 Open Access Article
Open Access ArticleEnzymatic acylation: assessing the greenness of different acyl donors
Monica
Paravidino
* and
Ulf
Hanefeld
Gebouw voor Scheikunde, Technische Universiteit Delft, Julianalaan, 136, 2628, BL, Delft, The Netherlands. E-mail: m.paravidino@tudelft.nl; u.hanefeld@tudelft.nl; Fax: +31 15278 1415; Tel: +31 15 278 5879
First published on 4th August 2011
Abstract
The hydrolase-catalyzed esterification of alcohols is the best established enzymatic transformation in today's organic chemistry, along with the corresponding ester hydrolysis. Over the years, various different acyl donors have been proposed to overcome the major limitation of the condensation of an alcohol and an acid, the unfavourable equilibrium. This review aims at screening the actual number of applications of the different acyl donors, and at assessing the “greenness” (or lack thereof) of the most applied among them. Indeed, the use of an enzyme to catalyze an esterification is often regarded as sufficient to define the whole transformation as “green”. However, this definition can easily be misinterpreted if the contribution of the acyl donor to the overall process is overlooked, as is often the case. Aiming at filling this gap, this contribution evaluates the advantages and disadvantages of the acyl donors, and assesses their green credentials using an efficient tool in strategic planning, a strengths-weaknesses-opportunities-threats (SWOT) analysis. A calculation of the atom economy and E-factor for representative acylations involving each donor is included, as well as an analysis of the adherence of each process to the twelve principles of Green Chemistry.
What is green about biocatalysis?
The use of biocatalysis in organic chemistry has come a long way, from academic curiosity a century ago to a standard practice even on an industrial scale today.1,2 One reason for this success is the recent trend towards Green Chemistry,3–6i.e. the need for more environmentally acceptable chemical processes. Indeed, biocatalysis performs well in the context of Green Chemistry, offering an environmentally benign catalyst (the enzyme), mild conditions and selectivity at different levels (chemo-, regio- and stereo-). As a result, enzymatic routes are often more attractive than the conventional counterparts from the environmental and economic standpoint.6 It is therefore no surprise that a significant number of publications describe enzymatic reactions as being “green”. However, the definition of every biocatalytic route as “green” per se is misleading. The use of an enzyme to catalyze a reaction is certainly a good starting point towards a more sustainable chemistry, but does not ensure the greenness of the whole process. Indeed, all aspects (amount and hazardousness of solvents, reagents and waste) have to be carefully taken into account.Hydrolases2,7,8 are a particularly well established class of enzymes and the reactions they catalyze (hydrolysis and formation of esters and amides) are often referred to as green and sustainable. However, while this is reasonably true for the hydrolysis, which utilises water as reagent/solvent, the same definition does not apply automatically to the ester and amide synthesis, which requires a suitable acyl donor9–11 and often an organic solvent. Indeed, the hydrolase-catalyzed formation of an ester from an alcohol and an acid (transesterification) is a reversible reaction. It is common practice to circumvent this problem, and drive the reaction to completion, by using activated acyl donors rather than acids. The leaving group X is in this case a weak nucleophile that cannot attack the ester formed (Scheme 1). Thus, a quantitative acylation of the alcohol is ensured.
