Low-temperature synthesis of highly hydrophilic Ti-containing mesoporous silica thin films on polymer substrates by photocatalytic removal of structure-directing agents
Yu
Horiuchi
,
Haruhisa
Ura
,
Takashi
Kamegawa
,
Kohsuke
Mori
and
Hiromi
Yamashita
*
Division of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University, 2-1, Yamada-oka, Suita, Osaka 565-0871, Japan. E-mail: yamashita@mat.eng.osaka-u.ac.jp; Fax: +81 6 6879 7457; Tel: +81 6 6879 7457
First published on 20th October 2010
Abstract
This article describes a novel preparation method for highly hydrophilic porous thin films via photocatalytic removal of a structure-directing agent (SDA) under UV-light irradiation at ambient temperature. Since this technique requires no calcination process, it can effectively coat surfaces that have low thermal resistance, such as polymer substrates. In the present study, Ti-containing mesoporous silica thin films (Ti-MSTF) were prepared on polycarbonate and quartz substrates using a newly developed method involving UV-light irradiation. The film surface wettabilities were investigated by water contact angle measurements. Complete removal of the SDA was confirmed by time-dependent changes in the FT-IR spectra under UV-light irradiation, which also suggested that Ti-oxide moieties in the mesoporous silica framework play a crucial role in the removal of the SDA. XRD, UV-vis and XAFS measurements revealed that the Ti-MSTF had a mesoporous structure and isolated Ti-oxide moieties. The water contact angle measurements indicated that the Ti-MSTF exhibited highly hydrophilic property after photocatalytic removal of the SDA, and maintained this property under dark conditions for several days.
Introduction
Recently, there has been increasing research on high functionalization of solid surfaces by surface modification techniques such as sol–gel coating, vapor deposition, plating and self-assembly.1–7 Surface wetting is a very important property for material design, because it strongly influences adhesion and joining. In addition, control of interface wettability between water and solid surfaces, particularly the fabrication of superhydrophilic surfaces, has received much attention for application to self-cleaning and antifogging materials.5,6,8–10 To fabricate a superhydrophilic surface, titanium dioxide (TiO2) thin films are often utilized because of their photoinduced superhydrophilicity under UV-light irradiation.11 Several approaches have been proposed for the enhancement of this surface property, such as the combination of silica with a TiO2 thin film, the formation of mesostructures and noble metal doping.12–14 Although considerable research on hydrophilic surface modification of thin-film materials has been reported, the target substrates have mainly been limited to inorganic materials such as quartz and silicon. The development of thin-film coating technologies on various material surfaces is an important issue for the practical use of this unique surface property. The low-temperature synthesis of such films could increase the number of applicable materials, because it enables the surface coating of materials with low thermal resistance, such as plastics.Our group has reported that single-site photocatalyst thin films, which consist of a mesoporous silica framework containing isolated transition metal oxide moieties, are highly hydrophilic, even under dark conditions.15–17 This fascinating surface property originates from the unique architecture of the films. The mesoporous silica framework has many silanol groups. The isolated transition metal oxide moieties serve as adsorption sites for water molecules due to charge localization. Moreover, the mesoporous structure induces a capillary penetration effect. Since water molecules are strongly attracted to the surface of the silica framework through these processes, single-site photocatalyst thin films exhibit high hydrophilicity.
Porous structures can be created by a sol–gel method using a surfactant as a template, in which high-temperature calcination is typically employed to remove the surfactant by combustion.18 As a consequence, it is difficult to synthesize these porous structures on substrates with low thermal resistance. In order to overcome this drawback, we propose a new synthesis route for a porous structure in a film based on photocatalytic removal of an organic template without calcination. Single-site photocatalyst thin films form a charge-transfer excited state, which is generated from tetrahedrally-coordinated metal oxide moieties, and exhibit photo-oxidation activity under UV-light irradiation.19–22 Thus, we anticipate that the porous structure can be created by the irradiation of UV-light without a calcination process due to their own abilities of photocatalytic decomposition.
Herein, Ti-containing mesoporous silica thin films (Ti-MSTFs), which are single-site photocatalyst thin films, were prepared on polycarbonate (PC) and quartz substrates by photocatalytic removal of the template without calcination. The surface wetting properties of these films were investigated using water contact angle measurements.
