Quality control of plant food supplements†
Elisabetta
Sanzini
*a,
Mihaela
Badea
b,
Ariana Dos
Santos
c,
Patrizia
Restani
c and
Hartwig
Sievers
d
aDept. Veterinary Public Health and Food Safety, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161, Rome, Italy. E-mail: elisabetta.sanzini@iss.it; Fax: +39 0649387101; Tel: +39 0649902408
bTransilvania University of Brasov, Faculty of Medicine, Str. Nicolae Balcescu Nr 56, 500019, Brasov, Romania. E-mail: mbadeaplantlibra@yahoo.com; Fax: +40 268412185; Tel: +40 724103073
cPhytoLab GmbH & Co. KG, 91487, Vestenbergsgreuth, Germany. E-mail: hartwig.sievers@phytolab.de; Fax: +49 9163 88-349; Tel: +49 9163 88-154
dDept. Pharmacological Sciences, Università degli Studi di Milano, via Balzaretti 9, 20133, Milano, Italy. E-mail: ariana.santos@unimi.it; patrizia.restani@unimi.it; Fax: +39 0250318284; Tel: +39 0250318350/371
First published on 17th August 2011
Abstract
It is essential to guarantee the safety of unprocessed plants and food supplements if consumers' health is to be protected. Although botanicals and their preparations are regulated at EU level, at least in part, there is still considerable discretion at national level, and Member States may choose to classify a product either as a food supplement or as a drug. Accurate data concerning the finished products and the plant used as the starting point are of major importance if risks and safety are to be properly assessed, but in addition standardized criteria for herbal preparation must be laid down and respected by researchers and manufacturers. Physiologically active as well as potentially toxic constituents need to be identified, and suitable analytical methods for their measurement specified, particularly in view of the increasing incidence of economically motivated adulteration of herbal raw materials and extracts. It remains the duty of food operators to keep up with the scientific literature and to provide sufficient information to enable the adaptation of specifications, sampling schemes and analytical methods to a fast-changing environment.
1 Introduction
In the European Community the main regulations that are relevant in this field are Directive 2002/46/EC1 on food supplements and Directive 2004/24/EC2 on traditional herbal medicinal products for human use.At present, botanicals and botanical preparations are partially harmonised at EU level (among others: Directive 2002/46/EC1 and EC Regulations 396/2005/EC,3 1881/2006/EC,4 and 1925/2006/EC5), but there is still considerable discretion at national level.
For the consumer, quality of a product means suitability for use, reliability, efficacy, and above all its safety. Elements that may affect the safety of plant food supplements (PFS) are:
– the presence of toxic compounds;
– the presence of pharmacologically active substances;
– the presence of addictive or psychotropic substances;
– adverse reactions to, and drug interactions with, otherwise non toxic substances;
– genetic variants among the plant species;
– differences in processing and manufacturing conditions.
Some other problems are addressed in this section:
– misidentification of the initial plant source;
– adulteration with other plants;
– environmental contamination (e.g., with heavy metals and pesticide or herbicide residues);
– biological contamination (mycotoxins, micro-organisms);
– the addition of illegal substances.
2 General safety considerations
Labelling of a herbal supplement as “natural” does not mean it is guaranteed not to have harmful effects. For example, the herbs kava (Piper methysticum) and comfrey (Symphytum officinale) have been linked to serious liver damage.6,7Data about the finished products and the quality of the botanical used for PFS are of major importance for risk assessment and safety evaluation. As not all the active ingredient(s) in herbal supplements have to be identified, it is important for researchers and manufacturers to have and to respect standardization criteria for PFS preparations. An important approach is to specify the constituents, which can be used as markers for monitoring quality. They must be measured using defined analytical methods.8 Even if a marker is not linked to bioactivity or a therapeutic effect, it can function as an index of product consistency and quality control.9
A large number of chemical compounds in the plant may be needed to provide the desired physiological effect in humans, while others may exert a number of undesirable effects, or none. Many botanical constituents are actually toxins synthesized by the plant in order to ward off predators and parasites; and some botanical chemical compounds may act in exact opposition to the principal active ingredient.
Botanical food supplements may be derived from secondary food sources (e.g., soy extracts containing isoflavones, tomato extracts rich in lycopene) or derived directly from herbs and spices (e.g. garlic oil, rosemary extracts, green tea extracts). The nature (species, part of the plant used), their preparation and conditions of use (length of time, periodicity of use) determine their impact on safety. For example, some PFS may be intended to be used only for short periods, e.g. for weight control or stimulation of the immune system in winter,8 and may be harmful if used longer.
