Antioxidative, antibrowning and antibacterial activities of sixteen floral honeys
Xin
Chang
a,
Jiehua
Wang
b,
Shaohui
Yang
b,
Shan
Chen
a and
Yingjin
Song
*b
aSchool of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072, China
bSchool of Agriculture and Bioengineering, Tianjin University, Tianjin 300072, China. E-mail: jiehuaw_tju@yahoo.com; Fax: +86-22-87401878; Tel: +86-22-87402171
First published on 22nd August 2011
Abstract
Commonly consumed honeys from sixteen different single floral sources were analyzed for their in vitroantioxidant capacities by several methods including DPPH, ABTS, FRAP, SASR and MDA assays. The total polyphenol contents varied among the tested honeys and were highly correlated to their antioxidant capacity values. The antioxidant capacity of Chinese milk vetch flower honeys was significantly higher than those of other flower honeys. All honeys tested were active in inhibiting the browning of apple homogenate and linden honey displayed the highest inhibition rate as 85%. When the antimicrobial activity of the investigated honeys was screened using Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli), clover honey exhibited the strongest antibacterial activity as 2.2 mg mL−1kanamycin equivalent inhibition.
Introduction
Honey is a sweet food made from nectar by honey bees through a process of regurgitation and is stored as a food source in wax honeycombs inside the beehive. As a mixture of sugars and other compounds, honey gets its sweetness mainly from fructose and glucose with the remaining carbohydrates including maltose, sucrose, and other complex carbohydrates.1 For at least 2700 years, honey has been used by humans to treat a variety of ailments through topical application, but only recently have the antiseptic and antibacterial properties of honey been chemically explained. China is the top producer of natural honey according to the reports of the Food and Agriculture Organization of the United Nations (FAO).Recent scientific evidence supports the antioxidant2–4 and antibacterial5–9 effectiveness of some honeys. It has been demonstrated that some constituents present in honey have antioxidant properties and these include phenolic acids and flavonoids,10 certain enzymes (glucose oxidase and catalase),11ascorbic acid, protein and carotenoids.12 A good correlation has been reported between the amino acid composition of honey and its radical scavenging activity.10,13 Honey has also been proven to be effective against deteriorative oxidation reactions in foods, such as lipid oxidation4,14 and enzymatic browning of fruits and vegetables.2
The present study is undertaken to evaluate the antioxidant potential of the main flower honeys consumed in China and determine which composition of honey has correlation with their antioxidant capacities. The antibrowning and antimicrobial effects of these honeys have been compared with the aims to contribute to the current knowledge about the beneficial effects of floral honeys for human health.
Materials and methods
Honey samples
Monofloral honeys were obtained from the Tongrentang (TRT) Pharmaceutical Cooperation, which was founded in the year of 1669 and has become an authentic honey producer in China. Microscopic analysis of honey sediment composition was performed for pollen grain morphometric measurements to confirm that the nominate monofloral type predominates. The floral sources of samples collected were: linden (Tilia tuan), Chinese wolfberry (Lycium barbarum), Chinese milk vetch (Astragalus sinicus L.), sunflower (Helianthus annus L.), lychee (Litchi chinensis), loquat (Eriobotrya japonica), cut-leaf chastetree (Vitex negundo var. heterophylla), orange (Citrus reticulata Banco), clover (Medicago sativa L.), codonopsis (Radix codonopsis), schisandra (Fructus schisandrae), black locust (Robinia pseudoacacia L.), milkvetch (Astragalus adsurgens), mother wort (Herba leonuri), longan (Dimocarpus longans Lour.) and jujube (Zizyphus jujuba Mill.). These samples were produced in the same year of 2009 and fresh honey samples weighing 50 g were sealed in amber glass bottles and stored at room temperature in the dark before the analyses were performed.Determination of polyphenolic and total flavonoid content
The Folin–Ciocalteu method was used to determine the total phenolic content (TPC) as reported.15 Each honey sample of 0.06 mL at 0.2 g mL−1 in distilled water was then mixed with 0.3 mL of Folin–Ciocalteu reagent and 0.2 mL of 7.5% sodium carbonate (Na2CO3). After incubation in the dark at 25 °C for 30 min, the absorbance of the reaction mixture was measured at 765 nm using a Beckman Du 640 spectrophotometer (Instruments Inc., USA). Gallic acid was used as standard to produce the calibration curve (0.05–0.3 g L−1). The total phenolic content was expressed in mg of gallic acid equivalents (mg GAE/100 g of honey).Total flavonoid content (TFC) was determined as described previously.16 Briefly, a sample of 0.15 mL of pure honey was mixed with 1.7 mL of 30% ethanol (v/v) in a test tube, followed by the addition of 0.075 mL of a 0.5 M sodium nitrite (NaNO2) solution and 0.075 mL Al(NO3)3. After 5 min, 1 mL of 1 M NaOH solution were added and mixed well. The absorbance was immediately measured against the blank (the same mixture without the sample) at 506 nm. Rutin was used as standard to produce the calibration curve (10–300 mg L−1). Total flavonoid content was expressed as mg of rutin equivalents (mg RE/100 g of honey).