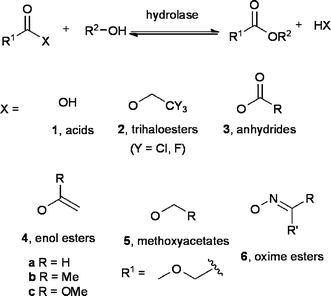 | ||
| Scheme 1 Illustrates the main acyl donors described in literature. | ||
Although important for determining the sustainability of the esterification, the impact of the acyl donor is usually neglected. With the aim to fill this gap, this contribution offers a guide to the choice of the most suitable acyl donors for esterification reactions for both the laboratory and industrial scale. The synthesis and applications of these acyl donors have already been described in some excellent reviews,9–11 to which the reader is referred. Here, the greenness of the most used of them (Table 1) is assessed in comparison with the baseline case, i.e. the condensation of an alcohol and an acid (Scheme 1, X = OH). In addition, the relative advantages and disadvantages of each reagent are evaluated. The green credentials of the acyl donors are evaluated against the relevant among the twelve principles3,4 of Green Chemistry, elegantly formulated by Anastas and Warner (Fig. 1). In particular, the 1st, 2nd, 3rd, 10th, and 12th principles apply to the use of acyl donors as reactant in an enzymatic esterification, and are therefore employed for the discussion here. Moreover, the life cycle assessment (LCA) for the different donors is reported whenever available, and additional criteria, viz. atom economy12 and E-factor,13 are introduced.
 | ||
| Fig. 1 The twelve principles of Green Chemistry. | ||
| Entry | Acyl donor | Number of applications |
|---|---|---|
| Data referred to the time-span 2007–present. Sources: Web of Science and Sci-Finder. | ||
| 1 | 1 | 4 |
| 2 | 2 | 2 |
| 3 | 3 | 22 |
| 4 | 4a | 152 |
| 5 | 4b | 9 |
| 6 | 4c | 1 |
| 7 | 5 | 7 |
| 8 | 6 | 1 |
Atom economy (or efficiency, eqn (1)) measures how many of the atoms of the starting material end up in the desired product, and is reported as a percentage. The higher the value, the better the economy. However, it should be noted that this parameter does not allow for the effective yield of a reaction nor the need for an excess of reagents, but rather assumes 100% yield, and is based solely on the stoichiometric equation.
 | (1) |
A clearer picture of the greenness of a process can be obtained by calculating its E-factor (eqn (2)), which defines all the compounds that are not product as waste. A truly green reaction has an E-factor close to 0, in accordance to the 2nd principle of Green Chemistry.3
 | (2) |
Finally, a strengths-weaknesses-opportunities-threats (SWOT)14 analysis is presented for each case. This tool provides an overview of the relative advantages and disadvantages of acyl donors and indicates potential directions for improving the process.
Baseline: carboxylic acids
The enzymatic condensation of an alcohol and an acid needs to be performed under continuous removal of the water, in order to shift the equilibrium towards the product. On a laboratory scale, this protocol (which requires obviously high temperatures) is scarcely applied, and the preference is given to the use of activated acyl donors generally under milder conditions. However, the use of carboxylic acids as acylating agents in enzymatic reactions find some very interesting applications on a large scale. One such example (Scheme 2)15 is the synthesis of polyester in preparative scale using glycerol and adipic acid. A feasibility study for the industrial production of this polymer showed that this enzymatic polymerization can be successfully performed at 60 °C and 20 mbar with only 3 wt% of Cal-B (Novozym 435). Conversions above 90% are obtained with a space time yield of 370 g d−1 L−1, which results in an extremely low E-factor (0.35). Moreover, the atom economy is extremely high (97%), as expected for a condensation leading to water as the only byproduct. | ||
| Scheme 2 | ||
The applicability of acids at industrial level was further demonstrated by the preparation of emollient esters such as myristyl myristate reported by Evonik researchers in collaboration with the group of Liese (Scheme 3).16 The esterification could be performed without solvent, mixing equimass amounts of reactants at 75 °C in the presence of Novozym 435. An outstanding space time yield of 6731 g d−1 L−1 was achieved for myristyl myristate.