The growing conditions (adherence to good agricultural practice, limitation of pesticide usage) and storage conditions (humidity, temperature) are also important as safety criteria. In complex combinations of PFS, herbs may interact with each other, or with other drugs or nutrients. Herbal supplements can, like drugs, cause medical problems if not used correctly or if taken in large amounts. Even then, some consumers can experience negative effects even when they follow the instructions on the PFS label. Allergic reactions may occur, which are hard to predict because they are idiosyncratic.
Other factors to be taken into account are the part of the plant used for preparation (root, leaves, flower, fruits), the details of extraction procedures (boiling water, lipophilic solvents, the length of time of extraction) and type of final product (pill, capsule, tablet, or liquid). The content and nature of biologically active compounds of PFS may be quite different if these factors are changed. A product may also contain, as contaminants, parts of other herbs not mentioned on the label, which may not accurately reflect the herbal content of the product. Microbiota have been identified in syrups or infusions made from dried plant blends.10
It is not possible to give a simple checklist of tests that will be appropriate for establishing the safety of botanical food supplements. Some authors have proposed a decision tree as an aid to safety evaluation.8
Crucial to a successful strategy for assessing quality is the isolation of purified reference standards of active ingredients, botanical adulterants and contaminants, and the standardization of accessible analytical techniques for specific identification. Fingerprints of plant extracts (using HPLC or LC-MS) are also recommended. These techniques provide both qualitative and quantitative information concerning primary active compounds, their secondary metabolites and indications of purity of the original source material. Unexpected peaks may indicate contamination or adulteration.9
However, the safety of botanical preparations should primarily be assessed on the grounds of toxicity and the effect on consumers of prolonged exposure.11Fig. 1 shows determinants of herbal food supplements as well as the assessment of efficacy.10,12
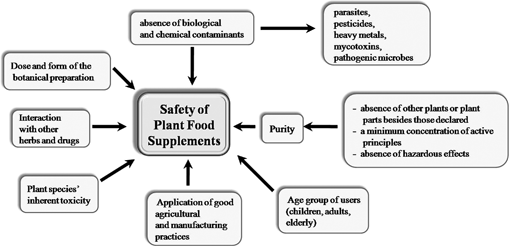 | ||
| Fig. 1 Determinants of safety and efficacy of herbal food supplements. | ||
There have been few randomized controlled studies of adverse effects of most of the herbal ingredients currently available, so clinicians are left to rely on case reports, passive surveillance, and small clinical trials if they are to give advice on the safe use of PFS.13
Due to the increasing pollution of air, water, soil, the herbal products may be contaminated; as a consequence their composition, quality and safety must be controlled.14,15
3 Correct identification of plant source
The production of safe botanical food supplements of high quality begins with plants of the correct species. Plants intended for use in food supplements should be cultivated and harvested using good agricultural practices, and field-collected material should be acquired using good collection practices. The WHO Programme on Traditional Medicines has published guidelines for good agriculture and collection practice in the acquisition of quality botanicals.16 If wild plant specimens are collected or if the plant material or extracts are purchased from suppliers without assurance of good agricultural practice, they should be assayed more carefully.First, the considered plant should be completely and accurately identified, as follows:15,17
– scientific name (plant family, genus, species with name of authority, and if relevant, variety, and chemotype);
– common name;
– part(s) of the plant used; and
– geographic origin.
Secondly, each batch of plants used in the production should be identified using taxonomic examination (macroscopic and/or microscopic) and/or a biochemical or chemical test. The several established methods for authentication, standardization, and quality assurance include plant taxonomic identification, morphological and microscopic examination, fingerprint chromatography, DNA molecular marker characterization, and immunoassay of species-specific proteins.12,18,19
Macroscopic identification is best done on the intact whole plant during collection or harvesting, when the appearance, characteristic smell or stinging properties can be examined and the presence of contaminants, such as sand, gravel or morphologically distinct plant material noted.20–26 However, macroscopic examination may not be sufficient to identify the species or distinguish subtle sub-species differences in chemotype or ecotype.