Antioxidant capacity and reducing power assay
The antioxidant capacity and reducing power was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), ferric reducing antioxidant power (FRAP) and superoxide radical scavenging activity (SRSA) assays.17–19 For DPPH assay, 0.5 mL of diluted honey (0.2 g mL−1) was mixed with 2 mL DPPH solution (2 × 10−4 M in methanol), and after 30 min of incubation in dark, the absorbance was read at 517 nm against a water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blank. For ABTS assay, the stable radical cation ABTS•+ was prepared by reacting 7 mM ABTS with same volume of 2.45 mM potassium persulfate in ddH2O and the solution was kept in dark at room temperature for 12 h before use. After being diluted with water until the absorbance at 734 nm got 0.7 ± 0.02, 2 mL of 0.2 g mL−1 honey was mixed with 2 ml ABTS•+ solution and votexed for 6 min before the absorbance at 734 nm was measured. For FRAP assay, 0.3 M acetate buffer (pH 3.6), 10 mM TPTZ (dissolved in 40 mM HCL) and 20 mM FeCl3 (10
1) blank. For ABTS assay, the stable radical cation ABTS•+ was prepared by reacting 7 mM ABTS with same volume of 2.45 mM potassium persulfate in ddH2O and the solution was kept in dark at room temperature for 12 h before use. After being diluted with water until the absorbance at 734 nm got 0.7 ± 0.02, 2 mL of 0.2 g mL−1 honey was mixed with 2 ml ABTS•+ solution and votexed for 6 min before the absorbance at 734 nm was measured. For FRAP assay, 0.3 M acetate buffer (pH 3.6), 10 mM TPTZ (dissolved in 40 mM HCL) and 20 mM FeCl3 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) were mixed and kept at 37 °C. Honey at 0.1 g mL−1 (1 mL) was mixed with TPTZ working solution (1.8 mL) and the mixture was incubated at 37 °C for 10 min before the absorbance was measured at 593 nm. For SRSA assay, superoxide radicals were generated in PMS–NADH system by oxidation of NADH and assayed by reduction of NBT. A 500 μl honey solution at 0.1 g mL−1 was mixed with 1 mL of 78 μM NADH and 1 ml 50 μM NBT in pH 8.0, 16 mM Tris·HCl. Then 500 μl PMS at 10 μM was added and after 10 min of incubation at 25 °C, the absorbance was measured at 560 nm. All the above assays were standardized using Trolox and the results were expressed as Trolox equivalents (Trolox equivalent antioxidant capacity, TEAC). The malondialdehyde (MDA) assay was performed by thiobarbituric acid (TBA) reaction using commercially available kit A003 (Nanjing Jiancheng BioEng. Co., China) and the results were expressed as MDA formation per 100 g honey.
1, v/v/v) were mixed and kept at 37 °C. Honey at 0.1 g mL−1 (1 mL) was mixed with TPTZ working solution (1.8 mL) and the mixture was incubated at 37 °C for 10 min before the absorbance was measured at 593 nm. For SRSA assay, superoxide radicals were generated in PMS–NADH system by oxidation of NADH and assayed by reduction of NBT. A 500 μl honey solution at 0.1 g mL−1 was mixed with 1 mL of 78 μM NADH and 1 ml 50 μM NBT in pH 8.0, 16 mM Tris·HCl. Then 500 μl PMS at 10 μM was added and after 10 min of incubation at 25 °C, the absorbance was measured at 560 nm. All the above assays were standardized using Trolox and the results were expressed as Trolox equivalents (Trolox equivalent antioxidant capacity, TEAC). The malondialdehyde (MDA) assay was performed by thiobarbituric acid (TBA) reaction using commercially available kit A003 (Nanjing Jiancheng BioEng. Co., China) and the results were expressed as MDA formation per 100 g honey.
