Polyphenols prevent lipid abnormalities and arterial dysfunction in hamsters on a high-fat diet: a comparative study of red grape and white persimmon wines
Jin-Hyang
Suh
a,
Anne
Virsolvy
b,
Aurélie
Goux
a,
Cécile
Cassan
b,
Sylvain
Richard
b,
Jean-Paul
Cristol
a,
Pierre-Louis
Teissèdre
c and
Jean-Max
Rouanet
*a
aUMR 204-Prévention des Malnutritions et des Pathologies Associées, Université Montpellier 1 & 2, Place E. Bataillon, CC 023, 34095 Montpellier cedex 5, France. E-mail: jm.rouanet@univ-montp2.fr; Fax: +33467 14 35 21; Tel: +33467 14 35 21
bInserm U637, Physiopathologie cardiovasculaire, Hôpital Arnaud de Villeneuve, Bât. André Crastes de Paulet, 371, rue du Doyen G. Giraud, 34295, Montpellier cedex 5, France
cUMR 1219 Oenologie, Institut des Sciences de la Vigne et du Vin Bordeaux-Aquitaine, Université Victor Segalen Bordeaux 2, 210 chemin de Leysotte, CS 50008, 33882, Villenave d'Ornon cedex, France
First published on 30th August 2011
Abstract
Scope: We compared the effects of two dealcoholized wines, persimmon (P) and Merlot (M), in hypercholesterolemic hamsters. Methods and results: Four groups of hamsters received a standard (ST) or an atherogenic diet (AT) for 12 weeks. AT animals received either dealcoholized persimmon wine (AT + P) or Merlot wine (AT + M) by gavage, while controls received water (AT and ST). Plasma cholesterol, triglycerides and glucose and paraoxonase activity were measured. Oxidative stress was assessed by aortic O2°− production, and vascular function was evaluated in aortic rings. The atherogenic diet led to higher plasma triglycerides (246%), total cholesterol (142%), LDL-cholesterol (91%) and HDL-cholesterol (49%). Aortic production of O2°− also increased (207%) and vascular reactivity was modified with altered endothelial function as assessed by acetylcholine-dependent vasorelaxation. The two wines partially prevented these alterations, reducing O2°− production and improving vascular reactivity without altering endothelial function. There was no difference between the P and M groups, although the procyanidin composition of the two dealcoholized fractions differed significantly, and only dimer concentrations were similar. Conclusion: These findings indicate that polyphenols are responsible, at least in part, for the antiatherogenic/antioxidant effects of wines.
1 Introduction
Epidemiological studies demonstrate that the consumption of red wine is beneficial in the prevention of cardiovascular diseases, a concept embodied by the term “French Paradox”.1 The cardioprotective effects of red wine have been attributed mainly to the antioxidant properties of a broad range of polyphenols,2 especially those of the procyanidin group,3 which are supposed to affect lipid metabolism and to have beneficial effects on cardiovascular function4 by reducing cholesterolemia and triglyceridemia. They are widely present in vegetables and fruits, including grapes and persimmons, and consist of polymeric catechin and epicatechin dimers, trimers, tetramers and oligomers of up to 8 units. They are abundant in many types of grape wine and their biological properties have been extensively reviewed.4 Several studies have shown the beneficial effects of procyanidins in the prevention of atherosclerosis both in humans and in animal models (see ref. 4 and 5). However, there is no correlation between antioxidant capacity and total phenolic content, in particular procyanidin composition and the degree of polymerization of procyanidin compounds.6 Oligomeric procyanidin dimers (group B) are thought to possess the best antioxidant properties7 and have been shown to reproduce the bioactivity of wine extracts with respect to glucose and lipid metabolism.8 However, despite the large number of reports on this subject, it is difficult to draw clear conclusions regarding the effectiveness or the most active forms of procyanidins.The persimmon (Diospyros kaki) is a widely available fruit in Korea, Japan and China, and is marketed throughout Europe. This fruit contains the richest source of condensed tannins, mainly those belonging to the proanthocyanidin B group,9 which exhibit high antioxidant potential.10 Studies using fresh and dry persimmons have revealed a decrease in plasma lipid levels and an increase in plasma antioxidant activity in rats.11 In addition, persimmon tannins increase life expectancy and reduce the incidence of stroke in hypertensive rats,10 an effect attributed to the fact that persimmon tannins are 20 times more potent than the antioxidant vitamin E. Gorinstein et al.11 have reported that the whole ripe fruit exhibits hypolipidemic properties in rats subjected to a cholesterol-supplemented diet, but that the antioxidant effect of this fruit is associated mainly with persimmon phenols. However, the effects of persimmon wine on the antioxidant status of hamsters administered a high fat diet have not been examined.