Examination under the microscope, on the other hand, can assess whole, fragmented, or powdered plant material, including characteristic hairs (e.g. glandular or stellate), cell types, fibres and granular objects (e.g.starch grain, calcium oxalate crystals), as well as minute floral and fruit characteristics. Microscopic characteristics of the most important plants have been described, but today professional microscopists trained in the analysis of botanical materials are rare, and published reference standards are not easy to access.27–29
‘Fingerprinting’ uses chromatographic and/or spectroscopic profiling: high performance liquid chromatography (HPLC), thin layer chromatography (TLC), high performance TLC (HPTLC), or gas chromatography (GC), or Fourier transform infrared (FTIR), near infrared (NIR), or nuclear magnetic resonance (NMR) spectrometry. ‘Chemical fingerprinting’ takes a group of chemicals, which are known to be present in and also absent from a particular plant and compares its profile with that of an extract from the plant (a) to identify and classify the plant or material, (b) to check the quality of material or product, or (c) to assess its purity and identify the adulterants; it may even quantify them.30
DNA fingerprinting has recently been strongly advocated: the genetic composition of each species is unique and is not affected by age, physiological conditions or environmental factors. DNA can be extracted from fresh or dried organic tissue. Molecular genetic tools, like barcoding, random amplified polymorphic DNA (RAPD) and sequence characterized amplified region (SCAR) markers are reliable in quality control of products. RAPD can be used to identify plant raw material but it is difficult to reproduce the fingerprints and they are better converted to SCAR markers. The limitations mean that DNA analysis has so far been confined to academia. Furthermore, with regard to PFS production, it is important to realise that the DNA fingerprint is the same in every part of the plant, but the phytochemical content is not, and this content also depends on the growing conditions and the environment of the plant in general.31,32
4 Quality control of adulterants and naturally occurring contaminants of concern
Among the large number of plant species marketed as health related products in the EU, few are cultivated on a large scale, but they account for 80% of the volume. Most commercialized herbs are gathered from the wild, but the resultant products constitute only 20% of the volume. Thus, in controlling the quality of botanical raw materials consideration should of course be given to the conditions of (controlled) cultivation, but even more attention should be paid to the less controllable conditions of wild collection besides the habitat, country of origin, storage and transport.The most important contaminants of herbal raw materials are pesticides, heavy metals, mycotoxins and microbiological contaminants, such as bacteria, moulds or yeasts. Recently, other groups of substances have been recognised as important: polyaromatic hydrocarbons (PAH). For some of these contaminants regulations are in place, limiting their content to technically achievable and toxicologically acceptable levels. Most contaminants are present in herbal raw materials only in μg kg−1 to mg kg−1 concentrations and dried herbal materials present a very challenging matrix, so that the most advanced analytical technology is needed to quantify them reliably. In addition, toxic substances may originate from different parts of the plant in question or from other species. Adequate and effective control of both contaminants and toxic plant substances requires adequate sampling schemes, and in the case of spot contaminants (e.g., mycotoxins and micro-organisms), extensive sampling schemes.
4.1 Pesticides
Regulation (EC) 396/2005 establishes a regime for setting and controlling maximum residue levels (MRLs) of pesticides in food and feedstuffs.3 In Annex I of the regulation, food is divided into categories. There is no specific category for herbal food supplements, but herbal raw materials may be assigned to one of the categories “Vegetables, fresh or frozen”, “Tea, Coffee, Cocoa, Herbal Infusions” or “Spices”, as appropriate, see Regulation (EC) 178/2006.33 Provisional harmonised MRLs for known active substances are promulgated in Annex III to the regulation on the basis of information about national MRLs submitted by member states. Assessments of the European Food Safety Authority (EFSA) will form the basis of proposals to change MRLs. A default MRL of 0.01 mg kg−1 applies to products for which no specific MRL has been set.34,35Analytical procedures used for pesticide analysis must be validated according to SANCO/10232/2006.36 In particular, they must satisfy the following criteria:
– the method is suitable for the pesticide residue/substance to be analysed and not susceptible to interference from co-extractives;
– natural occurrence of some constituents is considered in the interpretation of results;
– 70–110% of each pesticide should be recovered.
For routine pesticide analysis the standard methods EN 1239337 and EN 12396-338 may be used, which are commonly established in laboratories specialized in contaminant analyses. In assigning MRLs to specific herbs, until there are more than a few herb-specific MRLs in Annexes II and III, the allocation list of the European Herbal Infusions Association (EHIA) will provide a valuable tool.39
4.2 Heavy metals
Toxic metals found in herbal raw materials include lead (Pb), cadmium (Cd), mercury (Hg) and arsenic (As). Regulation (EC) No. 629/200840 sets maximum levels in the EU for Pb, Cd and Hg in food supplements. For most commercialized herbs these maximum levels can be observed. Higher levels may exceptionally be acceptable provided that product safety is assured.41,42Suitable measurement methods in herbal raw materials are provided in the European Pharmacopoeia:43atomic absorption spectroscopy (AAS) and inductively coupled plasma (ICP) techniques are the methods of choice in the herbal industry.14
4.3 Mycotoxins
Mycotoxins are chemically diverse compounds produced by moulds. Mould spores are present in all natural environments, but their germination and growth require highly specific conditions of humidity, light and temperature. Significant mould counts or elevated levels of mycotoxins in a product or plant source indicate inappropriate post-harvesting conditions either in drying, transport or storage processes. Since moulds and mycotoxins typically occur in isolated locations, reliable detection requires extensive sampling. For dried herbs and spices no commonly agreed or official sampling scheme is available. Those established for peanuts, cereals or cocoa beans may provide an approach.The most commonly found mycotoxins are the aflatoxins, which may be found in a variety of foods and herbal raw materials. Other mycotoxins like patulin are restricted to certain types of commodities: patulin is typical for apples and other fruits but is rarely found in leafy herbs. In the EU, fourteen mycotoxins have been accorded specific maximum levels in food, but only in a few herbal raw materials, namely ginger (Zingiber officinale), curcuma (Curcuma petiolata), licorice (Glycyrrhiza glabra) and capsicum species.4,44 There is no official method for the reliable quantification of all 14 EU-regulated mycotoxins, but the European Pharmacopoeia provides validated methods for aflatoxins B1, B2, G1, G2 and for ochratoxin A, which are widely used in the herbal industry.45,46
It remains the responsibility of the food operator to assess whether to test specific herbs for mycotoxins and, if found, whether the detected levels might represent a health risk to consumers in general, infants, pregnant women, or people with kidney or liver insufficiency. SCF and EFSA Scientific Opinions on the toxicological assessment of various mycotoxins provide helpful guidance.