Determination of browning inhibition and antimicrobial tests
A spectrophotometric assay was used to determine the browning inhibiting capacities of honey as previously described.2 Briefly, fresh juice of Red Delicious apples were incubated with or without honey at 1 g mL−1 concentration and the mixture was kept at room temperature for 10 min before the homogenate was extracted with aqueous methanol. The extract was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 5 min before its optical density was determined at 420 nm against an aqueous methanol blank. Data was represented as the change in the absorbance and the % of browning inhibition was calculated as (Ac-Aa-Ab)/Ac × 100, where Ac is the absorbance of apple homogenate without honey, Aa is the absorbance of apple homogenate with honey and Ab is the absorbance of honey itself.
000 g for 5 min before its optical density was determined at 420 nm against an aqueous methanol blank. Data was represented as the change in the absorbance and the % of browning inhibition was calculated as (Ac-Aa-Ab)/Ac × 100, where Ac is the absorbance of apple homogenate without honey, Aa is the absorbance of apple homogenate with honey and Ab is the absorbance of honey itself.
The antimicrobial efficacy of honey was tested using Gram-positive S. aureusESA 40 and Gram-negative E. coliDH5α, both of which were obtained from China General Microbiological Cultural Collection Centre, Beijing and maintained on LB media.8 Different concentrated solutions of kanamycin (Invitrogen, USA) (50–1000 μg mL−1) were used as standard.
Statistical analysis
Data were processed using SPSS 12.0 software for Windows. The Levene test was used to check equality of variances, and one-way ANOVA (LSD test) was used to estimate statistically significant differences (p < 0.05).Results and discussion
Phenolic acids and flavonoids have been considered as the potential markers of the honey botanical origins and they contribute to honey color, taste and flavor as well as to their beneficial effects on health.16 Based on the results of the colorimetric analysis using Folin–Ciocalteu reagent, the polyphenolic and flavonoid content of honeys from various floral sources were examined (Fig. 1). The phenolic concentrations of all honey varieties were estimated as milligrams of gallic acid equivalent (GAE) per 100 g honey. The total polyphenol content ranged from 100.8 ± 4.9 (Chinese milk vetch) to 60.5 ± 2.3 (Loquat) GAE/100 g honey and the total flavonoid content varied from 2.3 ± 0.4 (Chinese milk vetch) to 0.6 ± 0.2 (Jujube), as mg rutin per 100 g honey (Fig. 1A).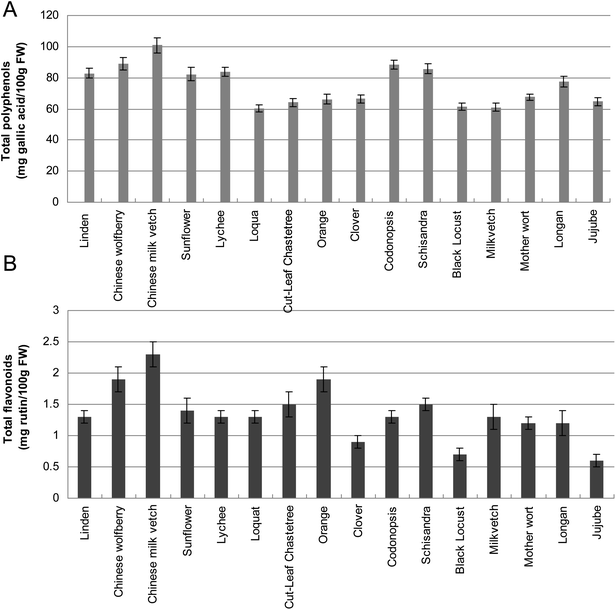 | ||
| Fig. 1 Contents of polyphenols (Fig. 1A) and flavonoids (Fig. 1B) in 16 floral honeys. Values are means of 3 replicates ± SD. FW, Fresh Weight. | ||
Considering the multifaceted aspects of antioxidants and their reactivity, several antioxidant assays including the DPPH, ABTS, FRAP and SRSA tests were applied to evaluate the antioxidant capacities and reducing power of various honeys. The free radical scavenging activity determined by DPPH of tested honeys varied from 101.2 ± 1.6 (Chinese milk vetch) to 56.0 ± 1.1 (Loquat) mg Trolox per 100 g fresh honey (Fig. 2A). It has been shown that in terms of radical scavenging capacity measured by DPPH method, honeydew honeys has a higher activity than floral honeys and blend honeys.13 It