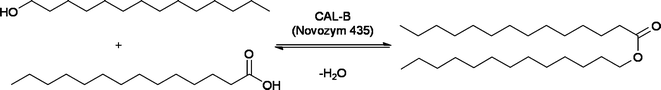 | ||
| Scheme 3 | ||
As summarized in the SWOT analysis (Fig. 2), carboxylic acids are more benign and environmentally friendly than other common acyl donors (e.g., trifluoroesters, vinyl acetate),17 as they are characterized by a lower flammability and explosion hazard, and do not form harmful waste. They show a good compliance to the 1st, 2nd, 3rd, and 10th principle of Green Chemistry, and should therefore be preferred to activated (and more toxic) acyl donors whenever possible. On the other hand, carboxylic acids suffer often from a limited solubility; therefore, the condensation with alcohols might require the use of an excess of alcohol or of a solvent.
 | ||
| Fig. 2 SWOT analysis for carboxylic acids. | ||
Trihaloesters
2,2,2-Trichloroethyl groups were introduced as activated acyl donors in the early 1990s in the first acylation studies in organic solvents.18 The activation is poor8 and transesterifications are therefore slow. As a consequence, only limited examples (none in the last five years) can be found in literature where this leaving group is used.2,2,2-Trifluoroethyl esters 2b are slightly more activated than their trichloro counterparts, but are still significantly less used than vinyl esters.19,20 Their synthesis is easily accomplished either viacarbodiimide coupling of acids and alcohols21,22 or using acid chlorides and the alcohol.19,23–25
A few years ago, the dynamic kinetic resolution of a series of allylic alcohols using 2,2,2-trifluoroethyl butanoate in the presence of Subtilisin-CLEC and a Ru complex as the racemization catalyst was reported.20 For the example reported in Scheme 4, an atom economy of 68.6% and an E-factor of 11.8 were obtained. Overall, the process is quite poor.
 | ||
| Scheme 4 | ||
The byproduct (trifluoroethanol) gets a relatively good score in the LCA (life cycle assessment) and has a low reactivity, but raises serious issues from the health and waste points of view (Fig. 3), as pointed out in a recent assessment of common organic solvents.17 Indeed, it is a harmful and non-biodegradable substance, which is against what the relevant principles of Green Chemistry advise. Therefore, its use and generation should be avoided, also in consideration of possible future health related regulations.
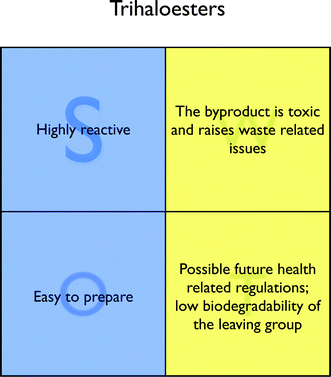 | ||
| Fig. 3 SWOT analysis for trihaloesters. | ||
Anhydrides
Although acyclic anhydrides have been applied early in enzymatic reactions,26 they find moderate application in enzyme-mediated ester synthesis (see Table 1). Their ability to acylate the enzyme, deactivating it, as well as the competing, unselective background reaction, and the occurrence of undesired side reactions related to the acid released account for their limited use.9–11 Moreover, anhydrides are irritating. The SWOT table summarizes all these aspects.Nonetheless, cyclic anhydrides (e.g., succinic anhydride) are more often used for the kinetic resolution of alcohols than their acyclic equivalent, even on an industrial scale.27 Indeed, they offer the advantage of an easy separation of the product monoester (through extraction with bases) from the unreacted alcohol. Moreover, acylations with cyclic anhydrides have an atom economy of 100%, although it must be taken into account that the hemiester obtained is normally not the desired final product, and an additional step (the hydrolysis) has to be performed.
One such example28 (Scheme 5) is given by the resolution of (R,S)-N-(tert-butoxycarbonyl)-3-hydroxymethylpiperidine with succinic anhydride and BCL.
 | ||
| Scheme 5 | ||
The (S)-hemisuccinic ester was easily separated and hydrolyzed to give 7, intermediate in the preparation of a tryptase inhibitor. Reiteration of the process allowed 7 to be obtained in 32% yield and 98.9% ee. A calculation of the E-factor for the first cycle only gives a value of 15, which makes the process quite poor.
