Binding of citrus flavanones and their glucuronides and chalcones to human serum albumin
Muhammad Kamran
Khan
ab,
Njara
Rakotomanomana
ab,
Claire
Dufour
ab and
Olivier
Dangles
*ab
aINRA, UMR408, Safety and Quality of Plant Products, 84000, Avignon, France. E-mail: Olivier.Dangles@univ-avignon.fr; Fax: (+33) 490 14 44 41; Tel: (+33) 490 14 44 46
bUniversité d'Avignon et des Pays de Vaucluse, UMR408, 84000, Avignon, France
First published on 28th September 2011
Abstract
Naringenin and hesperetin glycosides are the major polyphenols (flavanones) of citrus fruits and juices and are thought to participate in the cardioprotective effects of diets rich in plant products. Naringenin and hesperetin glucuronides (resulting from conjugation at the A- or B-ring) are the main circulating metabolites in humans and their binding to human serum albumin (HSA) is expected to modulate their half-life in plasma and tissue distribution. In this work, the binding of flavanone glucuronides to HSA was investigated by fluorescence spectroscopy. Binding constants in the range of 3–9 × 104 M−1 were estimated. The affinity of glucuronides for HSA is close to that of naringenin and hesperetin themselves. Competition experiments in the presence of the fluorescent probes dansylsarcosine and quercetin were used to gain information on the flavanone binding site. Naringenin and hesperetin chalcones were also included for comparison as their glucuronides too were detected in the general circulation. Naringenin and hesperetin chalcones spontaneously undergo cyclization back to the parent flavanones under neutral conditions. The cyclization was significantly slowed down by HSA but led to a racemic mixture of (2R) and (2S) flavanones in the absence or presence of HSA.
1. Introduction
Citrus fruits and juices are extensively consumed worldwide and are the richest source of specific polyphenols called flavanones (Fig. 1), which may protect against cardiovascular disease and cancers.1–4 Citrus flavanones are typically 7-β-O-D-rhamnoglucosides. However, after ingestion, deglycosidation by microbial enzymes takes place in the colon and flavanone aglycones thus formed are absorbed through the intestinal barrier by passive diffusion, then extensively conjugated in endothelial and hepatic cells.5–7 Consequently, dietary flavanones are mainly detected in plasma and urine as glucuronides and these conjugates, not the native glycosides and aglycones, are distributed to tissues and responsible for the effects on health. From these bioavailability data, it is now clear that cell studies on the bioactivity of flavanones must be validated with flavanone glucuronides (Fig. 1) to achieve full biological significance.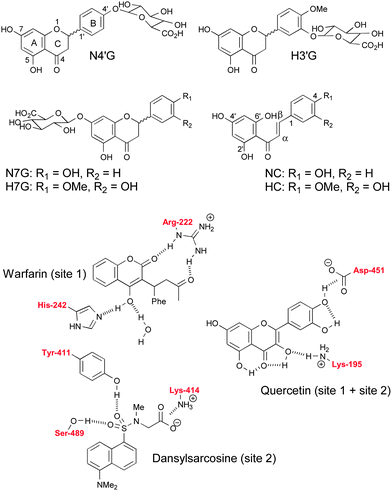 | ||
| Fig. 1 Chemical structures of HSA ligands. Some hydrogen bonding interactions between fluorescent probes and HSA residues are shown (see text for details). | ||
The delivery of the circulating flavanone metabolites to specific biological sites is still poorly documented. The interaction of flavonoids with human serum albumin (HSA) could be an important factor, controlling their half-life in plasma and transport to biological sites. Indeed, serum albumin is the major protein of blood plasma with a concentration as high as 0.6 mM. Beside its role in the maintenance of colloidal osmotic blood pressure and bodily detoxification, HSA transports fatty acids and a large variety of drugs8 and food components, including polyphenols.9 Interestingly, HSA has been shown to accumulate in solid tumors and in inflamed joints in arthritic disease and could thus favour the specific delivery of drugs at those sites.10 The binding to HSA of flavanone glucuronides is expected to provide a suitable model for investigating the transport of dietary flavanones in the blood circulation. Flavanone chalcones (Fig. 1), the biosynthetic precursors of flavanones,11 are also present in the diet. For instance, naringenin chalcone is one of the major flavonoids of tomato fruit and normally accumulates in the peel.12,13 Moreover, flavanones can be converted to their respective chalcones in gastrointestinal conditions, which may affect intestinal absorption.14Naringenin chalcone was also shown to display anti-inflammatory and anti-allergic activities.15,16
In a previous paper, we reported the chemical synthesis of the four major flavanone glucuronides circulating in blood after citrus consumption, hesperetin 3′- and 7-O-β-D-glucuronides and naringenin 4′- and 7-O-β-D-glucuronides (Fig. 1).17 In the present work, we wish to report the binding to HSA of these metabolites in comparison with the parent aglycones and their chalcone precursors. In addition, competition experiments are carried out to locate the binding site(s) and the influence of HSA on the chalcone–flavanone cyclization is assessed.
2. Experimental section
2.1 Materials
HSA (fraction V, 96–99%, MW = 66500 g mol−1), quercetin (98%), naringenin (95%), hesperetin (95%) and dansylsarcosine (DNSS, 99%) were purchased from Sigma-Aldrich (St Quentin Fallavier, France). Glucuronides of naringenin (4′-O-β-D-glucuronide (N4′G) and 7-O-β-D-glucuronide (N7G)) and hesperetin (3′-O-β-D-glucuronide (H3′G) and 7-O-β-D-glucuronide (H7G)) were chemically synthesized as already described.17 All solutions were prepared in deionized water or HPLC grade MeOH. Analytical TLC was performed on silica gel 60 F254 (Merck KGaA, Darmstadt, Germany). Silica gel 60 (40–63 μm, Merck KGaA) was used for column chromatography.2.2 NMR
1D 1H and 13C NMR spectra were recorded on a Bruker Advance DPX-300 apparatus. Chemical shifts (δ) in ppm, 1H–1H coupling constants (J) in Hz.2.3 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water mixture containing NaOH (2 g, 6 M). After heating at 80 °C on a water bath for about 5 min, the deep red solution was cooled to room temperature and neutralized by ice-cold 2 M HCl saturated with NaCl. The yellow precipitate (ca. 200 mg, 20%) thus obtained was filtered and dissolved in EtOAc for purification by chromatography on silica gel using cHex–EtOAc (4
1 ethanol–water mixture containing NaOH (2 g, 6 M). After heating at 80 °C on a water bath for about 5 min, the deep red solution was cooled to room temperature and neutralized by ice-cold 2 M HCl saturated with NaCl. The yellow precipitate (ca. 200 mg, 20%) thus obtained was filtered and dissolved in EtOAc for purification by chromatography on silica gel using cHex–EtOAc (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) as the eluent. Rf (cHex–EtOAc, 4
6) as the eluent. Rf (cHex–EtOAc, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 0.47; NMR, δH (300 MHz; CD3OD; Me4Si): 8.09 (1 H, broad d, J 15.6, β-H), 7.71 (1 H, broad d, J 15.6, α-H), 7.51 (2 H, d, J 8.6, 2-H, 6-H), 6.84 (2 H, d, J 8.6, 3-H, 5-H), 5.86 (2 H, s, 3′-H, 5′-H); δC (75 MHz; CD3OD; Me4Si): 194.56 (C
6) 0.47; NMR, δH (300 MHz; CD3OD; Me4Si): 8.09 (1 H, broad d, J 15.6, β-H), 7.71 (1 H, broad d, J 15.6, α-H), 7.51 (2 H, d, J 8.6, 2-H, 6-H), 6.84 (2 H, d, J 8.6, 3-H, 5-H), 5.86 (2 H, s, 3′-H, 5′-H); δC (75 MHz; CD3OD; Me4Si): 194.56 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.82, 166.42, 165.30 (C-2′, C-4′, C-6′), 159.44 (C-4), 144.03 (C-β), 131.71, 129.44 (C-1, C-2, C-6), 125.99 (C-α), 117.24, 116.73 (C-3, C-5), 103.75 (C-1′), 96.41 (C-3′, C-5′).