has also been reported that phenolic compounds in dark honey had higher activity than those obtained from clear honey.3,4,16 In this study, the honey with the darkest color is Chinese milk vetch and it also exhibited the highest level of DPPH antioxidant activity. The next four honeys with relatively darker color including codonopsis, orange, Chinese wolfberry and lychee, and they also exhibited higher antioxidant activities than other honey types in DPPH test (Fig. 2A). The values determined by ABTS assay ranged from 14.5 ± 0.3 (clover and milk vetch) to 10.1 ± 0.1 (sunflower) mg Trolox per 100 g fresh honey (Fig. 2B). In the ferric reducing antioxidant power (FRAP) assay, the ability of different types of honey to reduce Fe3+ to Fe2+ ranging from 14.9 ± 0.6 (Chinese milk vetch) to 7.0 ± 0.3 (milk vetch) mg Trolox per 100 g fresh honey (Fig. 2B). In order to measure the antioxidant capacities of honey to inhibit the lipid peroxidation, the malondialdehyde (MDA) assay was performed and the results showed that codonopsis and cut-leaf chastetree honey exhibited the highest and lowest activity in inhibiting the MDA production, respectively (Fig. 3A).
 | ||
| Fig. 2 Antioxidant activities of different honey types determined by DPPH and SRSA methods (2A); ABTS and FRAP methods (2B). | ||
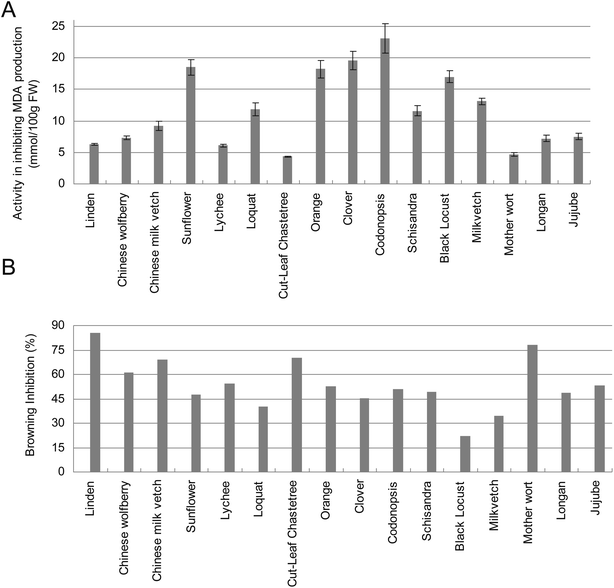 | ||
| Fig. 3 Antioxidant activities of different honey types determined by MDA (3A) and antibrowning methods (3B). | ||
Correlation analysis was used to explore the relationships amongst the different antioxidant variables. A correlation coefficient (R2) of 0.92, 0.95, 0.88 and 0.90 was established between the phenolic content and the antioxidative abilities obtained by the DPPH, ABTS, SRSA and FRAP methods, respectively. This correlation suggested that the phenolic compounds are, at least in part, responsible for the antioxidant effects of the tested honeys although there are probably other unknown components also contributing to their antioxidant actions. For example, the Chinese milk vetch honey possessed the highest level of total polyphenols in this study and it also exhibited the highest antioxidant activity revealed by DPPH, SRSA and FRAP assays (Fig. 2). It has been reported that the antioxidant capacity of honey is the result of the combined activity of a wide range of compounds, including phenolics, peptides, organic acids, enzymes, Maillard reaction products, and possibly other minor components.3,20 The results obtained from the partial identification of honey phenolic compounds in Northeast Portugal honey by HPLC showed that p-hydroxybenzoic acid, cinnamic acid, naringenin, pinocembrin and chrysin are the phenolic compounds present in most of the samples analyzed.15 Meanwhile, when the contents of proline and total free amino acids in honey samples were evaluated in another study, good correlation coefficients were obtained between the first two components and the radical scavenging activity.13 In contrast, no correlation has been identified between the content of flavonoid or protein and the antioxidant capacities in this study and no significant correlation between phenolic contents and MDA assay results was detected either. Good correlation coefficients were also detected among four antioxidative assays used in this study; however, no significant correlation was found between MDA and any of them.
Honey has been utilized for browning control in the processing of light raisins21 and in grape juice processing.22 It has been reported that honey could inhibit PPO activity in apple slices, grape juice, and in model systems.23 In this study, honeys from different floral sources exhibited various degree of browning inhibiting activity when incubated together with apple homogenates (Fig. 3B). The effectiveness of honeys in reducing browning was thought to be due to the protein components of honey which could form a complex with polyphenolic tannins;2,24 however, the clarification rate did not correlate well with any particular phenolic group of the juices.25 The linen honey exhibiting the highest antibrowning activity in this work only possessed a modest high level of total protein and polyphenol contents (Fig. 3B).
Antibacterial properties of honey have been related to the level of hydrogen peroxide determined by its relative levels of glucose oxidase, catalase26 and other nonperoxide factors such as lysozyme, phenolic acids and flavonoids.27,28Fig. 4 shows the antibacterial activities of floral honeys against selected pathogenic bacteria measured by the agar diffusion method. The strongest antibacterial activity of honey was recorded by clover honey in this study (Fig. 4). There are several studies attributing the inhibitory effect of plant extracts against bacterial pathogens to their phenolic composition29,30 and the inhibitory effect of these phenolics could be explained by its adsorption to cell membranes, interaction with enzymes, and substrate and metal ion deprivation.31 In a previous biological assay of Northeast Portugal honey, S. aureus has been shown to be the most sensitive microorganisms and B. subtilis, S. lentus, K. pneumoniae and E. coli were moderately sensitive to the antimicrobial activity of its phenolic compounds extracts.16 In this study, most tested honeys exhibited stronger inhibition capacity against S. aureus than E. coli except jujube and schisandra honeys (Fig. 4).
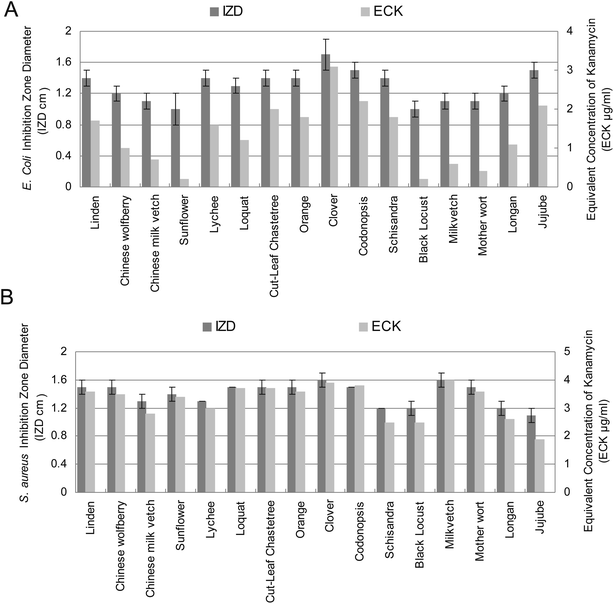 | ||
| Fig. 4 Antibacterial activities of sixteen floral honeys at concentrations (0.7 g mL−1) against E. coli and S. aureus. Inhibition zone calculated in diameter around the disc (mm ± SD). IZD, inhibition zone diameter; ECK, equivalent concentration of kanamycin. | ||
Conclusion
In conclusion, sixteen selected floral honeys traditionally consumed in China were evaluated for their total antioxidant capacities on the basis of multimechanistic antioxidant assays. The current information on the honey variety with different antioxidant potential would be useful in the choice for human health benefits and we believe in addition to antioxidants, more active components in honey will be characterized and used for different therapeutic purposes in the near future.References
- N. Gheldof, X. H. Wang and N. J. Engeseth, J. Agric. Food Chem., 2002, 50, 5870–5877 CrossRef CAS.
- L. Chen, A. Mehta, M. Berenbaum, A. R. Zangerl and N. J. Engeseth, J. Agric. Food Chem., 2000, 48, 4997–5000 CrossRef CAS.