In conclusion, cyclic anhydrides show a very good agreement with the 1st, 2nd, 3rd and 10th principle of Green Chemistry, and appear therefore as relatively green acyl donors (Fig. 4). On the other hand, acyclic anhydrides perform worse from the atom economy and waste generation standpoint, and are therefore a less convenient choice.
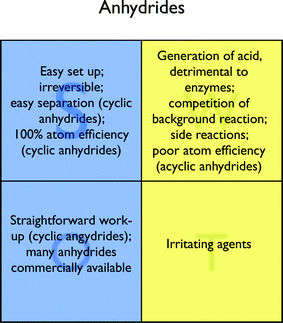 | ||
| Fig. 4 SWOT analysis for anhydrides. | ||
Enol esters
By far the best activated and most used acyl donors on a small scale (see Table 1) are enol esters such as vinyl acetate (VA, 4a, R = H), isopropenyl acetate (IPA, 4b R = Me) and ethoxyvinyl esters (4c, R = OMe).29–31 The leaving group is an enol that immediately tautomerizes to the ketoform (Scheme 6). Thus, no nucleophile remains and the reaction becomes irreversible. Some vinyl esters and IPA are commercially available, since they are building blocks in polymer chemistry and are used as acylating agents in non-enzymatic reactions as well. Pd- or acid catalyzed transesterifications ensure the conversion of these bulk chemicals into the desired vinyl or isopropenyl esters.11Alkoxyvinyl esters can also be synthesized from the corresponding acid and acetylene using different Ru catalysts.32,33 These acyl donors (especially the cheap VA) are often used in large excess, thus making the use of a solvent unnecessary. | ||
| Scheme 6 | ||
When comparing their SWOT analyses, VA emerges as the cheapest and the most reactive, but these advantages are severely undermined by a serious drawback, i.e. the generation of a stoichiometric amount of acetaldehyde. Not only can acetaldehyde deactivate some lipases (CRL and GCL) by formation of imine bonds with the lysine residues, it also presents major process obstacles,34 such as the low flash (−40 °C) and boiling (21 °C) points and the explosive properties of air–acetaldehyde mixtures.35 Moreover, acetaldehyde is an irritating agent36 that has been classified as an inhalation carcinogen37 (LD50 = 661 mg kg−1) a few years ago. Another safety hazard is represented by the potential of VA to undergo exothermic polymerization in the gas phase.38 This risk should be taken into account whenever VA is used in large excess and removed by distillation at the end of the reaction, as is the case, among others, in the synthesis of more hindered vinyl esters donors. For all these reasons, only very few reports on the scale-up of vinyl esters mediated acylations can be found in the literature.39,40 In one such example from Roche (Scheme 7),40 VA is used for the Chirazyme L-2 mediated acetylation of an intermediate for Vitamin A synthesis. At 100% conversion, 1.6 kg of desired product are prepared per day, corresponding to an acceptable E-factor of 8.12. The addition of EDTA and of ppm of an organic base and an antioxidant to the reaction mixture to protect the enzyme against degradation could not be taken into account in the calculation.
 | ||
| Scheme 7 | ||
When using IPA or ethoxyvinyl acetate,41 the byproducts are acetone and ethyl acetate, respectively. Since both compounds are unreactive towards lysine,9 no enzyme deactivation is observed. Moreover, they are significantly more benign than acetaldehyde.36 However, IPA and ethoxyvinyl acetate suffer from some of the same disadvantages (hazardous traditional synthesis, polymerization issue) as VA, and no scale up reaction have been reported so far. Although a relatively mild synthesis for alkoxyvinyl esters was proposed in 1993,33 no application on a large scale are known, probably due to the cost of the Ru catalyst.