O), 168.82, 166.42, 165.30 (C-2′, C-4′, C-6′), 159.44 (C-4), 144.03 (C-β), 131.71, 129.44 (C-1, C-2, C-6), 125.99 (C-α), 117.24, 116.73 (C-3, C-5), 103.75 (C-1′), 96.41 (C-3′, C-5′).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 0.38; NMR, δH (300 MHz; CD3OD; Me4Si): 8.08 (1 H, broad d, J 15.6, β-H), 7.66 (1 H, broad d, J 15.6, α-H), 7.11 (2 H, m, 2-H, 6-H), 6.97 (1 H, d, J 8.3, 5-H), 5.87 (2 H, s, 3′-H, 5′-H), 3.91 (3 H, s, OCH3); δC (75 MHz; CD3OD; Me4Si): 194.46 (C
6) 0.38; NMR, δH (300 MHz; CD3OD; Me4Si): 8.08 (1 H, broad d, J 15.6, β-H), 7.66 (1 H, broad d, J 15.6, α-H), 7.11 (2 H, m, 2-H, 6-H), 6.97 (1 H, d, J 8.3, 5-H), 5.87 (2 H, s, 3′-H, 5′-H), 3.91 (3 H, s, OCH3); δC (75 MHz; CD3OD; Me4Si): 194.46 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 171.06, 166.72, 166.47 (C-2′, C-4′, C-6′), 151.73, 148.37 (C-3, C-4), 143.95 (C-β), 130.67 (C-1), 126.89 (C-α), 123.58, 115.16, 112.94 (C-2, C-5, C-6), 106.32 (C-1′), 97.47, 96.42 (C-3′, C-5′), 56.79 (OCH3).
O), 171.06, 166.72, 166.47 (C-2′, C-4′, C-6′), 151.73, 148.37 (C-3, C-4), 143.95 (C-β), 130.67 (C-1), 126.89 (C-α), 123.58, 115.16, 112.94 (C-2, C-5, C-6), 106.32 (C-1′), 97.47, 96.42 (C-3′, C-5′), 56.79 (OCH3).
2.4. UV-spectroscopy
An Agilent 8453 UV-visible spectrometer equipped with a 1024-element diode-array detector was used to record the absorption spectra over the wavelength range 190–1100 nm. A water thermostated bath was used to control the cell temperature with an accuracy of ±0.1 °C. The spectroscopic measurements were carried out with a quartz cuvette of 1 cm optical pathlength.2.5 Kinetic analyses
Concentrated solutions of flavanone chalcones (2 mM) were prepared in MeOH to give final concentrations of about 50 μM in the cell. The cyclization was performed in a pH 7.4 phosphate buffer (50 mM Na2HPO4 + 100 mM NaCl) in the presence or absence of HSA (0–2 equiv.). Changes in the absorption spectra of chalcone and flavanone were monitored at 382 and 323 nm, respectively (maximal absorption) and recorded as a function of time (cycle time: 30 s). Furthermore, the effect of temperature (298, 304, 311 K) and HSA concentration (0–2 equiv) on the cyclization rate was determined. All experiments were carried out thrice.2.6 Fluorescence spectroscopy
Steady-state fluorescence spectra were recorded on a thermostated Safas Xenius spectrofluorometer. The excitation and emission slit widths were set at 10 nm. All studies were performed at 25 (± 1) °C. Four excitation–emission conditions were used: a) excitation at 295 nm (HSA Trp residue), emission light collected between 270 and 410 nm (310 and 370 nm in case of chalcones); b) excitation at 470 nm (naringenin chalcone - HSA complex), emission light collected between 530 and 590 nm; c) excitation at 370 nm (DNSS–HSA complex), emission light collected between 350 and 600 nm (460 and 520 nm in the case of chalcones); d) excitation at 450 nm (quercetin–HSA complex), emission light collected between 350 and 600 nm. Solutions were prepared daily by dissolving HSA in a pH 7.4 buffer (50 mM phosphate + 100 mM NaCl), while the concentrated solutions of ligands were prepared in MeOH. In all experiments, the cosolvent concentration was lower than 10%.2.7 Binding to HSA
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HSA–probe molar ratio) and aliquots (1–150 μL) of a 1–5 mM solution of the second ligand in MeOH.
1 HSA–probe molar ratio) and aliquots (1–150 μL) of a 1–5 mM solution of the second ligand in MeOH.
2.8 Binding data analysis
All calculations were carried out with the least-square regression program Scientist (MicroMath, Salt Lake City, USA). Beside the expression of the fluorescence intensity IF, the typical relationships used in the curve-fitting procedures were combinations of the mass law for the complexes and mass conservation for the ligand L, protein P and probe D (see below).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding, optimized values for the binding constant (K1) and the molar fluorescence intensity of the complex (fPL) were estimated by fitting the IFvs. Lt curves against eqn (1)–(3) where Lt is the total ligand concentration and Pt the total protein concentration. The molar fluorescence intensity of the free ligand (fL) was estimated from a linear plot of the fluorescence intensity vs. ligand concentration in the absence of HSA. The weak fluorescence intensity of free HSA (molar fluorescence intensity fP) detected in the absence of ligand was taken into account.
1 binding, optimized values for the binding constant (K1) and the molar fluorescence intensity of the complex (fPL) were estimated by fitting the IFvs. Lt curves against eqn (1)–(3) where Lt is the total ligand concentration and Pt the total protein concentration. The molar fluorescence intensity of the free ligand (fL) was estimated from a linear plot of the fluorescence intensity vs. ligand concentration in the absence of HSA. The weak fluorescence intensity of free HSA (molar fluorescence intensity fP) detected in the absence of ligand was taken into account.| IF = fL[L] + fP[P] + fPLK1[L][P] | (1) |
| Lt = [L](l + K1[P]) | (2) |
| Pt = [P](1 + K1[L]) | (3) |
| IF = fP[P]exp(−εLlLt) | (4) |
In eqn (4), εL stands for the sum of the molar absorption coefficients of the ligand at the excitation and emission wavelengths, and has been checked to be identical for the bound or free ligand. Its value is determined independently by UV-visible spectroscopy from a Beer's plot. Finally, l is the mean distance travelled by the excitation light at the site of emission light detection. For the spectrometer used in this work, l is estimated to be 0.65 cm.