Experimental
Materials
Polyethylene (20) stearyl ether (C18H37(OCH2CH2)20OH) described as Brij®78 was purchased from Sigma-Aldrich Co. Tetraethyl orthotitanate ((C2H5O)4Ti; TEOT) was purchased from Tokyo Kasei Kogyo Co., Ltd. Tetraethyl orthosilicate ((C2H5O)4Si; TEOS) was purchased from Wako Pure Chemical Ind., Ltd. 5 N hydrochloric acid (HCl) and ethanol (EtOH) were purchased from Nacalai Tesque Inc. All chemicals were used as received.Synthesis
Ti-MSTF was prepared on quartz and PC substrates by sol–gel/spin-coating, followed by UV-light irradiation for the photocatalytic removal of the structure-directing agent (SDA). Typically, a mixture of Brij®78 (0.25 mmol) as an SDA, TEOS (4.9 mmol) as a silica source, TEOT (0.1 mmol) as a titania source, HCl (0.07 mmol), EtOH (100 mmol) and H2O (25 mmol) was stirred in a Teflon bottle for 20 min at room temperature. The obtained clear sol was dropped onto each substrate, spread evenly and coated at a spinning rate of 4000 rpm for 1 min. After spin-coating, the film/plate samples were dried at 373 K for 10 h. Photocatalytic removal of the SDA was performed by the irradiation of UV-light from a 200 W mercury xenon lamp made by San-ei Electric Co., Ltd. at 40 mW cm−2 for several hours (1–8 h). During the irradiation, Ti-oxide moieties in the silica framework served as photocatalytic sites, and the SDA infiltrating the porous structure was decomposed. In the synthesis on PC substrates, argon ion beam irradiation was performed before coating to enhance adhesion. In comparison, pure silica MSTF was also prepared by the same method, except a titanium-free precursor solution was used. A schematic illustration of the synthetic procedure is shown in Fig. 1. | ||
| Fig. 1 Schematic illustration of the preparation of Ti-MSTF on quartz and PC substrates utilizing the photocatalytic removal of the SDA. | ||
Characterization
Fourier transform infrared (FT-IR) absorption measurements were performed at room temperature using a JASCO FT/IR-6100. The sample chamber was degassed under vacuum. Photon-stimulated desorption measurements were carried out using an ESCO PSD. Scanning electron microscope (SEM) images were obtained with a JEOL JSM-5600. Standard θ–2θX-ray diffraction (XRD) data were recorded on a Rigaku Ultima IV X-ray diffractometer at room temperature using Cu Kα radiation (λ = 1.5418 Å). Kr adsorption measurements were performed using a BELSORP-max (BEL Japan, Inc.) at 77 K. The sample was degassed under vacuum at 378 K for 3 h prior to data collection. The BET method was applied to determine the surface areas and pore volumes. Diffuse reflectance UV–vis absorption spectra were monitored by a Shimadzu UV-2450 spectrometer at room temperature. The reference sample was BaSO4, and the absorption spectra were obtained using the Kubelka–Munk function. In measurements, the thin-film samples prepared on quartz substrates were fixed in front of BaSO4, and their spectra were collected by subtracting that of quartz as background data. Ti K-edge X-ray absorption fine structure (XAFS) spectra were measured in the fluorescence mode at room temperature at the BL-7C facilities of the Photon Factory at the National Laboratory for High-Energy Physics, Tsukuba. A Si (111) double-crystal was used to monochromatize the X-rays from the 2.5 GeV electron storage ring. In a typical experiment, the sample was loaded into a plastic-windowed in situcell. The pre-edge peaks in the X-ray absorption near-edge structure (XANES) spectra were normalized for atomic absorption based on the average absorption coefficient of the spectral region.Evaluation of wettability
The wettabilities were examined by measuring the contact angle of pure water (3 μL) dropped onto the thin-film samples using a contact angle meter (DropMaster 300 of Kyowa Interface Science Co., Ltd.). A 10 mW He–Ne laser, a bandpass filter, a high-resolution (2000 × 1312 pixels) digital camera with a remote controller, a video and an image automatic transmission and processing system were used for the measurement. Because the band-pass filter removed all other wavelengths except for the laser beam (632 nm), the reflected light could be removed to obtain high definition drop profiles.Results and discussion