The atom economy of acylations with enol esters is generally poor (50–60%) when small alcohols are used (Fig. 5–7), but improves when increasing the molecular weight of the acyl acceptor. Obviously, it is higher for VA than for IPA and ethoxyvinyl esters.
 | ||
| Fig. 5 SWOT analysis for vinyl acetate. | ||
 | ||
| Fig. 6 SWOT analysis for isopropenyl acetate. | ||
 | ||
| Fig. 7 SWOT analysis for ethoxyvinyl esters. | ||
Alkyl methoxyacetates
Ethylmethoxyacetate was introduced by BASF researchers in the 1990s42 for the enzymatic resolution of chiral amines. Since then, esters of methoxyacetic acid have been playing an important role in the large-scale production of optically pure aliphatic and benzyl amines, and amino alcohols.27,43–45 The methoxy substituent remarkably enhances the carbonyl reactivity, so that the initial acylation rate for ethylmethoxyacetate is 100 times faster than the corresponding reaction with, for example, ethyl butyrate.46 Additionally, these acyl donors are unrivaled in terms of selectivity, which is especially increased when using esters of secondary alcohols like isopropyl methoxyacetate.44In a typical procedure (Scheme 8),42 equimolar amounts (165 mmol) of 1-phenylethylamine and ethylmethoxy acetate are converted in MTBE in the presence of 2 g of Burkholderia plantariilipase. At 52% conversion, the reaction affords 48% of (R)-amide in 93% ee. The procedure is characterized by a quite high atom economy (80.7%), and an E-factor as high as 11.1 (Fig. 8).
 | ||
| Scheme 8 | ||
 | ||
| Fig. 8 SWOT analysis for alkyl methoxyacetates. | ||
Oxime esters
Oxime esters, first reported in 1989,47 have scarcely been used in the last 5 years. They are generally considered irreversible acyl donors, even though a reversible behaviour has been sometimes reported.10 Besides the simple acetone oxime,48,49 more complex oxime esters have been recently proposed34 and proved even more successful. Their preparation involves the DCC coupling of oximes and acids or the condensation of oximes and acid chlorides.11An interesting example of industrial application of an oxime ester50 is the acylation of the primary alcohol of Ribavirin with Cbz-protected L-alanyl oxime ester in the presence of CAL-B (Chirazyme) applied by Schering-Plough on a pilot scale (Scheme 9).
 | ||
| Scheme 9 | ||
The acyl donor was prepared in situ by coupling acetone oxime and Cbz-Ala in the presence of di-tert-butyl dicarbonate in THF, and directly reacted with ribavirin. The atom economy for the esterification is 86.1%, but the E factor for the overall process is as high as 33.2, which reflects the generation of a large amount of waste (Fig. 9).
Conclusions
When performing an enzymatic esterification on a large scale, the simple carboxylic acid appears still as the best acyl donor from the Green Chemistry perspective. It has a very good atom economy, generates water as the only waste and shows a relatively good environmental impact. Besides, a very broad range of acids are readily available, which can be employed directly. However, this advantage is counterbalanced by the necessity to continuously remove the water formed in order to shift the unfavourable equilibrium.On a small scale, the use of an activated donor rather than the simple acid is still preferred, as witnessed by the current literature (Table 1). However, when assessing all the reagents available for their “greenness”, the popularity of vinyl acetate (and, to a lesser extent, of enol esters in general) seems largely undeserved. VA does not comply with any of the relevant Green Chemistry principles considered here. The safety issues related to its synthesis and, above all, the generation of the environmentally unfriendly and harmful acetaldehyde, in our opinion, call for the search for more sustainable alternatives. Equally serious drawbacks from a green perspective affect the use of trihaloesters.
Alkyl methoxyacetates emerge as a very good choice for the acylation of amines. The high reactivity and selectivity are accompanied by a satisfying atom economy, a relatively easy synthesis, and the formation of simple alcohols as byproducts. Unfortunately, the unfavourable equilibrium makes alkyl methoxyacetates inapplicable to the esterification of alcohols. In this case, cyclic anhydrides should be preferred whenever possible, as they are characterized by 100% atom economy and simplify the separation of the desired product from the unreacted alcohol. In this respect, they offer more advantages than oxime esters, too. Indeed, the latter are also characterized by a good atom economy and by the formation of relatively benign byproducts, but suffer from a laborious synthesis and the difficult separation of the oxime that is used in excess.