Assuming competition between probe D and ligand L and pure 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding, eqn (5) and (6) can be derived and used in the curve-fitting of the IFvs. Lt curve for the determination of optimized values for parameters K1 and fDP (after preliminary determination of KD in the absence of L and of fD in the absence of L and P).
1 binding, eqn (5) and (6) can be derived and used in the curve-fitting of the IFvs. Lt curve for the determination of optimized values for parameters K1 and fDP (after preliminary determination of KD in the absence of L and of fD in the absence of L and P).
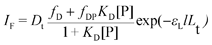 | (5) |
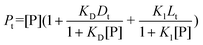 | (6) |
Assuming noncompetitive binding of probe D and ligand L, the quenching of the D–HSA complex fluorescence by ligand L was analyzed by considering that L binds to free HSA (P1) with binding constant K1 and to the D–HSA complex (P2) with binding constant K2. XB being the fraction of HSA bound to probe D (estimated from a DNSS–HSA binding constant of 156 × 103 M−1), eqn (7)–(10) were used in the curve-fitting procedure aimed at estimating K2, K1 being set constant at the value previously determined (quenching of HSA fluorescence):
| Lt = [L](l + K1[P1] + K2[P2]) | (7) |
| (1 − XB)Pt = [P1](1 + K1[L]) | (8) |
| XBPt = [P2](1 + K2[L]) | (9) |
| IF = fP2[P2]exp(−εLlLt) | (10) |
2.9. Chiral UPLC
Chiral separation of the flavanone enantiomers was carried out on the Acquity Ultra Performance LC™ (UPLC™) apparatus from Waters equipped with an UV-visible diode array detector. The chromatographic separation was conducted on a ChiralPAK AD-H (amylose tris-(3,5-dimethylphenylcarbamate) coated on 5 μM silica-gel) column with internal dimensions of 250 × 4.6 mm. The column was thermostated at 25 °C and eluted with cHex–iPrOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at a flow rate of 0.5 mL min−1.19 The wavelengths selected for flavanone and chalcone detection were 280 nm and 370 nm.
1) at a flow rate of 0.5 mL min−1.19 The wavelengths selected for flavanone and chalcone detection were 280 nm and 370 nm.
2.10. Molecular modeling
Semi-empirical quantum mechanics calculations were run on the HyperChem 5.1 software (Hypercube, Waterloo, Canada) with the AM1 program. Absorption spectra were simulated from single point calculations on optimized structures with configuration interaction.3. Results and discussion
3.1 Synthesis
Flavanone glucuronides (Fig. 1) are the main metabolites detected in the blood circulation after citrus consumption. For this work, glucuronides of naringenin (N4′G and N7G) and hesperetin (H3′G and H7G), the major flavanone aglycones in grapefruit and orange respectively, were chemically synthesized. The synthetic pathways and structural analyses were discussed in detail in our previous publication.17Naringenin and hesperetin chalcones (NC and HC, respectively) (Fig. 1) were prepared by C-ring opening (retro-Michael addition) in optimized strongly alkaline conditions.20,21 Upon acidification, chalcone anions are rapidly protonated and neutral chalcones precipitate. Their isolation in the solid state prevented their cyclization back to flavanones. The NMR data of naringenin chalcone are consistent with the literature.223.2 Binding to HSA
Serum albumin, the major protein in blood plasma, carries fatty acids,23drugs24 and dietary components, such as polyphenols.9 HSA consists of three helical domains I (1–195), II (196–383) and III (384–585) and each domain is further subdivided into two sub-domains A and B. The protein has an overall shape of a heart and its structure is stabilized by 17 disulfide bonds.25,26 The two primary binding sites for drugs and other xenobiotics are called site 1 and site 2 and are located in sub-domains IIA and IIIA, respectively. Both sites bind through hydrophobic cavities lined by positively charged amino acid residues (Arg, Lys) at the entrance of the pockets. Within each site, secondary binding pockets have been identified.The transport of drugs and dietary components in the circulatory system is an important step in the overall process of delivery to tissues. Several studies have been reported on drug delivery to tissues by HSA and strategies have been developed to enhance drug stability and half-life in plasma by strengthening drug–HSA binding.8,10 Thus, investigating interactions between HSA and polyphenol metabolites can provide a pertinent insight into the fate of circulating polyphenols in terms of rate of excretion or delivery to tissues. For instance, following the ingestion of 50 mg of polyphenols (aglycone equivalent), the mean elimination half-life of catechins and flavanones is 2–3 h, whereas it is 5–8 h for isoflavones and 18–20 h for flavonols.27 Interestingly, this ranking parallels the increasing affinity of the aglycones for HSA,9 which may also be translated in their metabolites.
The literature available on albumin–flavonoid interactions reports quantitative thermodynamic data (binding constants) and qualitative analyses aimed at locating the possible binding sites.9,28 In particular, a combination of fluorescence spectroscopy with Fourier-transformed infrared (FT-IR) and UV-visible spectroscopies was used to determine the binding constants and binding sites of hesperetin within HSA.29 However, although investigations with quercetin metabolites30 and hydroxycinnamic acid glucuronides18 were recently reported, most works have dealt with commercially available flavonoid aglycones and glycosides, not the true circulating metabolites that are actually expected to bind to HSA and be distributed to tissues to express biological activities.
In this work, the binding to HSA of citrus flavanones, their biosynthetic precursors (chalcones) and the true circulating flavanone metabolites in humans (glucuronides) was investigated by fluorescence spectroscopy. Flavanones and their derivatives in their free or HSA-bound forms are too poorly fluorescent to investigate the interaction by direct irradiation of ligands. In fact, only naringenin chalcone (NC) could be investigated by this method. Indeed, the fluorescence of NC is weak in a neutral buffer but becomes much stronger in the presence of HSA (Fig. 2). Surprisingly, in the presence or absence of HSA, NC displays an excitation spectrum that is shifted by ca. 90 nm to higher wavelengths compared to the absorption spectrum (Fig. 2). This shift indicates that a ground-state minor tautomeric or acid–base form of NC is specifically excited. A similar phenomenon was also observed with the flavonol quercetin,317-hydroxyflavone32 but not with hesperetin chalcone (HC) in the present work. Hence, it seems critically dependent on the acidic O4–H group of NC. Consistently, semi-empirical quantum calculations predicted a shift by ca. 90 nm of the low-energy absorption band of NC upon deprotonation of O4–H. We thus suggest that the fluorescence of NC and its enhancement upon binding to HSA is due to low concentrations of 4-oxy anion, which are slightly increased in the presence of HSA due to favorable electrostatic interactions. However, NC is only marginally deprotonated upon binding as its UV-absorption at ca. 380 nm is shifted by only 3 nm in the presence of HSA (2 equiv.).