The process of vinification is known to prevent polyphenol alterations and to increase their extraction rate from fruits. It has also been established that oenological practices and ageing affect phenolic content, composition and antioxidant activity, which also depend on the variety of grape and the vintage.12 Among French wines, the highest polyphenol concentrations are found in Cabernet-Sauvignon, Merlot and Pinot Noir wines.13
Here, we investigated the protective and antioxidant properties of two dealcoholized wines made from persimmons and Merlot grapes, respectively, in correlation to their polyphenol composition, in a well-characterized model of early atherosclerosis.14,15
2 Results
2.1 Wines and diets
As shown in Table 1, the polyphenol content and composition of P and M are fundamentally different. The level of total polyphenols in M (4427 ± 57 mg L−1) was 6.2 times as high as that in P (714 ± 13 mg L−1). Monomers, trimers and non determined polyphenol fractions differed significantly between the two wines. Monomers were about 10 times more concentrated in M than in P. Trimers were not detected in P whereas they were found at low concentrations in M (11.8 ± 0.3 mg L−1). Dimers were found at high levels in both M and P (313.4 ± 1.2 mg L−1 and 410.8 ± 1.4 mg L−1, respectively). The dimer fraction represented 57.5% of total polyphenol content in P and 7.2% in M. The determination of the dimer composition of the two wines revealed that in P, only the procyanidin dimer B2 was present, whereas the four procyanidins dimers, B1, B2, B3, and B4, were detected in M at similar concentrations, with B4 occurring at a slightly higher level.| (mg L−1) | Persimmon wine | Merlot wine | |
|---|---|---|---|
| a Values are means ± SEM from three measurements. ***: p < 0.001. b Total polyphenol content determined according to Singleton and Rossi27 and expressed as gallic acid equivalents (mg L−1). c Procyanidin composition (mg L−1) measured by high-performance liquid chromatography using UV detection at 280 nm, according to Chira et al.28 d Not detected. | |||
| Total polyphenolsb | 714.2 ± 35.9 | 4427.8 ±93.5*** | |
| Monomersc | Catechin | n.d. | 175.93 ± 2.21 |
| Epicatechin | 3.11 ± 0.08*** | 139.95 ± 1.01 | |
| Epicatechin-3-O-gallate | n.d. | 3.81 ± 0.06 | |
| Epigallocatechin-gallate | 29.44 ± 0.11 | 39.05 ± 0.29 | |
| 32.55 ± 0.19 | 358.7 ± 3.37 | ||
| Dimersc | B1 | n.d. | 85.84 ± 1.94 |
| B2 | 410.82 ± 3.89 | 36.37 ± 0.24 | |
| B3 | n.d. | 36.89 ± 0.63 | |
| B4 | n.d. | 152.10 ± 0.98 | |
| 410.82 ± 3.89 | 311.2 ± 0.54 | ||
| Trimersc | n.d. | 11.77 ± 0.08 | |
2.2 Effects of wines on body weight
The average energy intake was 65.3, 81.2, 75.8 and 74.9 kJ per day (data not shown) for ST, AT, AT + P, AT + M animals, respectively. Nevertheless, weight gain was 4.3 times greater in ST than in AT animals, while no difference was seen between the AT and the two wine groups. This can be attributed to the unbalanced AT diet. Abdominal adipose tissue weight was significantly greater (24%) in AT than in ST animals. The consumption of P and M reduced this effect by 22% and 9%, respectively (Table 2).| ST | AT diet | AT diet + Persimmon | AT diet +Merlot | |
|---|---|---|---|---|
| a Circulating levels of glucose, total cholesterol, HDL-C, LDL-C and triglycerides were measured in fasting plasma of hamsters. Superscripts refer to statistical comparisons vs.ST (*) and vs. AT groups (§). §: p<0.05; ** and §§: p<0.01; *** and §§§: p<0.001. | ||||
| Weight gain (g) | 29.42 ± 2.03 | 6.80 ± 3.34 *** | 3.30 ± 1.54 *** | 2.54 ± 3.57 *** |
| Fat/weight (%) | 1.57 ± 0.06 | 1.95 ± 0.07 ** | 1.52 ± 0.09 §§ | 1.78 ± 0.08 § |
| Glycemia (mmol L−1) | 8.35 ± 0.31 | 8.44 ± 0.72 | 6.98 ± 0.44 | 8.39 ± 0.43 |
| Total cholesterol (mmol L−1) | 3.02 ± 0.09 | 7.32 ± 0.45 *** | 5.58 ± 0.12 ***,§§§ | 5.80 ± 0.17 ***,§§§ |
| HDL-C (mmol L−1) | 1.86 ± 0.05 | 2.77 ± 0.15 *** | 2.77 ± 0.14 *** | 3.05 ± 0.09 *** |
| LDL-C (mmol L−1) | 0.89 ± 0.05 | 3.62 ± 0.28 *** | 2.39 ± 0.11 ***,§§§ | 2.23 ± 0.13 ***,§§§ |
| Triglycerides (mmol L−1) | 0.59 ± 0.04 | 2.04 ± 0.20 *** | 0.91 ± 0.04 §§§ | 1.12 ± 0.11 **,§§§ |
2.3 Effects of wines on total-, HDL- and LDL-cholesterol and triglycerides
At the end of the experimental period, plasma levels of TC, HDL-C, LDL-C and TG were significantly higher in the AT group than in ST animals (Table 2). Animals receiving either P or M had significantly lower cholesterolemia (5.58 ± 0.12 and 5.80 ± 0.17mmol L−1, respectively) than AT animals (7.32 ± 0.45mmol L−1). Wine treatment did not modify HDL-C levels. However, both wines led to higher HDL-C/TC ratios; HDL-C values represented 50% and 35% of TC in the P and M group, respectively. Administration of P or M also triggered a reduction in the increase of LDL-C. While the LDL-C level in AT animals was 200% of that in the ST group, it was only 126% and 118% in the AT + P and AT + M groups, respectively. The same was true of plasma TG values, where levels after P and M treatment were 154% and 190% of ST values, compared to AT animals in which they increased to 346%.2.4 Effect of wines on oxidative stress
The AT diet significantly lowered plasma paraoxonase activity (32%) compared to the ST diet. However, the consumption of P and M reduced the decrease in PON activity (to 19% and 6%, respectively; Fig. 1A). As shown in Fig. 2B, the aortic production of O2°− displayed a 210% increase in AT animals when compared to the ST group. However, hamsters consuming P or M wines exhibited a lower increase in O2°− production (55% and 21% respectively), not significantly different from levels seen in ST.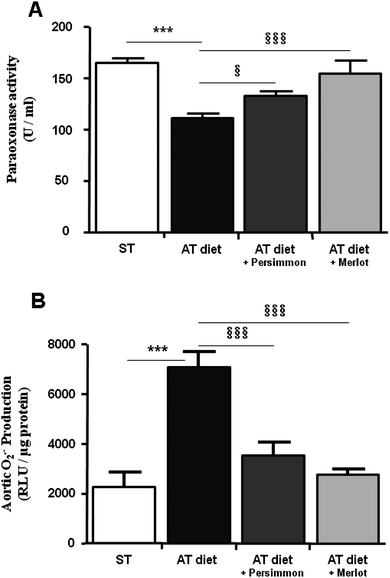 | ||
| Fig. 1 Persimmon and Merlot grape wines prevent the increase in oxidative stress. (A) PON1 activity measured in plasma from ST, AT, AT + P and AT + M groups. (B) O2− production in the aorta for the same groups. Values are expressed as relative light units (R.L.U)/μg protein. * and § refer to statistical comparisons vs.ST and AT respectively. §: p<0.05; *** and §§§: p < 0.001. | ||
 | ||
| Fig. 2 Persimmon and Merlot wines prevent vascular and endothelial dysfunctions. Vascular reactivity was evaluated in response to cumulative doses of various drugs. Left-hand panels: variations in isometric tension in the hamster aorta from the ST group; right-hand panels: dose-responses established for all groups, ST (❍), AT (●), AT + P (■) and AT + M (❑). (A) Contractile response to 1 to 80 mM KCl. (B) and (C) acetylcholine (ACh)- and sodium nitroprusside (SNP)-induced relaxation of hamster aorta. Aortic rings previously contracted with 1 μM phenylephrine (PE) were then relaxed with cumulative doses of ACh or SNP ranging from 1 nM to 10 μM. Values are expressed as percentage of relaxation and represent means ± SEM of 4 animals, with experiments performed in quadruplicate. * and § refer to statistical comparisons vs.ST and AT respectively. * and §: p < 0.05; **: p < 0.01; *** and §§§: p < 0.001. | ||
2.5 Wines and endothelial function
The characterization of the vascular reactivity of the aorta showed that the maximal contraction induced by the depolarizing agent KCl was lower in AT animals (1.2 ± 0.2 g) than in the ST group (2.1 ± 0.1 g, p < 0.05), corresponding to a 43% reduction in the contractile response. This reduction was not observed in AT animals receiving either P or M. The maximal responses, 2.0 ± 0.3 g and 1.6 ± 0.1 g, respectively, for these two groups, were different from those in animals on an AT diet (p < 0.05) but not from those in ST animals (Fig. 2A, Table 3). Additionally, KCl induced a concentration-dependent contraction of aortic rings with endothelium from all groups (Fig. 2A) The sensitivity to KCl was modified by the AT diet. The EC50 values, determined from dose-response curves (Fig. 2A), decreased from 21.9 ± 0.4 nM in ST hamsters to 11.5 ± 1.2 nM in the AT group. This decrease was prevented to a similar extent by polyphenol supplementation with P (25.8 ± 0.3 nM) and with M (23.9 ± 0.4 nM).| ST | AT diet | AT diet+ Persimmon | AT diet+ Merlot | |
|---|---|---|---|---|
| a For each group, values represent the maximal contraction induced by a maximally active concentration of KCl (80 mM) expressed in grams, and the EC50 for KCl expressed in mM and determined from dose-response curves after fitting with a non-linear function using GraphPad Prism® software. Superscripts refer to statistical comparisons vs.ST (*) and vs.AT(§) groups. * and §: p < 0.05; *** and §§§: p < 0.001. | ||||
| Max contraction (g) | 2.1 ± 0.1 | 1.2 ± 0.2 * | 2.0 ± 0.3 § | 1.6 ± 0.1 § |
| KCl EC50 (mM) | 21.9 ± 0.4 | 11.5 ± 1.2 *** | 25.8 ± 0.3 ***,§§§ | 23.9 ± 0.4 ***,§§§ |
| ACh relaxation (%) | 34.8 ± 3.4 | 23.8 ± 1.9 ** | 31.1 ± 2.8 § | 32.9 ± 3.8 § |
Endothelial dysfunction is a key early factor in the development of atherosclerosis. We therefore evaluated endothelial function in all groups by analyzing the response to acetylcholine (ACh) and using sodium nitroprusside (SNP) as a control for the contribution of smooth muscle contraction. The cumulative addition of ACh (1 nM to 10 μM) and SNP (1 nM to 10 μM) resulted in a concentration-dependent relaxation of hamster aortic rings with endothelium that had been previously contracted with 1 μM phenylephrine (PE) (Fig. 2B–2C). AT animals had reduced endothelium-dependent vasodilator responses to ACh as compared to ST animals. The maximal relaxation obtained represented 34.8 ± 3.4% of PE-induced contraction in ST hamsters versus 23.8 ± 1.9% in AT animals (p = 0.01; n = 10), which corresponds to a 32% decrease in the response to ACh in AT animals. With P and M administration, endothelial function was conserved and no difference was observed in the vasodilator response to ACh as compared to ST animals; relaxation represented 31.1 ± 2.8% of PE-induced contraction for P (p = 0.04; n = 10) and 32.9 ± 3.8% for M animals (p = 0.04; n = 10). Unlike ACh, the relaxant response elicited by SNP was identical in all groups (Fig. 2C).
3 Discussion
Many in vivo studies have demonstrated the antioxidant properties of wine polyphenols, but so far, none has identified the phenolic compounds potentially involved. In the present study, we correlated polyphenol composition to antioxidant properties in the dealcoholized fraction of two different wines, a red grape (Merlot) wine and a white persimmon wine. For the first time, we show that an atherogenic diet impairs vascular reactivity and that these alterations are prevented by wine polyphenols in a well-characterized animal model of atherosclerosis.16 We have previously shown that this atherogenic diet induces the development of atherosclerosis in association with hypercholesterolemia and O2°− overproduction in hamsters.14,15Paraoxonase activity and reactive oxygen species (ROS), including O2°−, are markers of oxidative stress. While low paraoxonase activity promotes LDL oxidation and has been linked to the progression of arteriosclerosis,17 the overproduction of O2°− could be responsible for the generation and/or maintenance of oxidative stress conditions.18 These events contribute to the initiation and progression of vascular and endothelial dysfunction. In our study, hamsters given the AT diet had increased plasma TC, HDL-C, LDL-C and TG levels, signifying dyslipidemia. Oxidative stress was induced in this group, as shown by the decrease in PON activity and the increased production of aortic O2°−, and there were corresponding alterations in contractile function, as demonstrated by the vascular reactivity of aortic rings. The fact that there was a decrease in the vasodilator response to acetylcholine but not to SNP suggests that these alterations are related to endothelial dysfunction. Together, these results substantiate the effectiveness of the atherogenic diet.We have previously shown that the consumption of polyphenol-rich foods, polyphenol extracts and polyphenol-enriched white wine or sparkling red Pinot Noir wine prevents the development of atherosclerosis in hamsters through a mechanism related to increased antioxidant availability15,19 and an improved serum lipid profile.14,15,19 Here, we observed the same beneficial effects with both P and M wines. They significantly prevented the alteration of the serum lipid profile and the overproduction of O2°−via the decreased activity of NADPH oxidase in animals given an atherogenic diet. Paraoxonase activity was maintained at levels identical to those seen in controls given a standard diet. PON has also been shown to attenuate the postprandial oxidative stress response, possibly due to its lipase-like activity on chylomicron triacylglycerols20 and reflected by reduced triglyceridemia. Accordingly, in hamsters that were given wine, triglyceridemia was significantly reduced as compared to AT animals. Additionally, the atherogenic index, reflected by the HDL-C/ TC ratio, increased from 1.62 in ST to 2.64 in AT animals, but improved in the P (2.01) and M (1.90) groups. Consequently, the vascular contractile response and endothelial function were not impaired. Surprisingly, while no difference was observed between the effects of P and M wines in experimental animals, the analysis of their polyphenol composition revealed substantial differences both at the qualitative and quantitative levels. Total polyphenol content was 6 times lower in P wine than in M wine. The concentrations of procyanidin monomers and trimers varied significantly and were at least 10 times lower in P wine. Only the levels of procyanidin dimers were comparable between the two. However, while all four procyanidin dimers (B1 to B4) were present in M wine, only B2 was found in P wine. Therefore, taking into account the improvement in atherosclerotic markers in AT hamsters with the two wines, our analysis suggests that the antioxidant properties of both P and M wines are related to the presence of procyanidin dimers. Consistent with these findings, it has been shown that antioxidant activity is not correlated to total polyphenol content but is instead dependent on polyphenol composition,21 particularly the composition of procyanidin compounds. Procyanidin dimers and trimers are the most powerful molecules when it comes to mimicking the effects of whole grape seed procyanidin extracts.22 Furthermore, procyanidin B2 might have beneficial properties, such as anti-atherosclerotic, anti-inflammatory and antihypertensive activity, in vitro and in vivo.23Procyanidin B2 has also been shown to be a major contributor to the antioxidant activity of cocoa24 and apples.25 Even if dimer B2 was found as the major dimer in persimmon wine composition, and that in such a case it should have an essential activity, in Merlot wine, a synergistic effect of all the dimers appears imaginable and in conjunction with anthocyanins (4427 ± 57 mg L−1, not shown in Table 1).In persimmon wine, epigallocatechin-gallate (29.4 mg L−1) could act synergistically with dimer B2.
In addition, the inhibition of lipid absorption by procyanidins is another mechanism that cannot be ruled out.26 However, in a previous study27 we used wine polyphenol extracts ExGrape Seed (EGS) that contained 46% procyanidins and 54% monomeric flavanols, and ExGrape Total (EGT) that contained 34% anthocyanins, 35% procyanidins, and 27% monomeric flavanols. EGS and EGT respectively induced 77% and 84% endothelium-dependent relaxation. In an older study,28 we demonstrated that a red wine phenolic extract, shown by others to induce an endothelium-dependent relaxation via an enhancement of endothelial NO synthesis29 and containing 42% procyanidins, 20% monomeric flavanols, 14% anthocyanins and 24% phenolic acids, prevented early atherosclerosis in hamsters. Moreover, it has been recently shown that both procyanidins and anthocyanins in red wine are able to stimulate the endothelial NO synthase.30 Thus, it is likely that, alongside procyanidins, anthocyanins also contribute to the beneficial effect observed in the present study. These findings indicate that polyphenols are responsible, at least in part, for the antiatherogenic/antioxidant effects of wines.