Time-dependent changes in the FT-IR spectra under UV-light irradiation confirmed the complete removal of the SDA. Fig. 2(a) shows FT-IR spectra of the Ti-MSTF prepared on a quartz substrate at each irradiation time in the scan range of 2500–3250 cm−1. As expected, two main peaks at 2855 and 2920 cm−1 corresponding to C–H stretching vibrations of alkyl groups in the SDA gradually decreased, and finally disappeared after 4 h. This result suggests that the SDA infiltrating the mesoporous structure was completely removed. Moreover, the complete removal of the SDA in Ti-MSTF was investigated by detecting CO2 generated under UV-light irradiation. The as-synthesized thin-film samples containing the SDA were placed into a quartz cell connected to a vacuum line system and irradiated with UV-light (40 mW cm−2) under an O2 atmosphere (10 Torr). The production of CO2 was monitored by GC analysis using a Shimadzu GC-14B equipped with Porapaq Q + T packed column. In comparison, the amount of CO2 generated under calcination in the presence of O2 (10 Torr) at 723 K for 2 h (heating rate was 6 K min−1) instead of UV-light irradiation was also measured. The amount of CO2 generated from one piece of as-synthesized Ti-MSTF (10 × 10 mm) under calcination was determined to be 1.0 μmol. In the case of UV-light irradiation, the generation of almost the same amount of CO2 was detected as the amount obtained under calcination from the un-irradiated sample. These results suggest that almost all SDAs infiltrating the mesoporous structure could be removed via this removal process. On the contrary, as shown in Fig. 2(b), these two peaks in the spectra of pure silica MSTF without Ti-oxide moieties remained nearly unchanged, indicating the presence of residual SDA in the pores. These results indicate that the SDA was not decomposed solely by UV-light irradiation, but that Ti-oxide moieties in the silica framework of the Ti-MSTF played a crucial role in the removal of the SDA. | ||
| Fig. 2 Time-dependent change in the FT-IR spectra of (a) Ti-MSTF and (b) pure silica MSTF under UV-light irradiation. | ||
To further clarify the role of the Ti-oxide moieties, photon-stimulated desorption measurements, in which molecular desorption from a sample is observed by continuously recording the mass change under light irradiation at specific wavelengths, were performed. The photon-stimulated desorption profiles of Ti-MSTF and pure silica MSTF were monitored at 15, 29 and 45 m/z, corresponding to the terminal –CH3, –CH2CH3 and –CH2CH2OH groups of the SDA, respectively. The desorption amounts of various terminal groups (m/z = 15, 29 and 45) of the SDA in Ti-MSTF and pure silica MSTF under irradiation at wavelengths of 200–280 nm are summarized in Fig. 3. These wavelengths correspond to the absorption wavelengths of tetrahedrally-coordinated TiO4 moieties in Ti-MSTF due to charge-transfer excitation, as discussed below. The desorption from pure silica MSTF was negligible, indicating an inability to break the chemical bonds. In contrast, for Ti-MSTF, there was significant desorption of each terminal group. These results indicate that Ti-oxide moieties excited by incident light were responsible for breaking a chemical bond of the SDAvia a photo-oxidation reaction.
 | ||
| Fig. 3 Desorption amount of various terminal groups (m/z = 15, 29 and 45) of the SDA from pure silica MSTF and Ti-MSTF under light irradiation with wavelengths of 200–280 nm. | ||
As can be seen in Fig. 4, the prepared Ti-MSTFs were transparent, and a background image was easily visible through these films. The cross-sectional SEM image of Ti-MSTF on a PC substrate is also shown in Fig. 4. The thickness of the Ti-MSTF was about 1.5 μm, and its surface was uniform and without cracks.
 | ||
| Fig. 4 Cross-sectional SEM image and a photograph (inset) of Ti-MSTF on a PC substrate after the catalytic removal of the SDA under UV-light irradiation. | ||
Fig. 5 shows low-angle XRD patterns of Ti-MSTFs on quartz and PC substrates before and after UV-light irradiation. All diffraction patterns exhibited a single intense peak around 2θ = 1.5–3.0 degrees assigned to a (100) reflection, which can be indexed to a two-dimensional hexagonal lattice. After UV-light irradiation, the diffraction peak shifted to a slightly higher value, indicating a contraction of d100 spacing due to removal of the SDA. A similar shift is commonly observed after SDA removal by calcination.23–26 These results suggest that a mesoporous structure was formed on both quartz and PC substrates after UV-light irradiation. To investigate the adhesion between the Ti-MSTF and each substrate, XRD measurements were performed before and after a peeling test. The peaks maintained their positions, without the slightest shift, even after the peeling test. Therefore, the Ti-MSTF demonstrated good adhesion to both substrates. Furthermore, no peaks corresponding to crystalline titanium dioxide phases were observed at higher angles in the XRD patterns. This revealed that the Ti-oxide moieties were highly dispersed within the silica framework.