For patulin and the fusarium toxins fumonosin B1, B2, zearalenone and deoxynivalenol, official EN methods are available, mostly for application to cereals or corn products. However, the basic approach, i.e., using standardized sample preparation, chromatographic conditions and detection method, has also been shown to be suitable for herbal raw materials, with any necessary adaptations for the specific matrix. Many methods to measure fusarium toxins have been published. Taken together, published methods allow adequate analytical control of the mycotoxins regulated for food content in the EU.
The absolute amount of herbal material or herbal preparations consumed in food supplements is usually small and poses only low risk. However, the spot-contaminant nature of mycotoxins needs to be taken into account in monitoring herbal sources.
4.4 Micro-organisms
Any plant growing in a natural environment is colonized with micro-organisms, including bacteria, microalgae, yeasts and moulds, so that micro-organisms detected on herbal raw materials are not necessarily contaminants in the strictest sense. The natural opportunistic microflora found on herbal raw materials does not normally contain pathogenic species. However, infestation of herbal raw materials or preparations with pathogenic species (e.g., Enterobacteriae, Salmonellae, E. coli) or potentially pathogenic levels of opportunistic species (e.g., Bacillus spec.) may occur during any of the post-harvesting stages, and microbiological quality needs to be assessed routinely in both herbal raw materials and preparations. At present, no legal limits have been set for herbal food supplements, or for that matter, the majority of food items. Because herbal food supplements and herbal medicines are similar with respect to raw materials, preparation and finished forms, the European Pharmacopoeia analytical methodology is fully applicable. The microbial limits specified in the European Pharmacopoeia47 are equally appropriate for herbal food supplements and their attainment has been shown to be feasible provided suitable germ-reducing processing steps are applied, e.g.water vapour treatment.4.5 Other contaminants
On the basis of extensive data collection in EU countries on PAH levels comprising ca. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 samples of different food items48 and an EFSA scientific opinion regarding polycyclic hydrocarbons in food,49 the European Commission has recently filed a draft regulation to set maximum levels for PAH in certain foods.50
000 samples of different food items48 and an EFSA scientific opinion regarding polycyclic hydrocarbons in food,49 the European Commission has recently filed a draft regulation to set maximum levels for PAH in certain foods.50
Regulation (EC)1881/20064 on PAH levels in certain foodstuffs is based exclusively on benzo(a)pyrene (BaP) levels, but EFSA's scientific opinion of 200848,49 concludes that benzo(a)pyrene should not be used as the sole indicator of PAH contamination. Instead, a group of four PAHs – (benzo(a)pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene) – has been proposed as a more reliable basis for assessing PAHs in food. Very few data are available on the occurrence of PAHs in herbal ingredients used in food supplements. On the basis of preliminary data from single member states, draft Commission Regulation SANCO/10616/2009 rev. 6 (28.3.2011)50 states that “High levels of PAH have been found in some food supplements. Nevertheless, the levels are variable and depend on the specific type of food supplements. Further data on food supplements are needed and should be collected. Once these data become available, the need for setting maximum levels for PAH in food supplements will be evaluated.”
While legal limits for PAHs in food supplements do not currently exist in the EU, food operators should assess the necessity of PAH testing on a case-by-case basis considering, e.g., geographic origin, processing (e.g., roasting) or concentration procedures if they involve lipophilic solvents.
5. Toxic plant compounds
Among contaminants, one must consider toxic substances naturally occurring in plant sources: compounds of the herbal raw material itself, coming from the use of the wrong parts of the same plant or from different plant species. Official methods described in pharmacopoieas cover the most typical examples of this type of adulteration. Because plant sourcing practices are rapidly changing, the likeliness of intentional or unintentional adulteration has strongly increased in recent years. This consideration remains a major responsibility of food operators.The amount of undesirable compounds present in the recommended daily intake of a given product may be affected by the choice of raw material, the mode of preparation (extraction solvent, extraction temperature and duration) and by the recommended dose and conditions of use. At any stage in the process from field to finished product, reliable analytical methods are needed to ensure adequate quality and safety. it should be borne in mind that different methods can produce widely different values.
