Utilising hardly-water soluble substrates as a second phase enables the straightforward synthesis of chiral alcohols†
Christina
Kohlmann
a,
Nora
Robertz
a,
Susanne
Leuchs
b,
Lasse
Greiner
*ab and
Shukralla
Na’amnieh
c
aInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringer Weg 1, 52074, Aachen, Germany
bDECHEMA e. V., Karl-Winnacker-Institut, Theodor-Heuss-Allee 25, 60486, Frankfurt am Main, Germany. E-mail: greiner@dechema.de
cX-Zyme GmbH, Merrowingerplatz 1a, Düsseldorf, Germany
First published on 28th September 2011
Abstract
So far, the alcohol dehydrogenase-catalysed conversion of longer chain aliphatic substrates has been challenging due to their low solubility in aqueous solution. However, by utilising the ketone directly as a second organic phase, the straightforward synthesis of long chain aliphatic chiral alcohols is enabled.
Chiral compounds play an important role in chemical and pharmaceutical industries, as they are building blocks for various pharmaceuticals, flavours, agrochemicals, food additives and chemical catalysts. It is supposed that 40% of all chiral centres in drugs are secondary or tertiary hydroxyl groups.1 Hence, enantiopure alcohols are of special importance among the available building blocks. Therefore, various methods for the synthesis of these alcohols exist.2 Firstly, classical methods like the chemical resolution of racemates, chromatography or chiral pool syntheses can be used. Secondly, asymmetric chemical methods like CBS reduction3 or transfer hydrogenation with chiral catalysts4 can be applied. Finally, enzymes or whole cells can also be suitable catalysts.5
Due to superior regio- and enantioselectivity, along with higher product purity, more and more biocatalytic processes are being established in industry. Prevalent alcohol dehydrogenases (ADH) are used to catalyse the synthesis of chiral alcoholsvia the reduction of prochiral ketones.6 Unfortunately, without strategies to overcome the frequently poor solubility of industrially attractive substrates, the application of these biocatalysts is limited due to the dependency on water. On a technical scale, almost insoluble and non-water soluble ketones are preferably converted into chiral alcoholsvia chemical methods that either involve transition metal catalysts, high pressures and high temperatures or hazardous borane and chiral oxazaborolidine.3,4 Since, biocatalysts work under mild reaction conditions (e.g. room temperature and ambient pressure), their application for the conversion of hardly-water soluble substrates could enable sustainable and competitive synthesis routes.
As a consequence, the potential of aqueous organic one-phase and two-phase systems has been explored in several examples to overcome solubility problems.7 In both cases, the selection of an adequate organic solvent is challenging. While in one-phase systems direct influences on the biocatalyst performance and cofactor stability have to be taken into account, in two-phase systems sufficient partitioning of the substrate and product in dependence of enzyme kinetics needs to be achieved to avoid mass transfer limitations. However, two-phase systems are of particular interest, since they may aid the overcoming of inhibitions, facilitate product separation, as well as catalyst and cofactor recycling.8
An interesting strategy is the application of a pure substrate as the second phase, since the use of a non-miscible solvent as an additional organic component can be avoided, which, in consequence, further simplifies product separation and reduces the amount of waste. Therefore this concept was investigated for the Lactobacillus brevisalcohol dehydrogenase (LbADH)9-catalysed reductions of hardly-water soluble ketones (3-octanone, 2-octanone, 2-nonanone and 2-decanone) to their corresponding (R)-alcohols. Regeneration of the cofactor NADPH was carried out by a glucose dehydrogenase (GDH) from Bacillus sp. (Fig. 1). The tested substrates showed only limited solubility in aqueous phosphate buffer (7.4 mmol L−1 for 3-octanone, 7.9 mmol L−1 for 2-octanone, 2.1 mmol L−1 for 2-nonanone and 0.6 mmol L−1 for 2-decanone) and were therefore suitable for application as a non-reactive phase. The product alcohols could be easily separated from the aqueous phase by decantation. For analysis, all small-scale samples were extracted with hexane to allow quantitative recovery.