- N. Gheldof and N. J. Engeseth, J. Agric. Food Chem., 2002, 50, 3050–3055 CrossRef CAS.
- J. McKibben and N. J. Engeseth, J. Agric. Food Chem., 2002, 50, 592–595 CrossRef CAS.
- D. J. Willix, P. C. Molan and C. G. Harfoot, J. Appl. Bacteriol., 1992, 73, 388–394 CAS.
- J. Irish, D. A. Carter, S. E. Blair and T. A. Heard, Int. J. Antimicrob. Agents, 2008, 32, 89–90 CrossRef CAS.
- E. Temaru, S. Shimura, K. Amano and T. Karasawa, Pol. J. Microbiol., 2007, 56, 281–285 Search PubMed.
- C. Basualdo, V. Sgroy, M. S. Finola and J. M. Marioli, Vet. Microbiol., 2007, 124, 375–381 CrossRef CAS.
- A. A. Al-Jabri, B. Nzeako, Z. Al Mahrooqi, A. Al Naqdy and H. Nsanze, Br. J. Biomed. Sci., 2003, 60, 1–4 CAS.
- A. Meda, C. E. Lamien, M. Romito, J. Millogo and O. G. Nacoulma, Food Chem., 2005, 91, 571–577 CrossRef CAS.
- P. C. Molan and J. A. Betts, J. Wound Care, 2004, 13, 353–356 CAS.
- J. M. Alvarez-Suarez, S. Tulipani, S. Romandini, E. Bertoli and M. Battino, Mediterr. J. Nutr. Met., 2010, 3, 15–23 CrossRef.
- R. A. Perez, M. T. Iglesias, E. Pueyo, M. Gonzalez and C. de Lorenzo, J. Agric. Food Chem., 2007, 55, 360–365 CrossRef CAS.
- S. Antony, J. R. Rieck and P. L. Dawson, Poult. Sci., 2000, 79, 1846–1850 CAS.
- L. Estevinho, A. P. Pereira, L. Moreira, L. G. Dias and E. Pereira, Food Chem. Toxicol., 2008, 46, 3774–3779 CrossRef CAS.
- R. Ksouri, W. Megdiche, A. Debez, H. Falleh, C. Grignon and C. Abdelly, Plant Physiol. Biochem., 2007, 45, 244–249 CrossRef CAS.
- M. R. McLellan, R. W. Kim, C. Y. Lee and T. M. Long, J. Food Process. Preserv., 1995, 19, 1–8 CrossRef CAS.
- C. Y. Lee, Am. Bee J., 1996, 136, 872–873 Search PubMed.
- J. L. Oszmianski and C. Y. Lee, J. Agric. Food Chem., 1990, 8, 1892–1895 CrossRef.
- C. Y. Lee, V. Kagan, A. W. Jaworski and S. K. Brown, J. Agric. Food Chem., 1990, 38, 99–101 CrossRef CAS.
- T. Wakayama and C. Y. Lee, Food Chem., 1987, 25, 111–116 CrossRef.
- R. J. Weston, Food Chem., 2000, 71, 235–239 CrossRef CAS.
- P. B. Olaitan, O. E. Adeleke and I. O. Ola, Afr. Health Sci., 2007, 7, 159–165 Search PubMed.
- J. A. Snowdon and D. O. Cliver, Int. J. Food Microbiol., 1996, 31, 1–26 CrossRef CAS.
- M. J. Rodriguez Vaquero, M. R. Alberto and M. C. Manca de Nadra, Food Control, 2007, 18, 93–101 CrossRef CAS.
- R. Ksouri, H. Falleh, W. Megdiche, N. Trabelsi, B. Mhamdi, K. Chaieb, A. Bakrouf, C. Magne and C. Abdelly, Food Chem. Toxicol., 2009, 47, 2083–2091 CrossRef CAS.
- A. Scalbert, Phytochemistry, 1991, 12, 3875–3883 CrossRef.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, Methods Enzymol., 1999, 299, 152–178 CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 72, 1231–1237 CrossRef.
- I. F. Benzie and Y. T. Szeto, J. Agric. Food Chem., 1999, 47, 633–636 CrossRef CAS.
- H. Y. Chen and G. C. Yen, Food Chem., 2007, 101, 686–694 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