Overall, the analysis performed here confirms that to define any enzymatic process as green without evaluating the actual impact of the reagents involved is misleading. As demonstrated here, greener alternatives to the frequently used enol esters are available. In particular, carboxylic acids represent a much more sustainable choice, provided that an efficient water removal protocol is applied. Clearly the key to implementing a truly green process resides not only in the underlying chemistry, but also in the process engineering. The combination of both fields is a powerful tool opening up novel opportunities towards Green Chemistry.
Acknowledgements
Dr V. R. Calderone is gratefully acknowledged for inspiring the graphical part of this article.Notes and references
- W. D. Fessner and T. Anthonsen, Modern Biocatalysis: stereoselective and environmentally friendly reactions, Wiley-VCH, Weinheim, 2009 Search PubMed.
- A. Liese, K. Seelback and C. Wandrey, Industrial Biotransformations, Wiley-VCH, Weinheim, 2006 Search PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A–101A CrossRef.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–693 CrossRef CAS.
- R. A. Sheldon, I. W. C. E. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, 2007 Search PubMed.
- U. T. Bornscheuer and R. J. Kazlauskas, Hydrolases in Organic Synthesis, Wiley-VCH, Weinheim, New York, Chichester, Brisbane, Singapore, Toronto, 2005 Search PubMed.
- H. Stecher and K. Faber, Synthesis, 1997, 1–16 CrossRef CAS.
- R. Chênevert, N. Pelchat and F. Jacques, Curr. Org. Chem., 2006, 10, 1067–1094 CrossRef.
- K. Faber and S. Riva, Synthesis, 1992, 895–910 CrossRef CAS.
- U. Hanefeld, Org. Biomol. Chem., 2003, 1, 2405–2415 CAS.
- B. M. Trost, Science, 1991, 254, 1471–1477 CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- R. C. Appleby, Modern Business Administration, Pearson Education Ltd, 1994 Search PubMed.
- C. Korupp, R. Weberskirch, J. J. Muller, A. Liese and L. Hilterhaus, Org. Process Res. Dev., 2010, 14, 1118–1124 CrossRef CAS.
- L. Hilterhaus, O. Thum and A. Liese, Org. Process Res. Dev., 2008, 12, 618–625 CrossRef CAS.
- R. K. Henderson, C. Jimenez-Gonzalez, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- A. M. Klibanov, Acc. Chem. Res., 1990, 23, 114–120 CrossRef CAS.
- C. M. Monteiro, N. M. T. Lourenço and C. A. M. Afonso, Tetrahedron: Asymmetry, 2010, 21, 952–956 CrossRef CAS.
- M.-J. Kim, H. K. Lee and J. Park, Bull. Korean Chem. Soc., 2007, 28, 2096–2098 CrossRef CAS.
- N. Baba, M. K. Alam, Y. Mori, S. S. Haider, M. Tanaka, S. Nakajima and S. Shimizu, J. Chem. Soc., Perkin Trans. 1, 2001, 221–223 RSC.
- T. Miyazawa, S. Nakajo, M. Nishikawa, K. Hamahara, K. Imagawa, E. Ensatsu, R. Yanagihara and T. Yamada, J. Chem. Soc., Perkin Trans. 1, 2001, 82–86 RSC.
- V. K. Aggarwal, D. E. Jones and A. M. Martin-Castro, Eur. J. Org. Chem., 2000, 2939–2945 CrossRef CAS.
- S. Gedey, A. Liljeblad, L. Lázár, F. Fülöp and L. T. Kanerva, Tetrahedron: Asymmetry, 2001, 12, 105–110 CrossRef CAS.
