 0.05 shown in Fig. 5. The zeolite modification had a minimal influence on CH4 adsorption because the spherical and nonpolar CH4 molecule is not sensitive to the surface acidity or extraframework ions in the temperature range of concern here. The adsorption selectivity of NH3 over CH4, βNH3/CH4, is much greater than βNH3/CO2 in the temperature range of 35–150 °C because the adsorbance of CH4 in the ZSM-5 zeolites is smaller than that of CO2. The βNH3/CH4 value was 53.8 in ZSM-5 and 56.7 in HZSM-5 at 35 °C. The βNH3/CH4 increased to 240.7 and to 373.4 in ZSM-5 and HZSM-5, respectively, at 150 °C.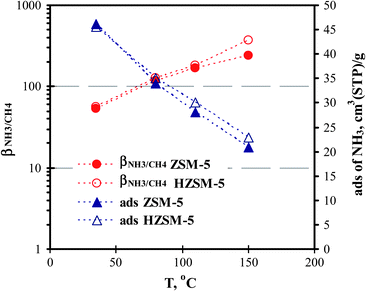 | ||
| Fig. 5 Temperature dependences of amount of NH3 adsorption and βNH3/CH4 in ZSM-5 and HZSM-5 zeolites. | ||
This observation can be explained by the fact that NH3 adsorption is similar in ZSM-5 and HZSM-5 at 35 °C where the total amounts of adsorption are both high due to physisorption; the NH3 adsorbance becomes largely different in the HZSM-5 and ZSM-5 at 150 °C where the adsorbance is dominated by the highly selective chemisorption with negligible physisorption. Thus, after modification, the enhancement of βNH3/CH4 is insignificant at low temperature but becomes significant at high temperature because the temperature-dependence of CH4 adsorption is not affected by the modification. The large value of βNH3/CH4 at 35 °C suggests the HZ-LPFG to be insensitive to CH4 and other very weakly adsorbing small gases as compared to CO2. In fact, the HZ-LPFG showed almost no response (i.e.ΔλR = 0 ± 0.05nm) to H2, CO, and CH4 even at room temperature when concentrations of these gases are below 5% as shown in Fig. 6.
 | ||
| Fig. 6 Response of HZ-LPFG to H2, CO, CH4 and NH3 at 23 °C and atmospheric pressure. | ||
Additional optical sensing experiments were performed for the HZ-LPFG with 10% CO2, 3% H2S, and 3% H2O in N2 at atmospheric pressure. The results are presented in Fig. 7. The responses to the 10% CO2 and 3% H2S are similar, both reached the baseline (i.e.ΔλR = 0 nm) at 50–65 °C. The response to the 3% H2O vapor was found to be stronger than those to CO2 and H2S due to the hydrophilic zeolite surface but weaker than that to NH3. The ΔλR for the 3% H2O vapor reached the baseline at ∼125 °C where the HZ-LPFG still exhibited a large ΔλR,NH3 of ca. −1.0 nm to the 5% NH3.
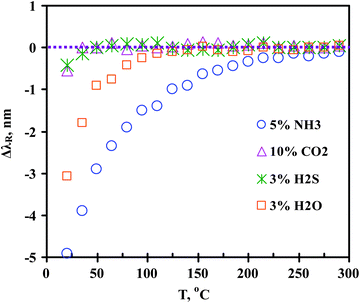 | ||
| Fig. 7 The HZ-LPFG responses to 10vol% CO2, 3vol% H2S, 3vol% H2O and 5vol% NH3 in N2 as function of temperature at atmospheric pressure. | ||
Effect of temperature on response speed and sensitivity
The above results demonstrate that the NH3 sensing selectivity by the HZ-LPFG can be greatly improved when operating at elevated temperatures. The operating temperature will also affect the speed of response and the detection sensitivity. The speed of response in gas sensing is expected to be faster at higher temperature because the endothermic desorption as well as diffusion of NH3 molecules in the microporous zeolite are facilitated at high temperature. Meanwhile, the NH3 adsorption in the zeolite is reduced at elevated temperature that decreases the magnitude of ΔλR,NH3 to lower the sensitivity.Single wavelength sensing experiments were performed to investigate the HZ-LPFG temporal response to switching sample gas flow between pure N2 and a mixture of 612 ppmv NH3 carried in N2. The dynamic response of transmission light intensity to the switch of gas composition was monitored at a fixed wavelength. The fixed single wavelength was specifically selected for each temperature such that the variation of the transmission light intensity would be the same if same amount of ΔλR occurs.† Therefore, the peak height of the transmission light intensity can qualitatively reflect the detection sensitivity. The experimental results are shown in Fig. 8.
 | ||
| Fig. 8 Temporal response of the HZ-LPFG when switching between pure N2 and 612 ppmv NH3 in N2 at various temperatures. | ||
Below 110 °C, the response time for switching from N2 to the 612 ppmv NH3, which involves exothermic NH3 adsorption, is much shorter than the reverse operation, which involves the endothermic NH3 desorption. At 22 °C, the output signal, i.e. the transmission light intensity, stabilized within 2 min after switching the sample gas from pure N2 to the NH3/N2 mixture but the signal stabilization took ∼120 min for the reverse process, i.e. switching back to pure N2 for regeneration. As temperature increased to 150 °C, the stabilization time had no obvious change for the NH3 sensing (adsorbing) step but was dramatically shortened to ∼2.5 min for the regeneration (desorption) step. It should be noted that the response time includes nearly 2 min needed for the sample gas (flow rate 10 cm3/min) to purge the 9.6-cm3 tube hosting the fiber. The stabilization time is determined by the time needed for the zeolite film to equilibrate the gas adsorption/desorption in the new gas environment. The results indicate that, at high temperature, the shortening of response time for regeneration is due primarily to the facilitated desorption of NH3 rather than the enhancement of NH3 diffusion in the microporosity because the kinetic size of NH3 (∼3Å) is much smaller than the MFI zeolite pore diameter (5.6Å).
It is also shown in Fig. 8 that the sensitivity of NH3 detection measured by the difference in output light intensities between 612 ppmv NH3 and pure N2, decreases with increasing temperature which is consistent with results in Fig. 4. This decrease of sensitivity is a result of the reduced amount of NH3 adsorption at elevated temperatures. Therefore, the enhancements of NH3 sensing selectivity and response speed at high temperature are achieved at a cost of lowered detection sensitivity.
Conclusion
ZSM-5 zeolite films with a low Si/Al ratio of ∼23 have been synthesized on LPFG optical fibers. The zeolite-coated LPFG (Z-LPFG) offers high sensitivity for gas sensing by monitoring the shift of the resonant wavelength upon molecular adsorption in the zeolite. The sensing selectivity of the Z-LPFG relies on the selectivity of gas adsorption in the zeolite pores. Zeolite surface modification combined with temperature control provides an effective way to enhance the gas sensing selectivity and response speed. However, operation at high temperature compromises the sensitivity. While the sensing performance in multicomponent mixtures is yet to be investigated, this study shows the feasibility of using zeolites as effective optical chemical sensing materials for construction of fiber optic sensors. The present work demonstrates that the optical response of HZ-LPFG to NH3 (ΔλR,NH3) has excellent quantitative correlations with NH3 concentration and temperature. Thus with temperature and concentration (CNH3) calibration data, a mathematic model of ΔλR,NH3 = f(T, CNH3) can be readily established and built into the computer program for quantitative measurement. There exist a large number of zeolitic materials with distinct crystallographic structures, pore sizes, material chemistry, and capacities for surface modification. This offers an excellent opportunity to develop a new class of zeolite-enabled fiber optic chemical sensors for applications in many important areas such as energy production processes, environmental monitoring, and chemical and biological threat detection. The zeolite-fiber integrated device is also potentially useful for studying molecular sorption and diffusion mechanisms in zeolites because of its high sensitivity and temporal resolution.Acknowledgements
This material is based upon work supported by the National Science Foundation (Grant CBET-0854203) and the U.S. Department of Energy NETL University Coal Research program (Grant No. DE-NT0008062).References
- K. Hoffmann and F. Marlow, In Handbook of Zeolite Science and Technology; S. M. Auerbach, K. A. Carrado, P. K. Dutta, ed.; Marcel Dekker: NewYork, 2003, p 921–949 Search PubMed.