 | ||
| Fig. 1 LbADH-catalysed reduction of prochiral ketones with GDH-catalysed cofactor regeneration (for details of R1 and R2, see text). | ||
Initial batch experiments were carried out with substrate amounts corresponding to a concentration of 80 mmol L−1 (assuming the substrate would be dissolved in the overall reaction volume) and compared on the basis of space time yields (STY = amount of product produced per litre of reaction volume per day), turnover numbers (TON = amount of product per amount of catalyst or cofactor, respectively) and environmental factors (E factor = kg waste per kg product) (see Table 1). The time course of the reactions is presented in Fig. 2.
| (R)-3-Octanol | (R)-2-Octanol | (R)-2-Nonanol | (R)-2-Decanol | |
|---|---|---|---|---|
| STY/mmol L−1d−1 | 74.6 | 77.8 | 88.1 | 82.9 |
| STY/g L−1d−1 | 9.72 | 10.1 | 12.7 | 13.0 |
| TONADH/103 | 713 | 743 | 842 | 792 |
| TONGDH/103 | 16.3 | 17.0 | 19.3 | 18.1 |
| TONNADP+ | 677 | 705 | 799 | 752 |
| E factor | 117 | 112 | 89 | 87 |
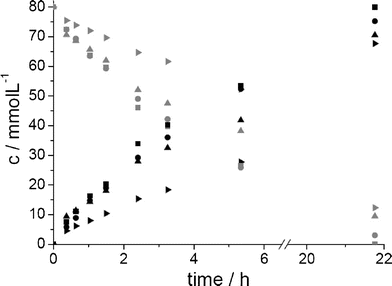 | ||
Fig. 2 Reaction progress for the LbADH-catalysed syntheses of chiral alcohols utilizing the substrate as the second phase (3-octanone , (R)-3-octanol ▶, 2-octanone , (R)-3-octanol ▶, 2-octanone , (R)-2-octanol ▲, 2-nonanone , (R)-2-octanol ▲, 2-nonanone , (R)-2-nonanol ■, 2-decanone , (R)-2-nonanol ■, 2-decanone and (R)-2-decanol ●). and (R)-2-decanol ●). | ||
Even though LbADH showed product inhibitions for all the alcohols,10 it was possible to achieve conversions of 84.6% for 3-octanone, 88.2% for 2-octanone, 99.9% for 2-nonanone and 94.0% for 2-decanone. Notably, only for 2-octanone was a substrate surplus inhibition found.10 This is most probably the reason for the low conversions compared to the other 2-ketones. Within all experiments, STYs of 75 to 83 mmol L−1d−1, respectively, and enantioselectivities (enantiomeric excess, ee) of > 99.9% were reached. Remarkably, the achieved STYs are similar to those obtained for syntheses with comparable TONs of about 90 mmol L−1d−1, where a biocompatible ionic liquid was applied as a solubiliser.10 However, when applying the substrate as a second phase, an additional organic component can be avoided; hence, facilitating downstream processing and thereby leading to reduced amounts of waste. When comparing the obtained E factors of 87 to 117 to those found in industry, a high potential for the development of an environmentally friendly process is apparent.11
Subsequently, for the synthesis of (R)-2-nonanol, reactions with 10-times increased biocatalyst concentrations and higher substrate concentrations (80, 100 and 150 mmol L−1) were carried out (Table 2). Within these batch experiments, conversions of at least 99.4% and ee values of >99.9% were achieved. Due to increased biocatalyst concentration, reduced TONs for both enzymes were found; nevertheless, the achieved values are still promising. Moreover, it was possible to improve the STY and TONNADP+ to reach industrially relevant values. Also, the E factors could be improved; by increasing the substrate concentration to 150 mmol L−1, an E factor of 54 was possible, which is within an acceptable range for fine chemical production.10
| c 2-nonanone /mmol L−1 | 80 | 100 | 150 |
|---|---|---|---|
| STY/mmol L−1d−1 | 881 | 889 | 814 |
| STY/g L−1d−1 | 127 | 128 | 117 |
| TONADH/103 | 83.8 | 105 | 157 |
| TONGDH/103 | 1.92 | 2.40 | 3.60 |
| TONNADP+ | 795 | 994 | 1 490 |
| E factor | 101 | 81 | 54 |
Conclusions
In conclusion, the application of the substrate itself as a second phase for biocatalytic reactions represents a straightforward method to enable environmentally friendly conversions of hardly-water soluble substrates. Applying this strategy to the LbADH-catalyzed syntheses of chiral aliphatic alcohols led to promising STYs, E factors and TONs for both the biocatalyst and the nicotinamide cofactor. Reaction engineering could further improve those syntheses.Acknowledgements
The authors thank the Federal Ministry of Economics and Technology via ZIM and DECHEMA for funding, as well as Prof. W. Leitner (RWTH Aachen University) for fruitful discussions.Notes and references
- H. P. Meyer, A. Kiener, R. Imwinkelried and N. Shaw, Chimia, 1997, 51, 287–289 CAS.