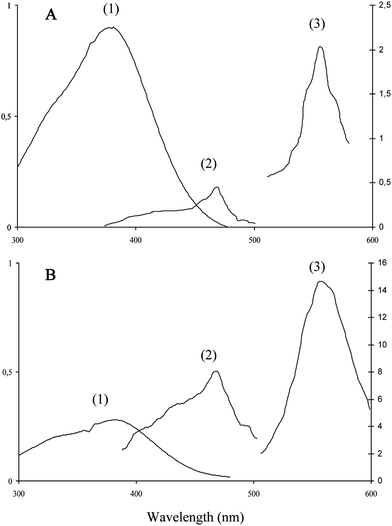 | ||
| Fig. 2 Absorption (1), excitation (2) and emission (3) spectra of naringenin chalcone in the absence (A) or presence (B) of HSA (20 μM) in pH 7.4 phosphate buffer, 25 °C. Excitation and emission wavelengths are set at 470 and 560 nm, respectively. Chalcone concentrations: 50 μM (A), 15 μM (B). | ||
Owing to the HSA-induced enhancement of the fluorescence of NC, a binding isotherm could be constructed from which the binding constant (pure 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding assumed) was extracted (Fig. 3): K1 = 46 × 103 M−1.
1 binding assumed) was extracted (Fig. 3): K1 = 46 × 103 M−1.
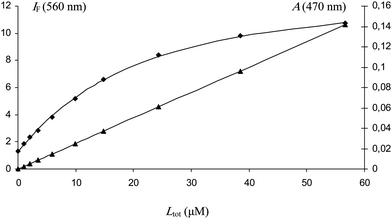 | ||
| Fig. 3 Changes in the fluorescence intensity of naringenin chalcone at 560 nm (λex = 470 nm) as a function of its concentration. Initial HSA concentration = 5 μM (pH 7.4 phosphate buffer, 25 °C). Fluorescence of HSA-bound ligand (♦), absorbance of free ligand at 470 nm (▲). K = 46 (± 3) ×103 M−1 (r = 0.9992). | ||
For other ligands, we had to consider the HSA intrinsic fluorescence due to its single Trp residue (Trp-214), which is located in sub-domain IIA where small negatively charged aromatic ligands are most likely to bind.8,24 The signal intensity and its sensitivity to quenching by sub-domain IIA ligands make it possible to use low protein and ligand concentrations. However, all the selected ligands absorb at the excitation (295 nm) and emission (340 nm) wavelengths so that a correction of the fluorescence intensity at 295 nm and 340 nm for this inner filter effect has to be applied in the data treatment (see experimental part).33 The investigation of chalcone–HSA binding was complicated by the instability of chalcones, which on the one hand are readily cyclized into flavanones in the buffer medium (see below) and on the other hand are sensitive to photo-induced (Z)–(E) isomerization. In this case, the most critical point was to operate rapidly enough to avoid substantial ring closure during data acquisition. Hence, fluorescence emission spectra were collected in a very narrow range (310–370 nm when Trp is excited, 530–590 nm when the chalcone ligand is excited). Satisfactory curve-fittings were achieved by assuming the formation of nonfluorescent complexes of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. 4, Table 1). The order of magnitude for the K1 values of flavanones and their derivatives is in good agreement with previous reports.29,34 The binding constants of naringenin 4′-O-β-D-glucuronide and hesperetin 3′-O-β-D-glucuronide are slightly lower than those of naringenin 7-O-β-D-glucuronide and hesperetin 7-O-β-D-glucuronide, respectively. Compared with naringenin and hesperetin, it can be noted that glucuronidation only weakly destabilizes the flavanone–HSA complexes, the effect being slightly stronger with B-ring glucuronidation. Moreover, bathochromic shifts in Trp-214 emission band are observed with aglycones and B-ring glucuronides but not with A-ring glucuronides (Fig. 5). The flavanone–chalcone conversion (C-ring opening) increases the affinity to HSA in the case of naringenin only.
1 stoichiometry (Fig. 4, Table 1). The order of magnitude for the K1 values of flavanones and their derivatives is in good agreement with previous reports.29,34 The binding constants of naringenin 4′-O-β-D-glucuronide and hesperetin 3′-O-β-D-glucuronide are slightly lower than those of naringenin 7-O-β-D-glucuronide and hesperetin 7-O-β-D-glucuronide, respectively. Compared with naringenin and hesperetin, it can be noted that glucuronidation only weakly destabilizes the flavanone–HSA complexes, the effect being slightly stronger with B-ring glucuronidation. Moreover, bathochromic shifts in Trp-214 emission band are observed with aglycones and B-ring glucuronides but not with A-ring glucuronides (Fig. 5). The flavanone–chalcone conversion (C-ring opening) increases the affinity to HSA in the case of naringenin only.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) complex in a pH 7.4 phosphate buffer at 25 °C (n = 2)
1) complex in a pH 7.4 phosphate buffer at 25 °C (n = 2)
| Ligand | K 1 (×103, M−1) | ε L (M−1 cm−1) | |||
|---|---|---|---|---|---|
| Trp-214 | Fluorescent probe | 295 nm | 340 nm | 370 nm | |
| a noncompetitive binding with quercetin. b probe = DNSS, competitive binding. c no influence on quercetin–HSA fluorescence. d not applicable with DNSS because chalcone absorption is too strong at 370 nm. e probe = quercetin, competitive binding. f the marginal excitation of NC at 450 nm is neglected. | |||||
| Nara | 77 (±1) | 30 (±0)b | 9910 | 11330 | 330 |
| N4′G c | 36 (±3) | 14 (±1)b | 10020 | 10450 | 360 |
| N7G c | 46 (±2) | 22 (±1)b | 11390 | 3510 | 1150 |
| NCd | 165 (±6) | 69 (±19)e,f | 5970 | 13340 | 18140 |
| Hespa | 85 (±1) | 18 (±1)b | 10570 | 11940 | 330 |
| H3′G | 40 (±4) | 8 (±1)b | 9450 | 9980 | 310 |
| H7G | 61 (±1) | 26 (±1)b | 12270 | 3900 | 1070 |
| HCd | 80 (±9) | 64 (±16)e | 3710 | 8480 | 12130 |
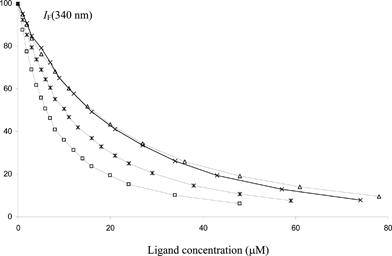 | ||
| Fig. 4 Changes in the relative fluorescence intensity of HSA at 340 nm (λex = 295 nm) as a function of the total flavanone concentration (pH 7.4 phosphate buffer, 25 °C). Initial HSA concentration = 2 μM. Ligands: Nar (*), N4′G (×), N7G (△), NC (□). | ||
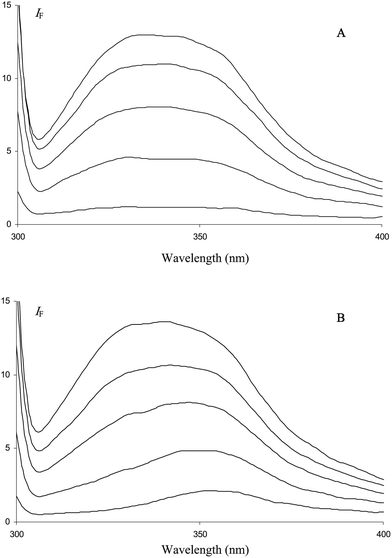 | ||
| Fig. 5 Quenching of Trp214 fluorescence by H7G (A) and H3′G (B). Initial HSA/ligand ratio = 1, final HSA/ligand ratio = 20. | ||
Fluorescent probes dansylsarcosine (DNSS) and the common dietary flavonol quercetin (Fig. 1) were used in competition experiments with the flavanones to gain information about the binding sites.