- X. G. Li and L. T. Kanerva, Org. Lett., 2006, 8, 5593–5596 CrossRef CAS.
- D. Bianchi, P. Cesti and E. Battistel, J. Org. Chem., 1988, 53, 5531–5534 CrossRef CAS.
- A. Schmid, J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts and B. Witholt, Nature, 2001, 409, 258–268 CrossRef CAS.
- A. Goswami, J. M. Howell, E. Y. Hua, K. D. Mirfakhrae, M. C. Soumeillant, S. Swaminathan, X. H. Qian, F. A. Quiroz, T. C. Vu, X. B. Wang, B. Zheng, D. R. Kronenthal and R. N. Patel, Org. Process Res. Dev., 2001, 5, 415–420 CrossRef CAS.
- M. Degueil-Castaing, B. De Jeso, S. Drouillard and B. Maillard, Tetrahedron Lett., 1987, 28, 953–954 CrossRef CAS.
- Y. F. Wang, J. J. Lalonde, M. Momongan, D. E. Bergbreiter and C. H. Wong, J. Am. Chem. Soc., 1988, 110, 7200–7205 CrossRef CAS.
- S. Raucher and B. L. Bray, J. Org. Chem., 1987, 52, 2332–2333 CrossRef CAS.
- T. Mitsudo, Y. Hori, Y. Yamakawa and Y. Watanabe, Tetrahedron Lett., 1986, 27, 2125–2126 CrossRef CAS.
- Y. Kita and S. Akai, Chem. Rec., 2004, 4, 363–372 CrossRef CAS.
- T. Storz, J. Gu, B. Wilk and E. Olsen, Tetrahedron Lett., 2010, 51, 5511–5515 CrossRef CAS.
- L. G. Britton, American Institute of Chemical Engineers, 1998, pp. 138–148.
- http://chemiekaarten.sdu.nl/chkonline/ .
- R. Gomes and M. E. Meek, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2001, 19, 1–21 Search PubMed.
- H. Reiss and M. A. Chowdhury, J. Phys. Chem., 1984, 88, 6667–6670 CrossRef CAS.
- B. Orsat, B. Wirz and S. Bischof, Chimia, 1999, 53, 579–584 CAS.
- W. Bonrath, R. Karge and T. Netscher, J. Mol. Catal. B: Enzym., 2002, 19–20, 67–72 CrossRef CAS.
- D. Klomp, K. Djanashvili, N. C. Svennum, N. Chantapariyavat, C.-S. Wong, F. Vilela, T. Maschmeyer, J. A. Peters and U. Hanefeld, Org. Biomol. Chem., 2005, 3, 483–489 CAS.
- F. Balkenhohl, K. Ditrich, B. Hauer and W. Ladner, J. Prakt. Chem., 1997, 339, 381–384 CrossRef CAS.
- M. Höhne and U. T. Bornscheuer, ChemCatChem, 2009, 1, 42–51 CrossRef.
- K. Ditrich, Synthesis, 2008, 2283–2287 CrossRef CAS.
- M. Breuer, K. Ditrich, T. Habicher, B. Hauer, M. Keßeler, R. Stürmer and T. Zelinski, Angew. Chem., Int. Ed., 2004, 43, 788–824 CrossRef CAS.
- M. Cammenberg, K. Hult and S. Park, ChemBioChem, 2006, 7, 1745–1749 CrossRef CAS.
- A. Ghogare and G. S. Kumar, J. Chem. Soc., Chem. Commun., 1989, 1533–1535 RSC.
- R. Pulido and V. Gotor, Carbohydr. Res., 1994, 252, 55–68 CAS.
- F. Moris and V. Gotor, J. Org. Chem., 1993, 58, 653–660 CrossRef CAS.
- M. Tamarez, B. Morgan, G. S. K. Wong, W. Tong, F. Bennett, R. Lovey, J. L. McCormick and A. Zaks, Org. Process Res. Dev., 2003, 7, 951–953 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |