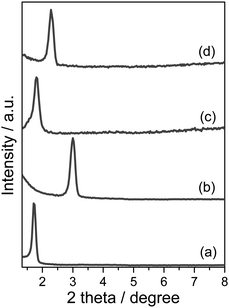 | ||
| Fig. 5 XRD patterns of Ti-MSTFs on (a, b) quartz and (c, d) PC substrates before (a, c) and after (b, d) the catalytic removal of the SDA under UV-light irradiation. | ||
Kr adsorption measurements offer several pieces of important structural information about the pore diameter, surface area and pore volume.27,28 The structural parameters obtained from the Kr adsorption are summarized in Table 1. The remarkable increase of the surface areas and pore volumes after UV-light irradiation revealed the formation of mesopores due to the removal of the SDA.
| Thin-film sample | Average pore diameter (nm) | BET surface area (cm2/cm2) | Pore volume (cm3/cm2) |
|---|---|---|---|
| Ti-MSTF before removal | — | 2.4 | 4.3 × 10−8 |
| Ti-MSTF after removal | 2.2 | 13.9 | 8.3 × 10−7 |
UV–vis absorption measurements and XAFS analysis also suggested the presence of highly dispersed Ti-oxide moieties. Fig. 6 shows UV–vis absorption spectra of the Ti-MSTF on a quartz substrate, and those of TiO2 powder and an SDA film as reference samples. In the TiO2 powder spectrum, an absorption band was observed at 200–400 nm due to bandgap excitation. The Ti-MSTF spectrum only exhibited absorption in the UV-wavelength region below 280 nm, which is assignable to the ligand-to-metal charge-transfer (LMCT) transition from O2− to Ti4+ ions in the tetrahedral geometry.29–32 Additionally, the Ti K-edge XANES spectrum of the Ti-MSTF showed a single pre-edge peak at 4968 eV corresponding to a dipole-allowed transition from the 1s to t2 molecular levels built from the 3d and 4p metal orbitals and a neighboring orbital.32–34 These results clearly indicate that Ti-oxide moieties in Ti-MSTF exist in isolated and tetrahedrally-coordinated states, without any aggregated species. No significant change in the absorption edge of UV–vis spectra of Ti-MSTFs before and after photocatalytic removal of SDAs was observed, indicating that the SDA was not directly in contact with tetrahedrally-coordinated Ti-sites. Thus, it can be considered that active oxygen species, such as HO• and O2•−, generated at Ti(IV)-sites under UV-light irradiation diffuse and react with the SDA. Moreover, the UV-vis spectrum of Ti-MSTF did not show the peak below 200 nm observed in the SDA film spectrum, indicating the complete removal of the SDA.