DNSS was reported to bind to site 2 of HSA.35,36 More recently, the binding sites of dansyl aminoacids were fully clarified by X-ray crystallography.37 While dansyl amino acids with polar or charged side chains (e.g., dansyl-L-Asn, dansyl-L-Glu, dansyl-L-Arg) bind preferentially to site 1, those with hydrophobic side chains (dansyl-L-Phe, dansyl-L-norvaline) or derived from amino acids having a secondary α-amino group (L-proline, sarcosine) are specific for site 2. In particular, unlike the dansyl amino acids that bind to site 1, DNSS is unable to form a hydrogen bond to the carbonyl oxygen of Ala-291 through its α-amino group. Moreover, the dansyl moiety of site 2 ligands such as DNSS is pinned between the side-chains of Asn-391 and Phe-403 on one side and Leu-453 on the other.
The fluorescence of the DNSS–HSA complex was gradually quenched by increasing flavanone concentrations. The quenching curves could be analyzed within the hypothesis of pure competitive 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for both the probe and ligand and by taking into account a very weak inner filter effect due to absorption of the ligand at the excitation wavelength (Table 1). However, the quenching of DNSS–HSA fluorescence by all ligands gave K1 values significantly weaker than those determined from the quenching of HSA fluorescence, which is indicative of noncompetitive binding. Hence, a new model was devised in which two sub-populations of HSA particles are considered: free HSA, which binds the flavanones with binding constant K1, and the HSA–DNSS complex, which binds the flavanones with binding constant K2 (formation of a ternary HSA–DNSS–flavanone complex). Given the HSA–DNSS binding constant (KD = 156 × 103 M−1), the concentration of HSA–DNSS complex lies in the range 34–46% of the total HSA concentration in our experimental conditions. From Table 2, it is clear that K2 values are typically 5–10 times as low as the corresponding K1 values. We thus propose that hesperetin, naringenin and their glucuronides bind to HSA noncompetitively with site 2 probe DNSS. However, the preliminary binding of DNSS severely weakens the affinity of the flavanones for HSA, which suggests that the two binding sites are close.
1 binding for both the probe and ligand and by taking into account a very weak inner filter effect due to absorption of the ligand at the excitation wavelength (Table 1). However, the quenching of DNSS–HSA fluorescence by all ligands gave K1 values significantly weaker than those determined from the quenching of HSA fluorescence, which is indicative of noncompetitive binding. Hence, a new model was devised in which two sub-populations of HSA particles are considered: free HSA, which binds the flavanones with binding constant K1, and the HSA–DNSS complex, which binds the flavanones with binding constant K2 (formation of a ternary HSA–DNSS–flavanone complex). Given the HSA–DNSS binding constant (KD = 156 × 103 M−1), the concentration of HSA–DNSS complex lies in the range 34–46% of the total HSA concentration in our experimental conditions. From Table 2, it is clear that K2 values are typically 5–10 times as low as the corresponding K1 values. We thus propose that hesperetin, naringenin and their glucuronides bind to HSA noncompetitively with site 2 probe DNSS. However, the preliminary binding of DNSS severely weakens the affinity of the flavanones for HSA, which suggests that the two binding sites are close.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) complex in a pH 7.4 phosphate buffer at 25 °C
1) complex in a pH 7.4 phosphate buffer at 25 °C
| Ligand | K 1 (×103, M−1)a | DNSS, HSA (μM) | X B b | K 2 (×103, M−1)c | εL (M−1 cm−1) 370 nm |
|---|---|---|---|---|---|
| a HSA - ligand L binding constant (from Table 1). b fraction of HSA bound to probe (using a DNSS - HSA binding constant of 156 × 103 M−1). c binding constant of ligand L to DNSS – HSA complex, SD from curve-fitting. | |||||
| Nar | 77 | 10, 10 | 0.46 | 10.4 (± 0.5) | 330 |
| 5, 5 | 0.34 | 14.3 (± 0.7) | |||
| N4′G | 36 | 10, 10 | 0.46 | 4.6 (± 0.1) | 360 |
| 5, 5 | 0.34 | 7.7 (± 0.2) | |||
| N7G | 46 | 10, 10 | 0.46 | 8.0 (± 0.3) | 1150 |
| 5, 5 | 0.34 | 10.5 (± 0.3) | |||
| Hesp | 85 | 10, 10 | 0.46 | 5.9 (± 0.2) | 330 |
| 5, 5 | 0.34 | 8.7 (± 0.4) | |||
| H3′G | 40 | 10, 10 | 0.46 | 3.0 (± 0.1) | 310 |
| 5, 5 | 0.34 | 4.7 (± 0.2) | |||
| H7G | 61 | 10, 10 | 0.46 | 9.2 (± 0.3) | 1070 |
| 5, 5 | 0.34 | 12.3 (± 0.4) |
Quercetin is a convenient probe for the identification of HSA binding sites due to its specific fluorescence properties. Indeed, quercetin is essentially nonfluorescent in its free state but becomes strongly fluorescent once bound to HSA.9,31 Interestingly, fluorescence emission is maximal when the complex is excited at 450 nm (well above its wavelength of maximal UV-visible absorption), i.e. at a wavelength for which interference with protein and other ligands is generally negligible. The primary binding site of quercetin and other flavones and flavonols is generally assumed to be site 19,28 due to their structural similarity with the coumarin warfarin (Fig. 1), whose binding site in sub-domain IIA was well established by X-ray cristallography.38 In particular, the roof and floor of the main pocket are delimited by Ala-291 and Leu-238, respectively, and warfarin binds to the bottom of this chamber (furthest from the entrance) so that an additional side pocket, involving for instance Leu-219 and the aliphatic portion of Arg-222, remains available to accommodate hydrophobic portions of other site 1 ligands. Docking experiments suggest that quercetin and warfarin can simultaneously bind to site 1, quercetin being partly located into the side pocket with its A-ring in van der Waals contact with Ala-291, Leu-238, Leu-219 and Arg-222.28 This prediction is consistent with our observation that warfarin does not alter the binding of quercetin to HSA.9 Docking experiments also suggest that the B-ring of quercetin protrudes into sub-domain IIIA with possible van der Waals contact with Pro-447 and hydrogen bonding between O4′–H and the side chain carboxylate of Asp-451.28
With the citrus flavanones and their derivatives, experiments in the presence of quercetin proved to be more discriminating than the ones involving DNSS (Fig. 6). In particular, it was observed that the flavanone aglycones and glucuronides bind noncompetitively with respect to quercetin as a strong residual fluorescence is observed at high flavanone/quercetin molar ratios (indicative of the formation of fluorescent flavanone–quercetin–HSA complexes). By contrast, flavanone chalcones totally quenched the fluorescence of the quercetin–HSA complex in an apparently competitive mode.