The substances that need to be measured may be divided into four groups.
First, the physiologically active substances providing the desired effects. In some cases, the compounds may exert adverse effects at high intake levels or in certain target populations, and upper limits may be appropriate.
Secondly, components of the same herb that are not related to the desired action but may cause adverse effects. This is the largest group, since it comprises any substance contained in the respective herb/preparation other than physiologically active ones. Toxicological effects and dose/toxicity relations have been established for some of the substances, which allows reasonable limits to be derived.
Thirdly, toxic compounds resulting from confusion or adulteration with other herbs or unwanted parts of the same herb. This group is becoming increasingly important for several reasons: the sourcing and trade of herbs and herbal preparations has become more international with an increasingly diversified structure of trade relations; the number of herbs/preparations traded internationally has steeply increased, creating more potential for unintentional mix-up; and the increasing global demand and price level of certain herbs encourages wilful adulteration with surrogate plant material, including potentially toxic species.
Fourth, increased knowledge and understanding of the phytochemical composition of herbs and herbal preparations has created new possibilities of targeted phytochemically designed adulteration, especially for enriched herbal extracts with a specified content of active compounds, e.g., Ginkgo biloba (flavonoids), Panax ginseng (ginsenosides) or Oxycoccus palustris/cranberry (proanthocyanidins).51–53 For more details see below (Identification of intentional adulteration).
Despite the large number of potentially occurring contaminants, there is no call for hysteria or for herbal products in particular to be suspected of being at risk of contamination or adulteration. However, examples like ochratoxin A in liquorice show that certain herbal materials may be seriously affected by particular contaminants.
It remains the duty of food operators to follow the scientific literature and market information and adapt the scope of specifications, sampling schemes and methods to a fast-changing environment and state of knowledge.
6 Intentional adulteration
Adulterants may be intentionally added to products containing botanical ingredients to increase the product bulk, to reduce manufacturing costs or for some other deceptive or illegal purpose.54 The added substance is usually not included in the labelling, which is not sufficiently strictly controlled.55Several undeclared active ingredients in products marketed as dietary supplements have been found, including anticoagulants (e.g., warfarin), anticonvulsants (e.g., phenytoin), HMG-CoA reductase inhibitors (e.g., lovastatin), phosphodiesterase type 5 inhibitors (e.g., sildenafil), nonsteroidal anti-inflammatory drugs (NSAIDs; e.g., indomethacin), and beta blockers (e.g., propranolol). A report on Chinese herbal medicines indicated that among 260 ‘Asian’ (no further details provided) patent medicines collected from Californian outlets, 7% contained undeclared pharmaceuticals.56
A systematic review briefly summarized the evidence for the adulteration of Chinese herbal medicines by synthetic therapeutic substances.57 One fatality and at least six potentially life-threatening events were presented. Phenylbutazone, phenytoin, glibenclamide and corticosteroids were some of the adulterants associated with serious adverse events. The concerns were heightened by the ready availability of these products through shops and therapists as well as by mail order and over the Internet.
Among the adulterant substances, those promoting weight loss, body-building, and sexual performance enhancement are the most frequently detected.
The use of performance-enhancing drugs in sport (so-called doping) is common, even though the practice is considered unethical by most international sports organizations and especially the International Olympic Committee, and it can be seriously detrimental to health. Anabolic steroids affect cardiovascular and mental health and are associated with an increased risk of cancer.57–59 Dietary supplements containing ephedra alkaloids have been linked to hypertension, tachycardia, stroke, seizures and death.60 The peptide hormones, so-called “sports-designer drugs”, are thought to be the most dangerous, while the combination of amphetamines, anabolic steroids or antihypertensives with intense physical activity has been shown to be responsible for severe adverse effects.
Ephedra alkaloids have produced health problems not only in athletes but also in subjects who took Ephedra-based food supplements in order to lose weight. A fatal case in a young, apparently healthy male college student drew attention to the dangers associated with ephedrine-containing products. The student regularly consumed a product containing ephedrine and caffeine known as Ripped Fuel. The official autopsy report and death certificate recorded “patchy myocardial necrosis associated with ephedrine toxicity from protein drink containing ma huang extract.” The blood and urine ephedrine levels suggested that the death was not caused by acute poisoning but was the result of chronic consumption.61,62
Because of widespread reports of adverse events associated with ephedrine use, FDA and European Countries banned Ephedra derivatives from the food supplements formulated for sports.
6.1 Detection and quantification of added drugs
Toxicological analysis aims at controlling prohibited substances and sometimes may be limited to qualitative analysis; however, it is often necessary to quantify the active substances in order to assess the significance of the adulteration for human health. Reliable risk assessment requires the development and/or application of an efficient analytical method. The analyst must select the most appropriate method of analysis among those available.When adulteration is suspected, the first objective is the identification of the class of molecules to be searched for. The chemical nature, as well as the toxicokinetic and toxicodynamic aspects of the hypothesized agent, must be assessed, since all these factors affect the analytical procedure, and the laboratory involved must be suitably equipped and staffed by trained personnel to allow rapid and valid analyses.
Several analytical approaches have been developed. Screening is an essential first step, and more than one screening method must be applied because thousands of chemical substances are involved. The putative adulterant should be classified according to its acidic, neutral or alkaline nature and separated with appropriate solvent systems and reagents. Thin-layer chromatography (TLC) and its improved version High Performance TLC (HPTLC) are widely used in screening to detect or exclude whole classes of substances, and is particularly suitable in screening for hormones.
More sensitive and specific analytical techniques must be used to quantify the contaminant, e.g.gas chromatography–mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC) plus mass spectrometry (HPLC-MS) using different ion sources and analyzers, and capillary electrophoresis (CE).
Knowledge of symptoms can limit the search to a few classes of compounds, and clinical data and pharmacological activity are available for some common food supplements, but even when sophisticated analytical techniques are used, the identification and quantification of adulterants requires time, expensive procedures and the availability of purified standards.
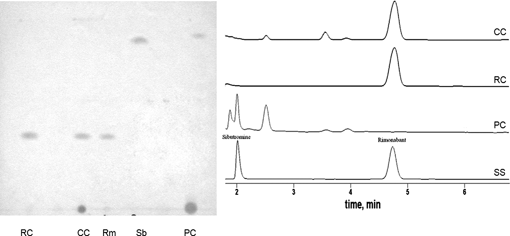 | ||
| Fig. 2 HPTLC (left) and HPLC chromatograms (right) of standard sibutramine and rimonabant and food supplements (modified from Kananet al.63).RC = Red Capsule; CC = Creamy Capsule; PC = Phytoslim Capsule; Rm = Rimonabant; Sb = Sibutramine. | ||
Fig. 2 (left) shows the same samples analysed by HPTLC. Two samples (Red Capsule and Creamy Capsule) had Rf values perfectly matching those of purified rimonabant (Rm), while Phytoslim Capsule showed a component with an Rf value similar to that of standard sibutramin (Sb). Thus, the results obtained by HPTLC agreed well with those obtained by HPLC.63
The herbal products had therefore been laced with synthetic weight-loss promoters. Rimonabant and sibutramine are prescribed for use in low dose and for a short time. Their inclusion in food supplements constitutes a severe danger for consumers who could easily exceed the safe dosage.
7 Conclusions
The quality control of plant materials is critical whether a botanical product is to be used as a medical treatment or as a plant food supplement. For the ultimate protections of the consumers, quality control should be applied throughout the various processing stages, from the raw material to the finished product. Unfortunately, there is no single or superior method to assure 100% quality control of a product. Instead it takes a mixture of techniques used in the correct manner, and applied to the proper tissue, to achieve this goal.Acknowledgements
The writing of this review was funded by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no 245199. It has been carried out within the PlantLIBRA project (website: http://www.plantlibra.eu). This report does not necessarily reflect the Commission views or its future policy on this area.Notes and references
- European Union Directive 2002/46/EC of 10 June 2002, Official Journal of the European Communities L. 183/51–57, 12 July 2002.
- European Union Directive 2004/24/EC of 31 March 2004, Official Journal of the European Communities L. 136/85–90, 30 April 2004.
- EC Regulation No. 396/2005 of 23 February 2005, Official Journal of the European Communities L. 70/1–16, 16 March 2005.
- EC Commission Regulation n. 1881/2006 of 19 December 2006, Official Journal of the European Communities L. 364/5–24, 20 December 2006.
- EC Commission Regulation n. 1925/2006 of 20 December 2006, Official Journal of the European Communities L. 404/26–38, 30 December 2006.
- F. Stickel, H.-M. Baumuller, K. Seitz, D. Vasilakis, G. Seitz, H. K. Seitz and D. Schuppan, J. Hepatol., 2003, 39, 62–67 CrossRef CAS.
- A. R. Mattocks, Lancet, 1980, 316, 1136–1137 CrossRef.
- R. Walker, Toxicol. Lett., 2004, 149, 187–195 CrossRef CAS.
- C. A. Swanson, Am. J. Clin. Nutr., 2002, 75, 8–10 CAS.
- F. Rossi, E. Gaio and S. Torriani, Food Microbiol., 2010, 27, 356–362 CrossRef CAS.
- B. Carratù, E. Federici, F. R. Gallo, A. Geraci, M. Guidotti, G. Multari, G. Palazzino and E. Sanzini, Ann. Ist. Super. Sanita, 2010, 46, 370–388 Search PubMed.
- R. B. van Breemen, H. H. S. Fong and N. R. Farnsworth, Chem. Res. Toxicol., 2007, 20, 577–582 CrossRef CAS.
- P. A. Cohen and E. Ernst, Safety of Herbal Supplements: A Guide for Cardiologists, Cardiovasc. Ther., 2010, 28, 246–253 CrossRef.
- S. K. Singh, S. K. Jha, A. Chaudhary, R. D. Yadava and S. B. Rai, Pharm. Biol., 2010, 48, 134–41 CrossRef CAS.
- B. Schilter, C. Andersson, R. Anton, A. Constable, J. Kleiner, J. O'Brien, A. G. Renwick, O. Korver, F. Smit and R. Walker, Food Chem. Toxicol., 2003, 41, 1625–1649 CrossRef CAS.
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal plants, Geneva, Switzerland, 2003. Available at: http://whqlibdoc.who.int/publications/2003/9241546271.pdf Search PubMed.
- L. Oelker, Ann. Ist. Super. Sanita, 2005, 41, 43–48 Search PubMed.
- N. P. Yadav and V. K. Dixit, Int. J. Integr. Biol., 2008, 2, 195–203 CAS.
- H. Tanaka, N. Fukuda and Y. Shoyama, Phytochem. Anal., 2006, 17, 46–55 CrossRef CAS.
- H. A. Hoppe, Drogen Kunde, Walter de Gruyter, Berlin-New York, 1975 Search PubMed.
- R. Hegnauer, Chemotaxonomie der Pflanzen, Basel-Boston-Berlin, Birkhäuser Verlag, 1992 and 2001 Search PubMed.
- Indian Ministry of Health and Family Welfare, Department of Indian Systems of Medicine & Homoeopathy, The Ayurvedic Pharmacopoeia of India, New Delhi: National Institute of Science Communication (CSIR), 2001 Search PubMed.
- J. Zhou, G. Xie, and X. Yan, Traditional Chinese Medicines, Molecular Structures, natural sources and application, ed. G. W. A. Milne, Ashgate Publishing Ltd, Hampshire-Burlington, 2nd edn, 2003 Search PubMed.
- J. A. Duke, Handbook of Medicinal Herbs, Boca Raton: CRC Press, 2000 Search PubMed.
- L. D. Kapoor, Handbook of Ayurvedic Medicinal Plants, Boca Raton: CRC Press, 2000 Search PubMed.
- J. Gruenwald, T. Brendeler, C. Jaenicke, and T. Fleming, Physicians' Desk Reference (PDR) for Herbal Medicines, Medical Economics Company, Montvale-Ew Jersey, 2nd edn, 2000 Search PubMed.
- N. G. Bisset, and M. Wichtl, Herbal Drugs and Phytopharmaceuticals, Medpharm Scientific Publisher, Stuttgart, 1994 Search PubMed.
- World Health Organization, Quality control methods for medicinal plants. Document N. WHO/PHARMA/92.559/rev1. Geneva, Switzerland, 1998 Search PubMed.
- American Herbal Pharmacopoeia (AHP), Botanical Pharmacognosy: Microscopic Characterization of Botanical Medicines, CRC Press Inc., 2011 Search PubMed.
- P. P. Fu, H. M. Chiang, Q. Xia, T. Chen, B. H. Chen, J. J. Yin, K. C. Wen, G. Lin and H. Yu, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2009, 27, 91–119 CAS.
- N. Techen, S. L. Crockett, I. A. Khan and B. E. Scheffler, Curr. Med. Chem., 2004, 11, 1391–1401 CAS.
- G. Heubl, Planta Med., 2010, 76, 1963–1974 CrossRef CAS.
- EC Regulation No. 178/2006 of 1 February 2006, Official Journal of the European Communities L 29/3–24, 2 February 2006.
- EC Regulation No. 149/2008 of 29 January 2008, Official Journal of the European Communities L 58/1–398, 1 March 2008.
- EC Regulation No. 839/2008 of 31 July 2008, Official Journal of the European Communities L 234/1–216, 30 August 2008.
- SANCO , Quality Control Procedures for Pesticide Residues Analysis, Document No. SANCO/10232/2006, dated 24 March 2006. Available at: http://ec.europa.eu/food/plant/resources/qualcontrol_en.pdf Search PubMed.
- Csn EN 12393-1-Foods of plant origin - Multiresidue methods for the gas chromatographic determination of pesticide residues - Part 1: General considerations, 2008 Search PubMed.
- Csn EN 12396-3-Non-fatty foods - Determination of dithiocarbamate and thiuram disulfide residues - Part 3: UV spectrometric xanthogenate method, 2000 Search PubMed.