 | ||
| Fig. 6 UV–vis spectra of Ti-MSTF (solid line), TiO2 powder (dotted line) and SDA film (dashed line). | ||
The surface wettability of Ti-MSTFs prepared on quartz and PC substrates was evaluated by water contact angle measurements. Fig. 7 shows photographs of water droplets on an original PC substrate and PC substrates coated with Ti-containing silica thin film without mesopores and with Ti-MSTFs before and after the catalytic removal of the SDA under UV-light irradiation. The water contact angle of the original PC substrate was 97°, indicating its hydrophobicity. After coating with Ti-MSTF, the surface wetting property changed from hydrophobic to hydrophilic (Fig. 7c). Moreover, the Ti-MSTF covering PC substrate was highly hydrophilic after the photocatalytic removal of the SDA, whose water contact angle was approximately 5° (Fig. 7d). On the other hand, the water contact angle of Ti-containing silica thin film without mesopores was 58° (Fig. 7b), suggesting that the formation of the porous structure enhanced its hydrophilicity. This enhancement was due to the effects of both capillary penetration and an increased number of silanol groups and isolated Ti-oxide moieties exposed on the inner surface of the porous silica framework, which acted as adsorption sites for water. In comparison, a solvent extraction method which is a typical SDA removal process under mild conditions was applied. The thin-film samples containing the SDA were kept in acidified ethanol (0.8 ml of 35 wt% HCl in 100 ml ethanol) at 353 K for 24 h, followed by washing with ethanol and drying in air at 373 K.35 The same process was repeated three times. The thus-obtained Ti-MSTF on PC substrate showed moderate hydrophilicity whose water contact angle was 39°. This is due to a negative effect of the adsorbed and residual solvent on its hydrophilicity. Fig. 8 shows the time-dependent change of water contact angles during SDA removal under UV-light irradiation. Ti-MSTFs on quartz and PC substrates were moderately hydrophilic before UV-light irradiation, similar to pure silica MSTF on a quartz substrate. While the contact angles of pure silica MSTF remained unchanged, those of Ti-MSTFs on quartz and PC substrates gradually decreased under UV-light irradiation, finally reaching a lower limit after 4 h. This result is in excellent agreement with the time required for the complete removal of the SDA according to the FT-IR measurements, and confirms the strong relationship between the formation of the porous structure and hydrophilic behavior. Furthermore, to investigate the stability of the hydrophilic surfaces, Ti-MSTFs after UV-light irradiation were stored in dark conditions. Their hydrophilicity was maintained for at least 3 days, with no significant increase in their water contact angles. Therefore, the high hydrophilicity was not caused by the UV-light irradiation, but their own characteristics due to the specific architecture formed by photocatalytic removal of the SDA. Because Ti-MSTFs exhibit high hydrophilic properties after the formation of the designed structure, and their surface properties remain stable for prolonged periods, the proposed technique could provide an effective highly hydrophilic surface modification technique for various practicable substrates.
 | ||
| Fig. 7 Photographs of water droplets on (a) an original PC substrate and (b–d) PC substrates coated with (b) Ti-containing silica thin film without mesopore and with (c and d) Ti-MSTFs before (c) and after (d) the catalytic removal of the SDA under UV-light irradiation. | ||
 | ||
| Fig. 8 Time-dependent change of water contact angles with Ti-MSTFs on quartz (filled squares) and PC (filled circles) substrates, and that of pure silica MSTF on quartz (filled triangles) during photocatalytic removal of the SDA under UV-light irradiation. | ||
Conclusions
In summary, we developed a novel low-temperature synthesis of porous thin-film materials utilizing photocatalytic removal of the SDA instead of calcination. By this method, a highly hydrophilic surface was successfully formed on a PC substrate. The SDA infiltrating the pore was efficiently photocatalytically decomposed by Ti-oxide moieties in the silica framework under UV-light irradiation, and the removal was complete after 4 h. The obtained Ti-MSTF was highly hydrophilic because of capillary penetration due to the porous structure and also because of high water adsorption by the many silanol groups and isolated Ti-oxide moieties present. The proposed method is applicable to the formation of highly hydrophilic coatings on various substrates, especially those with low thermal resistance.Acknowledgements
This study is financially supported by Grants-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 21656207). Y. H. thanks the JSPS Research Fellowship for Young Scientists. Y. H. also expresses his special thanks for Priority Assistance for the Formation of Worldwide Renowned Center of Research. The Global COE Program (Project: Center of Excellence for Advanced Structural and Function Materials Design) from Ministry of Education, Culture, Sports, Science and Technology of Japan.References
- J. van den Bergh, A. Tihaya and F. Kapteijn, Microporous Mesoporous Mater., 2010, 132, 137 CrossRef CAS.
- Y. Kuwahara, K. Maki, Y. Matsumura, T. Kamegawa, K. Mori and H. Yamashita, J. Phys. Chem. C, 2009, 113, 1552 CrossRef CAS.
- Y. Kuwahara, T. Kamegawa, K. Mori and H. Yamashita, Chem. Commun., 2008, 4783 RSC.
- N. Nishiyama, J. Kaihara, Y. Nishiyama, Y. Egashira and K. Ueyama, Langmuir, 2007, 23, 4746 CrossRef CAS.
- X. Zhang, F. Shi, J. Niu, Y. Jiang and Z. Wang, J. Mater. Chem., 2008, 18, 621 RSC.
- Y. Jiang, Z. Wang, X. Yu, F. Shi, H. Xu, X. Zhang, M. Smet and W. Dehaen, Langmuir, 2005, 21, 1986 CrossRef CAS.