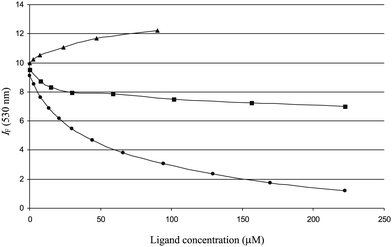 | ||
| Fig. 6 Influence of Nar (■), N4′G (▲) and NC (•) on the quercetin–HSA fluorescence. | ||
In summary, quercetin is a site 1 ligand closer to site 2 than the typical site 1 probe warfarin. It is significantly displaced by flavanone chalcones only. Indeed, chalcones, unlike flavanones, display largely planar conformations (see below) and an extended electron delocalization, which are two characteristics they share with quercetin. Flavanones and their glucuronides do not displace quercetin from its binding site, which is close to the entry of site 1. It is thus proposed that they bind to site 2. The binding pocket is distinct from but close to the one of DNSS, as DNSS significantly lowers the affinity of flavanones to HSA.
3.3. Chalcone–flavanone isomerization
Under mildly alkaline conditions, 2′-hydroxychalcones are cyclized into flavanones through intramolecular Michael addition involving an enolate intermediate. The mechanism was studied in details.20,39,40 However, the influence of HSA, the typical carrier of dietary polyphenols in the blood circulation, on this reaction is not known. Considering that glucuronidation of flavanones does not markedly alter their binding to HSA, we may also assume that the same applies to the parent chalcones and that data obtained from chalcone aglycones can be extrapolated to the circulating glucuronides. Thus, chalcones, obtained from the opening of the flavanone C-ring in highly alkaline conditions, were cyclized in a pH 7.4 phosphate buffer in the absence or presence of HSA. The reactions were followed by UV-visible spectroscopy at different temperatures (298 K, 304 K, 310 K) and for different HSA/chalcone molar ratios (0–2 equiv.). Spectroscopic monitoring (Fig. 7) shows a gradual increase in the characteristic flavanone band (323 nm) and concomitant decrease in the characteristic chalcone band (382 nm). It is noteworthy that the final absorbance at 382 nm is essentially zero in the absence or presence of HSA, which means that the chalcones are completely converted into the corresponding flavanones. Hence, no equilibrium is achieved in neutral conditions. The first-order rate constants of cyclization were estimated (Table 3). Based on the selective monitoring of chalcone depletion at 382 nm, cyclization of naringenin chalcone appears slower by a factor 7–10 in the presence of HSA (2 equiv.) depending on the selected temperature. As for hesperetin chalcone, the slowing down is smaller (a factor 4–6). Moreover, the rate constants increase by a factor 3–4 when the temperature is raised from 298 to 310 K. As expected, the rates of chalcone depletion and flavanone formation are close to each other.| HSA/chalcone molar ratio, T | Naringenin chalcone (×10−4, s−1) | Hesperetin chalcone (×10−4, s−1) | ||
|---|---|---|---|---|
| 382 nm | 323 nm | 382 nm | 323 nm | |
| 0, 25 °C | 17.4 (± 0.7) | 17.7 (± 0.9) | 13.8 (± 0.2) | 17.2 (± 0.5) |
| 0, 31 °C | 27.9 (± 1.1) | 28.8 (± 1.1) | 25.6 (± 0.6) | 28.4 (± 2.1) |
| 0, 37 °C | 50.0 (± 0.4) | 49.4 (± 1.5) | 42.5 (± 2.5) | 42.7 (± 0.7) |
| 0.4, 25 °C | 6.0 (± 0.1) | 6.1 (± 0.2) | 6.9 (± 0.7) | 7.3 (± 1.9) |
| 0.8, 25 °C | 3.4 (± 0.1) | 3.0 (± 0.3) | 4.9 (± 0.3) | 3.8 (± 0.6) |
| 1.2, 25 °C | 2.8 (± 0.1) | 2.6 (± 0.3) | 3.6 (± 0.1) | 3.2 (± 0.2) |
| 1.6, 25 °C | 2.2 (± 0.1) | 1.9 (± 0.3) | 2.7 (± 0.1) | 2.4 (± 0.1) |
| 2, 25 °C | 1.8 (± 0.1) | 1.7 (± 0.1) | 2.5 (± 0.1) | 2.3 (± 0.1) |
| 2, 31 °C | 3.9 (± 0.1) | 3.5 (± 0.1) | 5.1 (± 0.1) | 4.8 (± 0.1) |
| 2, 37 °C | 7.5 (± 0.2) | 6.9 (± 0.2) | 9.7 (± 0.2) | 9.3 (± 0.2) |
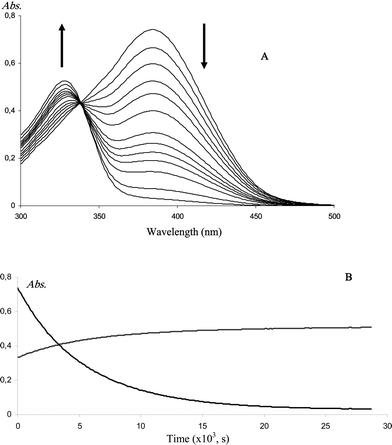 | ||
| Fig. 7 A: UV-visible spectral changes during the cyclization of naringenin chalcone. B: kinetic monitoring at 382 nm and 323 nm. HSA/chalcone molar ratio = 2; T = 25 °C. | ||
A gradual decrease in the cyclization rate constants was observed with increasing HSA concentrations (Table 3). This reflects the increase in the fraction of bound chalcones, which appear less prone to cyclization than free chalcones. However, flavanones by far remain the most stable isomers, either in their free or bound form. In particular, even in the presence of HSA, no chalcone is retained in neutral solution. This higher stability of flavanones in comparison with their chalcone precursors is also suggested by molecular modeling experiments (Fig. 8). It can also be noted that HSA slows down the cyclization of NC more strongly than that of HC, in agreement with NC having a higher affinity for HSA than HC (Table 1).