4 Experimental
4.1 Wines
The Merlot wine (M) (2008 vintage) was produced in Bordeaux (France), under the Appellation Pessac-Léognan (Château Seguin). The Persimmon wine (P) (2008 vintage) was produced in Gyeongbuk Province (Korea) (Cheongdo Wine Co. Ltd., Korea). Ethanol was removed by vacuum evaporation at 18 °C for 20 min under reduced pressure and an equivalent volume of distilled water was subsequently added to reconstitute the ethanol-free fraction.4.2 HPLC analysis
Total phenolic content was determined for each wine according to the method of Singleton and Rossi,31 and expressed in mg L−1 as gallic acid equivalents.For each wine, the separation of polyphenol compounds was performed by high-performance liquid chromatography (HPLC) using UV detection at 280 nm. The amounts of the monomers C, EC, ECG and EGCG, the dimers B1, B2, B3 and B4, and trimers were determined. A Finnigan ternary pump coupled to an Xcalibur data treatment system and a Finnigan UV-vis detector (UV-vis 200), and a Finnigan autosampler were used for solvent and sample delivery and detection. Separations were performed on reversed-phase Agilent Nucleosil C18 columns (250 mm × 4 mm, 5 μm particle size) eluted at a flow rate of 1 mL min−1. The solvents used were as follows: solvent A, 50 mM dihydrogen ammonium phosphate adjusted to pH 2.6 with orthophosphoric acid; solvent B, 20% A with 80% acetonitrile; solvent C, 0.2 M orthophosphoric acid adjusted with ammonia to pH 1.5.32 Calibration curves were constructed with standards of each compound and data were expressed as mg per liter of wine.
4.3 Animals, diets, and experimental design
Forty male golden Syrian hamsters (Janvier, Le Genest-St-Isle, France) weighing 90–100 g were randomly assigned to four groups (n = 12/group) with free access to food and water. Animals were handled according to the guidelines of the committee for Animal Care at the University of Montpellier (France) and NIH guidelines no 85–123 (Washington, DC, 1985). Hamsters were fed with either a standard diet (ST) or an atherogenic diet (AT) for a 12-wk period, and AT animals were administered tap water (AT), dealcoholized P (AT + P) or dealcoholized M (AT + M) daily by gavage during this period. The volume of the liquid administered was established by extrapolating from two glasses of wine per meal per day for a 70 kg human. This represents a daily volume of 7.14 mL kg−1 body weight. ST animals received tap water by gavage. The composition of the diet was identical to that previously described by Décordé et al.33 To be precise, the atherogenic diet contained 15% lard and 0.5% cholesterol, and the mineral and vitamin mixes did not contain selenium, vitamin C or vitamin E.4.4 Analytical procedures
At the end of the 12-wk period hamsters were deprived of food overnight. Then, animals were anesthetized with an intraperitoneal injection of pentobarbital (150mg kg−1) and fasting blood was collected by cardiac puncture. Plasma concentrations of total cholesterol (TC), HDL- and LDL-cholesterol were determined using commercially available kits (CH 200 and CH 203 respectively, Randox Laboratories LTD, Crumlin, UK) on a Pentra 400 automated analyzer (HORIBA ABX Montpellier, France). Plasma glucose and triglyceride (TG) levels were measured enzymatically (KonePro, KoneLab, Evry-Les-Lys, France) using reagents from the Thermo Electron Corporation (Cergy Pontoise, France). The thoracic aorta was excised and immersed in ice-cold buffer as required for the following procedures (superoxide anion determination and vascular reactivity).Paraoxonase (PON) activity was determined according to the method of Jaouad et al.34
NADPH oxidase activity was measured as superoxide anion production and was evaluated in the thoracic aorta immersed and equilibrated in Krebs buffer containing lucigenin (10 μM). The intensity of luminescence was measured on a luminometer (Perkin Elmer Wallac, Victor, Turku, Finland). Results were expressed as relative light units (RLU)/μg tissue.