- Available at: http://www.ehia-online.org/documents/Allocation_List_EHIA_Final_SEP2008.pdf.
- EC Regulation No. 629/2008 of 2 July 2008, Official Journal of the European Communities L 173/6–9, 3 July 2008.
- U. Gasser, B. Klier, A. V. Kühn, B. Steinhoff, Current Finding on the Heavy Metal Content in Herbal Drugs, Pharmeuropa Scientific Notes, 2009-1, pp. 37–50 Search PubMed.
- L. Kabelitz, Eur. J. Herb. Med., 1998, 4, 25–29 Search PubMed.
- Ph.Eur. 7.0, Heavy metals in herbal drugs and fatty oils, 2.4.27, pp. 129–130 Search PubMed.
- EC Regulation No. 105/2010 of 5 February 2010, Official Journal of the European Communities L 35/7–8, 6 February 2010.
- Ph.Eur 7.0, Determination of Aflatoxin B1 in herbal drugs, 2.8.18, pp. 244–246 Search PubMed.
- Ph.Eur 7.0, Determination of Ochratoxin A in herbal drugs, 2.8.22, pp. 249–250 Search PubMed.
- Ph.Eur 7.0, Microbiological quality of herbal medicinal products for oral use, 5.1.8, pp. 519 Search PubMed.
- EFSA, Findings of the EFSA Data Collection on Polycyclic Aromatic Hydrocarbons in Food, EFSA/DATEX/002 (revision 1), 31 July 2008 Search PubMed.
- EFSA, Polycyclic Aromatic Hydrocarbons in Food, Scientific Opinion of the Panel on Contaminants in the Food Chain, (Question No EFSA-Q-2007-136), adopted 9 June 2008, The EFSA Journal, 2008, 724, 1–114. Available at: http://www.efsa.europa.eu/en/efsajournal/doc/s724.pdf Search PubMed.
- SANCO/10616/2009 rev. 7 (11.4.2011) Draft. COMMISSION REGULATION (EU) No …/… amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Available at: http://members.wto.org/crnattachments/2011/sps/EEC/11_1634_00_e.pdf.
- S. Dentali, Simple Authentication Methods for Herbal Ingredient Integrity in the Face of EMA, Public Meeting on Economically Motivated Adulteration, May 1, 2009, Docket No: FDA-2009-N-016 http://www.fda.gov/NewsEvents/MeetingsConferencesWorkshops/ucm163619.htm Search PubMed.
- M. Tawab, M. Krzywon and M. Schubert-Zsilavecz, Pharm. Ztg., 2010, 155, S62–67 Search PubMed.
- R. Roth-Ehrang, 8th Annual Oxford International Conference on the Science of Botanicals; Oxford, Mississippi, April 4–6, 2009, Published in Planta Medica, 2009, 75, Issue 04 Search PubMed.
- J. Fang Deng, Toxicology Volumes, 2002, 181–182, 571–576 Search PubMed.
- Available at: http://www.who.int/bulletin/bulletin_board/82/whonews08041/en/.
- R. J. Ko, N. Engl. J. Med., 1998, 339, 847 CrossRef CAS.
- R. J. Ko, J. Intern. Med., 2002, 252, 107–113 CrossRef.
- M. Parssinen and T. Seppala, Sports Med., 2002, 32, 83–94 CrossRef.
- A. J. Gruber and H. G. Pope, Psychotherapy and Psychosomatics, 2000, 69, 19–26 CrossRef CAS.
- C. A. Haller and N. L. Benowitz, N. Engl. J. Med., 2000, 3343, 1833–1838 CrossRef.
- M. E. Powers, J. Athletic Training, 2001, 36, 420–424 CAS.
- FDA. Federal Register Notice - 65 FR 17510, April 3, 2000-Dietary Supplements Containing Ephedrine Alkaloids. Available at: http://www.fda.gov/Food/DietarySupplements/GuidanceComplianceRegulatoryInformation/RegulationsLaws/ucm079601.htm.
- S. Kanan, I. A. Abu-Yousef, C. Gunasekar, N. Abdo and S. Narasimhan, Eur. J. Scientific Research, 2009, 34, 348–357 Search PubMed.
- J. Vaysse, S. Balayssac, V. Gilard, D. Desoubdzanne, M. Malet-Martino and R. Martino, Food Additives & Contaminants: Part A, 2010, 27, 903–916 CAS.
- A. Vannacci, F. Lapi, R. Baronti, E. Gallo, L. Gori, A. Mugelli and F. Firenzuoli, Br. J. Clin. Pharmacol., 2009, 67, 473–474 CrossRef CAS.
Footnote |
| † This paper forms part of the themed issue on Plant Food Supplements: regulatory, scientific and technical issues concerning safety, quality and efficacy. |
| This journal is © The Royal Society of Chemistry 2011 |