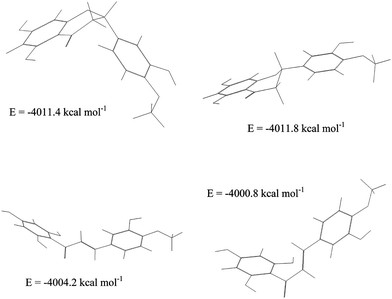 | ||
| Fig. 8 Low-energy conformations of hesperetin (top) and its chalcone (bottom). | ||
The kinetic data were also analyzed according to the Eyring equation (eqn (7)) so as to estimate the activation enthalpy and entropy of cyclization (Table 4).
| ln(kc/T) = ΔS≠/R + ln(kB/h) − ΔH≠/RT | (7) |
| ΔH≠ (kJ mol−1) | ΔS≠ (J K−1 mol−1) | |
|---|---|---|
| Naringenin chalcone | 65.0 (± 4.9) | −80 (± 16) |
| Naringenin chalcone - HSA | 88.5 (± 2.8) | −19.3 (± 9.3) |
| Hesperetin chalcone | 69.5 (± 3.3) | −66 (± 11) |
| Hesperetin chalcone - HSA | 85.1 (± 1.4) | −28.3 (± 4.7) |
From Table 4, it is clear that the HSA environment slows down the cyclization of chalcones by markedly raising the activation enthalpy. Molecular modeling shows that chalcones display low-energy s-cis and s-trans conformations (Fig. 8). While the extended s-cis conformation is more stable than s-trans, the latter only is involved in the cyclization. It can thus be suggested that HSA more strongly binds s-cis, thereby lowering the concentration of reactive s-trans conformation. This HSA-induced restriction in the conformational flexibility of chalcones would be partly released in the transition state in agreement with a less unfavorable activation entropy for the cyclization in the presence of HSA.
Flavanones resulting from the cyclization of HSA-bound chalcones were analyzed by chiral UPLC to look for possible enantioselective ring closure promoted by the chiral environment of the chalcone binding site. During flavonoid biosynthesis, chalcone isomerase catalyzes the intramolecular cyclization of chalcones into the corresponding (2S)-flavanones. It was demonstrated that chalcone isomerase locks the chalcone 2′-oxyanion substrate into a constrained conformation, permitting the cyclization to take place near the diffusion-controlled limit.11 As for HSA, the chiral separation of the flavanone enantiomers showed that the cyclization was not enantioselective and actually resulted in a racemic mixture (data not shown). Thus, while HSA itself may also induce restrictions in the conformational flexibility of chalcones, these changes favour inert conformations and do not orient the attack of 2′-OH on one of the two sides of the enone moiety.
On the basis of their half-life at 37 °C in the presence of HSA (∼10–15 min), it can be concluded that dietary chalcones must be at least in part converted to flavanones during absorption and transport, i.e. before reaching tissues to express biological activity. This is consistent with the detection of naringenin chalcone-2′-O-β-D-glucuronide but also N4′G and N7G in the urine of rats administered naringenin chalcone22 while only chalcone-2′-O-β-D-glucuronide was found in the plasma. Indeed, considering that the 6′-OH group is blocked by a strong intramolecular H bond with the keto group and that a free 2′-OH group is no longer available in chalcone-2′-O-β-D-glucuronide, it can be concluded that this specific chalcone conjugate cannot undergo cyclization (or at a much slower rate than the aglycone).
4. Conclusion
Our study shows that flavanone and their derivatives (chalcones and glucuronides) are moderate HSA ligands in agreement with their relatively high rate of clearance from plasma.27 Unlike chalcones, which bind to site 1 in competition with quercetin, flavanone aglycones and glucuronides are proposed to bind to site 2 close to the DNSS binding cavity. The moderate affinity of flavanone glucuronides for HSA should be large enough to ensure substantial in vivo binding given the high concentration of HSA in plasma (ca. 0.6 mM) and the low concentrations of circulating flavanone metabolites (<1 μM). For instance, taking a mean binding constant of 2 × 104 M−1 and a 1 μM concentration of flavanone glucuronides, it can be calculated that more than 90% of metabolites are bound to HSA in physiological conditions if interactions with any other plasma protein are neglected. Moreover, HSA was shown to bind the chalcones and slow down their cyclization into flavanones without influencing the stereochemical outcome (formation of a racemic mixture).Acknowledgements
This work was carried out within the AGRUVASC program funded by the French National Research Agency (ANR). The Higher Education Commission of Pakistan is gratefully acknowledged for providing a grant to MKK.References
- A. Crozier, D. Del Rio and M. N. Clifford, Bioavailability of dietary flavonoids and phenolic compounds, Mol. Aspects Med., 2010, 31, 446–467 CrossRef CAS.
- O. Benavente-Garcia and J. Castillo, Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity, J. Agric. Food Chem., 2008, 56, 6185–6205 CrossRef CAS.
- I. Erlund, Review of the flavonoids quercetin, hesperetin, and naringenin: Dietary sources, bioactivities, bioavailability, and epidemiology, Nutr. Res., 2004, 24, 851–874 CrossRef CAS.
- E. Tripoli, M. L. Guardia, S. Giammanco, D. Di Majo and M. Giammanco, Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review, Food Chem., 2007, 104, 466–479 CrossRef CAS.
- C. Manach, C. Morand, A. Gil-Izquierdo, C. Bouteloup-Demange and C. Remesy, Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice, Eur. J. Clin. Nutr., 2003, 57, 235–242 CrossRef CAS.
- C. Felgines, O. Texier, C. Morand, C. Manach, A. Scalbert, F. Regerat and C. Remesy, Bioavailability of the flavanone naringenin and its glycosides in rats, Am. J. Physiol. Gastrointest. Liver Physiol., 2000, 279, G1148–G1154 CAS.
- H. Matsumoto, Y. Ikoma, M. Sugiura, M. Yano and Y. Hasegawa, Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma, J. Agric. Food Chem., 2004, 52, 6653–6659 CrossRef CAS.
- A. Varshney, P. Sen, E. Ahmad, M. Rehan, N. Subbarao and R. H. Khan, Ligand binding strategies of human serum albumin: How can the cargo be utilized?, Chirality, 2010, 22, 77–87 CrossRef CAS.
- C. Dufour and O. Dangles, Flavonoid-serum albumin complexation: determination of binding constants and binding sites by fluorescence spectroscopy, Biochim. Biophys. Acta, Gen. Subj., 2005, 1721, 164–173 CrossRef CAS.
- F. Kratz, Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles, J. Controlled Release, 2008, 132, 171–183 CrossRef CAS.
- J. M. Jez and J. P. Noel, Reaction mechanism of chalcone isomerise, J. Biol. Chem., 2002, 277, 1361–1369 CrossRef CAS.