4.5 Aortic preparation and mounting for vasorelaxation studies
The thoracic aorta, immersed in phosphate buffered saline pH 7.4 containing 140 mmol L−1NaCl, 5 mmol L−1KCl, 1 mmol L−1MgCl2, 0.5 mmol L−1KH2PO4, 0.5 mmol L−1Na2HPO4, 2.5 mmol L−1CaCl2, 10 mmol L−1HEPES and 10 mmol L−1glucose, was cleaned of fat and connective tissue and cut into 2–3 mm-wide rings. Aortic rings were mounted between two stainless steel hooks placed in a conventional organ bath chamber filled with 5 mL of Physiological Saline Solution (PSS), maintained at 37 °C and continuously bubbled with O2. Changes in isometric tension were recorded as previously described35 using an IT1-25 force transducer and an IOX computerized system (EMKA Technologies, Paris, France). Each arterial (aortic) segment was subjected to a 60 min equilibration period at the predetermined optimal point of its active length–tension curve established at 1 g by measuring the response to 30 mM KCl at different levels of stretch. The contractile function of each arterial segment was assessed with 1 μM phenylephrine (PE). Acetylcholine causes endothelium-dependent vasorelaxation. Thus the presence of endothelium was confirmed by the application of acetylcholine (ACh, 1 μM), and vasorelaxation was assessed after PE-induced contraction. After wash-out and a 20–30 min period of stabilization, dose responses to the depolarizing agent KCl were evaluated by cumulative increases in the concentration of KCl (1–80 mM range). Endothelial function was assessed by studying the relaxing effects of ACh in arteries contracted using a maximally active concentration of PE (10 μM) inducing similar submaximal contractile level in all groups. Dose-response relaxation curves were then generated by cumulative increases in the concentration of ACh (1 nM–10 μM range). Each protocol was performed in quadruplicate in tissue from 4 different animals per group.4.6 Statistical analysis
Data are shown as means ± SEM. Statistical analysis was carried out using StatView IV software (Abacus Concepts, Berkeley, CA), by one-way ANOVA followed by Fisher's protected least significant difference test. Differences were considered significant at a P < 0.05.5 Conclusion
Our findings regarding oxidative stress, plasma lipoprotein distribution, vascular reactivity and endothelial function and their correlation to the phenolic composition of wines highlights the contribution of procyanidins to the antioxidant biological activity of wine extracts. To confirm these results, a similar study using fractions extracted from different wines is in progress.6 References
- S. Renaud and M. Lorgeril, Wine, alcohol, platelets, and the French paradox for coronary heart disease, Lancet, 1992, 339, 1523–6 CrossRef CAS.
- E. N. Frankel, J. Kanner, J. B. German, E. Parks and J. E. Kinsella, Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine, Lancet, 1993, 341, 454–7 CrossRef CAS.
- R. Corder, W. Mullen, N. Q. Khan, S. C. Marks, E. G. Wood, M. J. Carrier and A. Crozier, Oenology: red wine procyanidins and vascular health, Nature, 2006, 444, 566 CrossRef CAS.
- C. Blade, L. Arola and M. J. Salvado, Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms, Mol. Nutr. Food Res., 2010, 54, 37–59 CAS.
- M. I. Covas, P. Gambert, M. Fit and R. Torre, Wine and oxidative stress: Up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans, Atherosclerosis, 2010, 208, 297–304 CrossRef CAS.
- I. Spranger, B. Sun, A. M. Mateus, V. O. D. Freitas and J. M. Ricardo-da-Silva, Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds, Food Chem., 2008, 108, 519–32 CrossRef CAS.
- M. A. Soobrattee, V. S. Neergheen, A. Luximon-Ramma, O. I. Aruoma and T. Bahorun, Phenolics as potential antioxidant therapeutic agents: mechanism and actions, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 579, 200–13 CrossRef CAS.
- G. Montagut, I. Baiges, J. Valls, X. Terra, J. M. del Bas, X. Vitrac, T. Richard, J. M. Maillon, L. Arola, M. Blay, C. Bladé, J. Fernedez-Larrea, G. Pujadas, J. Salvado and A. Ardevol, A trimer plus a dimer-gallate reproduce the bioactivity described for an extract of grape seed procyanidins, Food Chem., 2009, 116, 265–70 CrossRef CAS.
- H. F. Gu, C. M. Li, Y. J. Xu, W. F. Hu, M. H. Chen and Q. H. Wan, Structural features and antioxidant activity of tannin from persimmon pulp, Food Res. Int., 2008, 41, 208–17 CrossRef CAS.