- N. K. Mal, M. Fujiwara and Y. Tanaka, Nature, 2003, 421, 350 CrossRef CAS.
- S. Hu, X. Cao, Y. Song, C. Li, P. Xie and L. Jiang, Chem. Commun., 2008, 2025 RSC.
- Q. Zhang, F. Xia, T. Sun, W. Song, T. Zhao, M. Liu and L. Jiang, Chem. Commun., 2008, 1199 RSC.
- J. Lahann, S. Mitragotri, T.-N. Tran, H. Kaido, J. Sundaram, I. S. Choi, S. Hoffer, G. A. Somorjai and R. Langer, Science, 2003, 299, 371 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Nature, 1997, 388, 431 CrossRef CAS.
- F. Meng and Z. Sun, Appl. Surf. Sci., 2009, 255, 6715 CrossRef CAS.
- K. R. Denison and C. Boxall, Langmuir, 2007, 23, 4358 CrossRef CAS.
- M. Machida, K. Norimoto, T. Watanabe, K. Hashimoto and A. Fujishima, J. Mater. Sci., 1999, 34, 2569 CrossRef CAS.
- Y. Horiuchi, H. Ura, H.-j. Yoo, T. Kamegawa, K. Mori, N. Nishiyama and H. Yamashita, ISIJ Int., 2010, 50, 255 Search PubMed.
- Y. Horiuchi, T. Kamegawa, K. Mori, H. Yamashita and N. Nishiyama, e-J. Surf. Sci. Nanotechnol., 2009, 7, 141 Search PubMed.
- Y. Horiuchi, K. Mori, N. Nishiyama and H. Yamashita, Chem. Lett., 2008, 37, 748 CrossRef CAS.
- K. Mori, Y. Kondo, S. Morimoto and H. Yamashita, J. Phys. Chem. C, 2008, 112, 397 CrossRef CAS.
- Y. Horiuchi, M. Shimada, T. Kamegawa, K. Mori and H. Yamashita, J. Mater. Chem., 2009, 19, 6745 RSC.
- K. Mori, Y. Miura, S. Shironita and H. Yamashita, Langmuir, 2009, 25, 11180 CrossRef CAS.
- H. Yamashita, K. Mori, S. Shironita and Y. Horiuchi, Catal. Surv. Asia, 2008, 12, 88 CrossRef CAS.
- H. Yamashita and K. Mori, Chem. Lett., 2007, 36, 348 CrossRef CAS.
- H. Miyata, Y. Kawashima, M. Itoh and M. Watanabe, Chem. Mater., 2005, 17, 5323 CrossRef CAS.
- C. Song and G. Villemure, Microporous Mesoporous Mater., 2001, 44–45, 679 CrossRef CAS.
- H. Miyata and K. Kuroda, J. Am. Chem. Soc., 1999, 121, 7618 CrossRef CAS.
- H. Miyata and K. Kuroda, Chem. Mater., 1999, 11, 1609 CrossRef CAS.
- C.-Y. Chiu, A. S. T. Chiang and K.-J. Chao, Microporous Mesoporous Mater., 2006, 91, 244 CrossRef CAS.
- T. Takei and M. Chikazawa, J. Ceram. Soc. Jpn., 1998, 106, 353 CAS.
- K. Mori, K. Sugihara, Y. Kondo, T. Takeuchi, S. Morimoto and H. Yamashita, J. Phys. Chem. C, 2008, 112, 16478 CrossRef CAS.
- P. Wu and T. Tatsumi, Catal. Surv. Asia, 2004, 8, 137 CrossRef CAS.
- L. Marchese, E. Gianotti, V. Dellarocca, T. Maschmeyer, F. Rey, S. Colucciab and J. M. Thomase, Phys. Chem. Chem. Phys., 1999, 1, 585 RSC.
- T. Blasco, A. Corma, M. T. Navarro and J. P. Pariente, J. Catal., 1995, 156, 65 CrossRef CAS.
- J. M. Thomas and G. Sankar, Acc. Chem. Res., 2001, 34, 571 CrossRef CAS.
- G. Sankar, J. M. Thomas, C. R. A. Catlow, C. M. Barker, D. Gleeson and N. Kaltsoyannis, J. Phys. Chem. B, 2001, 105, 9028 CrossRef CAS.
- K. Kailasam, A. Fels and K. Müller, Microporous Mesoporous Mater., 2009, 117, 136 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