- E. Capanoglu, J. Beekwilder, D. Boyacioglu, R. Hall and R. de Vos, Changes in antioxidant and metabolite profiles during production of tomato paste, J. Agric. Food Chem., 2008, 56, 964–973 CrossRef CAS.
- R. Slimestad, T. Fossen and M. J. Verheul, The flavonoids of tomatoes, J. Agric. Food Chem., 2008, 56, 2436–2441 CrossRef CAS.
- A. Gil-Izquierdo, M. I. Gil, F. A. Tomas-Barberan and F. Ferreres, Influence of industrial processing on orange juice flavanone solubility and transformation to chalcones under gastrointestinal conditions, J. Agric. Food Chem., 2003, 51, 3024–3028 CrossRef CAS.
- S. Hirai, Y. Kim, T. Goto, M. S. Kang, M. Yoshimura, A. Obata, R. Yu and T. Kawada, Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages, Life Sci., 2007, 81, 1272–1279 CrossRef CAS.
- C. Iwamura, K. Shinoda, M. Yoshimura, Y. Watanabe, A. Obata and T. Nakayama, Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells, Allergol. Int., 2010, 59, 67–73 CrossRef CAS.
- M. K. Khan, R. Rakotomanomana, M. Loonis and O. Dangles, Chemical synthesis of flavanone glucuronides, J. Agric. Food Chem., 2010, 58, 8437–8443 CrossRef CAS.
- S. Galland, N. Rakotomanomana, C. Dufour, N. Mora and O. Dangles, Synthesis of hydroxycinnamic acid glucuronides and investigation of their affinity for human serum albumin, Org. Biomol. Chem., 2008, 6, 4253–4260 CAS.
- S. Caccamese, C. Caruso, N. Parrinello and A. Savarino, High-performance liquid chromatographic separation and chiroptical properties of the enantiomers of naringenin and other flavanones, J. Chromatogr., A, 2005, 1076, 155–162 CrossRef CAS.
- E. A. Gonzalez, M. A. Nazareno and C. D. Borsarelli, Enthalpy-entropy compensation effect in the chalcone formation from naringin in water-ethanol mixtures, J. Chem. Soc., Perkin Trans. 2, 2002, 2052–2056 RSC.
- S. A. Andujar, M. A. Filippa, F. H. Ferretti and S. E. Blanco, Isomerization of 4'-methoxyflavanone in alkaline medium. Determination of the enolate formation constant, J. Mol. Struct., 2003, 636, 157–166 CrossRef CAS.
- M. Yoshimura, A. Sano, J. Kamei and A. Obata, Identification and quantification of metabolites of orally administered naringenin chalcone in rats, J. Agric. Food Chem., 2009, 57, 6432–6437 CrossRef CAS.
- I. Petitpas, T. Grune, A. A. Bhattacharya and S. Curry, Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids, J. Mol. Biol., 2001, 314, 955–960 CrossRef CAS.
- J. Ghuman, P. A. Zunszain, I. Petitpas, A. A. Bhattacharya, M. Otagiri and S. Curry, Structural basis of the drug-binding specificity of human serum albumin, J. Mol. Biol., 2005, 353, 38–52 CrossRef CAS.
- X. M. He and D. C. Carter, Atomic structure and chemistry of human serum albumin, Nature, 1992, 358, 209–215 CrossRef CAS.
- S. Sugio, A. Kashima, S. Mochizuki, M. Noda and K. Kobayashi, Crystal structure of human serum albumin at 2.5 Ǻ resolution, Protein Eng., Des. Sel., 1999, 12, 439–446 CrossRef CAS.
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Remesy, Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies, Am. J. Clin. Nutr., 2005, 81(suppl), 230S–42S CAS.
- F. Zsila, Z. Bikadi and M. Simonyi, Probing the binding of the flavonoid, quercetin to human serum albumin by circular dichroism, electronic absorption spectroscopy and molecular modelling methods, Biochem. Pharmacol., 2003, 65, 447–456 CrossRef CAS.
- M. Xie, X. Xu and Y. Wang, Interaction between hesperetin and human serum albumin revealed by spectroscopic methods, Biochim. Biophys. Acta., 2005, 1724, 215–224 CrossRef CAS.
- K. Murota, A. Hotta, H. Ido, Y. Kawai, J. H. Moon, K. Sekido, H. Hayashi, T. Inakuma and J. Terao, Antioxidant activity of albumin-bound quercetin metabolites after onion consumption in humans, J. Med. Invest., 2007, 54, 370–374 CrossRef.
- O. Dangles, C. Dufour and S. Bret, Flavonol-serum albumin complexation. Two-electron oxidation of flavonols and their complexes with serum albumin, J. Chem. Soc., Perkin Trans. 2, 1999, 737–744 RSC.
- A. Banerjee, K. Basu and P. K. Sengupta, Interaction of 7-hydroxyflavone with human serum albumin: A spectroscopic study, J. Photochem. Photobiol. B, 2008, 90, 33–40 CAS.
- D. Epps, T. J. Raub, V. Caiolfa, A. Chiari and M. Zamai, Determination of the affinity of drugs toward serum albumin by measurement of the quenching of the intrinsic tryptophan fluorescence of the protein, J. Pharm. Pharmacol., 1999, 51, 41–48 CrossRef CAS.
- W. He, Y. Li, J. Liu, Z. Hu and X. Chen, Specific interaction of chalcone-protein: Cardamonin binding site II on the human serum albumin molecule, Biopolymers, 2005, 79, 48–57 CrossRef CAS.
- D. E. Epps, T. J. Raub and F. J. Kezdy, A general, wide range spectrofluorometric method for measuring the site specific affinities of drugs towards human serum albumin, Anal. Biochem., 1995, 227, 342–350 CrossRef CAS.
- U. Mathias and M. Jung, Determination of drug–serum protein interactions via fluorescence polarization measurements, Anal. Bioanal. Chem., 2007, 388, 1147–1156 CrossRef CAS.
- A. J. Ryan, J. Ghuman, P. A. Zunszain, C. Chung and S. Curry, Structural basis of binding of fluorescent, site-specific dansylated amino acids to human serum albumin, J. Struct. Biol., 2011, 174, 84–91 CrossRef CAS.
- I. Petitpas, A. A. Bhattacharya, S. Twine, M. East and S. Curry, Crystal structure analysis of warfarin binding to human serum albumin, J. Biol. Chem., 2001, 276, 22804–22809 CrossRef CAS.
- C. O. Miles and L. Main, Kinetics and mechanism of the cyclisation of 2‘,6’-dihydroxy-4,4‘-dimethoxychalcone; influence of the 6’-hydroxy group on the rate of cyclisation under neutral conditions, J. Chem. Soc., Perkin Trans. 2, 1985, 1639–1642 RSC.
- N. S. Nudelman and J. J. P. Furlong, Conversion of flavanones into chalcones in alkaline medium. Kinetic and spectroscopic studies, J. Phys. Org. Chem., 1991, 4, 263–270 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