- M. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Kesseler, R. Sturmer and T. Zelinski, Angew. Chem., Int. Ed., 2004, 43, 788–824 CrossRef CAS.
- P. Galatsis, in Named Reactions for Functional Group Transformations, ed. J. J. Li and E. J. Corey, John Wiley & Sons, New Jersey, 2007, pp. 2–21 Search PubMed; A. Hirau, J. Chem. Soc. Chem. Commun., 1981, 315–317 Search PubMed; E. J. Corey, R. K. Bakshi, S. Shibata, C.-P. Chen and V. K. Singh, J. Am. Chem. Soc., 1987, 109, 7925–7926 RSC.
- T. Ohkuma, M. Koizumi, K. Muniz, G. Hilt, C. Kabuto and R. Noyori, J. Am. Chem. Soc., 2002, 124, 6508–6509 CrossRef CAS; T. Ohkuma, M. Koizumi, H. Doucet, T. Pham, M. Kozawa, K. Murata, E. Katayama, T. Yokozawa, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1998, 120, 13529–13530 CrossRef CAS; R. Noyori, Angew. Chem., Int. Ed., 2002, 41, 2008–2022 CrossRef CAS; R. Noyori and T. Ohkuma, Angew. Chem., Int. Ed., 2001, 40, 40–73 CrossRef CAS.
- K. Goldberg, K. Schroer, S. Lütz and A. Liese, Appl. Microbiol. Biotechnol., 2007, 76, 237–248 CrossRef CAS; K. Goldberg, K. Schroer, S. Lütz and A. Liese, Appl. Microbiol. Biotechnol., 2007, 76, 249–255 CrossRef CAS.
- T. Daussmann, T. C. Rosen and P. Dünkelmann, Eng. Life Sci., 2006, 6, 125–129 CrossRef; W. Hummel, Trends Biotechnol., 1999, 17, 487–492 CrossRef CAS.
- C. Kohlmann, L. Greiner, W. Leitner, C. Wandrey and S. Lütz, Chem.–Eur. J., 2009, 15, 11692–11700 CrossRef CAS; G. de Gonzalo, I. Lavandera, K. Durchschein, D. Wurm, K. Fabera and W. Kroutil, Tetrahedron: Asymmetry, 2007, 18, 2541–2546 CrossRef CAS; M. Eckstein, M. Villela Filho, A. Liese and U. Kragl, Chem. Commun., 2004, 1084–1085 RSC; H. Gröger, W. Hummel, C. Rollmann, F. Chamouleau, H. Hüsken, H. Werner, C. Wunderlich, K. Abokitse, K. Drauz and S. Buchholz, Tetrahedron, 2004, 60, 633–640 CrossRef CAS; H. Gröger, W. Hummel, S. Buchholz, K. Drauz, T. Van Nguyen, C. Rollmann, H. Husken and K. Abokitse, Org. Lett., 2003, 5, 173–176 CrossRef; W. Hussain, D. J. Pollard, M. Truppo and G. J. Lye, J. Mol. Catal. B: Enzym., 2008, 55, 19–29 CrossRef CAS.
- M. Eckstein, T. Daussmann and U. Kragl, Biocatal. Biotransform., 2004, 22, 89–96 CrossRef CAS.
- S. Leuchs and L. Greiner, Chem. Biochem. Eng. Q., 2011, 25, 267–281 Search PubMed.
- C. Kohlmann, N. Robertz, S. Leuchs, Z. Dogan, S. Lütz, K. Bitzer, S. Na’amnieh and L. Greiner, J. Mol. Cat. B, 2010, 67, 143–157 CrossRef.
- R. A. Sheldon, Chem. Commun, 2008, 3352–3365 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and analysis details. See DOI: 10.1039/c1gc15823f |
| This journal is © The Royal Society of Chemistry 2011 |