- J. L. Meinershagen and T. J. Bein, J. Am. Chem. Soc., 1999, 121, 448 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- P. Kortunov, L. Heinke, M. Arnold, Y. Nedellec, D. J. Jones, J. Caro and J. Kärger, J. Am. Chem. Soc., 2007, 129, 8041 CrossRef CAS.
- R. J. Kavanagh, K. K. Iu and J. K. Thomas, Langmuir, 1992, 8, 3008 CrossRef CAS.
- J. Zhang, X. Tang, J. Dong, T. Wei and H. Xiao, Opt. Express, 2008, 16, 8317 CrossRef CAS.
- J. Zhang, J. Dong, M. Luo, H. Xiao, S. Murad and R. A. Normann, Langmuir, 2005, 21, 8609 CrossRef CAS.
- J. Zhang, M. Luo, H. Xiao and J. Dong, Chem. Mater., 2006, 18, 4 CrossRef CAS.
- J. Zhang, X. Tang, J. Dong, T. Wei and H. Xiao, Sens. Actuators, B, 2009, 135, 420 CrossRef.
- A. M. Vengasarkar, P. J. Lemaire, J. B. Judkins, V. Bhatia, T. Erdogan and J. E. Sipe, J. Lightwave Technol., 1996, 14, 58 CrossRef.
- Y. Li, T. Wei, J. A. Montoya, S. V. Saini, X. Lan, X. Tang, J. Dong and H. Xiao, Appl. Opt., 2008, 47, 5296 CrossRef CAS.
- T. Erdogan, J. Lightwave Technol., 1997, 15, 12774.
- N. D. Rees, S. W. James, R. P. Tatam and G. J. Ashwell, Opt. Lett., 2002, 27, 686 Search PubMed.
- A. Cusano, P. Pilla, L. Contessa, A. Iadicicco, S. Campopiano, A. Cutolo, M. Giordano and G. Guerra, Appl. Phys. Lett., 2005, 87, 234105 CrossRef.
- Z. Gu, Y. Xu and K. Gao, Opt. Lett., 2006, 31, 2405 Search PubMed.
- X. Tang, K. Remmel, X. Lan, J. Deng, H. Xiao and J. Dong, Anal. Chem., 2009, 81, 7844 CrossRef CAS.
- V. Bhatia, Opt. Express, 1999, 4, 457 CrossRef CAS.
- R. Hou, Z. Hassemlooy, A. Hassan, C. Lu and K. P. Dowker, Meas. Sci. Technol., 2001, 12, 1709 CrossRef CAS.
- Y. J. Rao, Opt. Lasers Eng., 1999, 31, 297 CrossRef.
- U. Schemmert, J. Karger, C. Krause, R. A. Rakoczy and J. Weitkamp, Europhys. Lett., 1999, 46, 204 CrossRef CAS.
- U. Schemmert, J. Karger and J. Weitkamp, Microporous Mesoporous Mater., 1999, 32, 101 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Schematic illustration of the zeolite film coated LPFG. Experimental setup Z-LPFG test. LPFG transmission spectra and resonant wavelength as functions of film synthesis time. Figure showing selection of single wavelength for the test of sensing response. Adsorption isotherms of CO2 and NH3 in ZSM-5 and HZSM-5 at different temperatures. Adsorption isotherms for CH4 and NH3 in ZSM-5 and HZSM-5 zeolites at various temperatures. See DOI: 10.1039/c0jm02523b |
| This journal is © The Royal Society of Chemistry 2011 |