- S. Uchida, H. Ohta, M. Niwa, A. Mori, G. Nonaka, I. Nishioka and M. Ozaki, Prolongation of life span of stroke-prone spontaneously hypertensive rats (SHRSP) ingesting persimmon tannin, Chem. Pharm. Bull. (Tokyo), 1990, 38, 1049–52 CAS.
- S. Gorinstein, E. Bartnikowska, G. Kulasek, M. Zemser and S. Trakhtenberg, Dietary persimmon improves lipid metabolism in rats fed diets containing cholesterol, J. Nutr., 1998, 128, 2023–7 CAS.
- T. R. Rathel, R. Samtleben, A. M. Vollmar and V. M. Dirsch, Activation of endothelial nitric oxide synthase by red wine polyphenols: impact of grape cultivars, growing area and the vinification process, J. Hypertens., 2007, 25, 541–9 CrossRef.
- N. Landrault, P. Poucheret, P. Ravel, F. Gasc, G. Cros and P. L. Teissedre, Antioxidant capacities and phenolics levels of French wines from different varieties and vintages, J. Agric. Food Chem., 2001, 49, 3341–8 CrossRef CAS.
- C. Auger, J. M. Rouanet, R. Vanderlinde, A. Bornet, K. Décordé, N. Lequeux, J. P. Cristol and P. L. Teissedre, Polyphenols-enriched Chardonnay white wine and sparkling Pinot Noir red wine identically prevent early atherosclerosis in hamsters, J. Agric. Food Chem., 2005, 53, 9823–9 CrossRef CAS.
- T. Sutra, K. Décordé, J. Riss, C. Dallas, J. P. Cristol and J. M. Rouanet, A commercial extract of fruits and vegetables, Oxxynea, acts as a powerful antiatherosclerotic supplement in an animal model by reducing cholesterolemia, oxidative stress, and NADPH oxidase expression, J. Agric. Food Chem., 2007, 55, 4258–63 CrossRef CAS.
- A. Nistor, A. Bulla, D. A. Filip and A. Radu, The hyperlipidemic hamster as a model of experimental atherosclerosis, Atherosclerosis, 1987, 68, 159–73 CrossRef CAS.
- N. Gupta, K. Gill and S. Singh, Paraoxonases: structure, gene polymorphism & role in coronary artery disease, Indian J. Med. Res., 2009, 130, 361–8 CAS.
- E. Schulz, E. Anter and J. F. Keaney Jr, Oxidative stress, antioxidants, and endothelial function, Curr. Med. Chem., 2004, 11, 1093–1104 CAS.
- K. Décordé, A. Agne, D. Lacan, J. Ramos, G. Fouret, E. Ventura, C. Feillet-Coudray, J. P. Cristol and J. M. Rouanet, Preventive effect of a melon extract rich in superoxide scavenging activity on abdominal and liver fat and adipokine imbalance in high-fat-fed hamsters, J. Agric. Food Chem., 2009, 57, 6461–7 CrossRef.
- B. Fuhrman, N. Volkova and M. Aviram, Postprandial serum triacylglycerols and oxidative stress in mice after consumption of fish oil, soy oil or olive oil: possible role for paraoxonas-1 triacylglycerol lipase-like activity, Nutrition, 2006, 22, 922–30 CrossRef CAS.
- J. Sun, Y. F. Chu, X. Wu and R. H. Liu, Antioxidant and antiproliferative activities of common fruits, J. Agric. Food Chem., 2002, 50, 7449–54 CrossRef CAS.
- A. Serra, A. Macia, M. P. Romero, J. Valls, C. Bladé, L. Arola and M. J. Motilva, Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models, Br. J. Nutr., 2009, 103, 944–52 CrossRef.
- J. Yamakoshi, S. Kataoka, T. Koga and T. Ariga, Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits, Atherosclerosis, 1999, 142, 139–49 CrossRef CAS.
- S. Baba, N. Osakabe, M. Natsume, A. Yasuda, T. Takizawa, T. Nakamura and J. Terao, Cocoa powder enhances the level of antioxidative activity in rat plasma, Br. J. Nutr., 2000, 84, 673–80 CAS.
- R. Tsao, R. Yang, S. Xie, E. Sockovie and S. Khanizadeh, Which polyphenolic compounds contribute to the total antioxidant activities of apple?, J. Agric. Food Chem., 2005, 53, 4989–95 CrossRef CAS.
- A. Yasuda, M. Natsume, K. Sasaki, S. Baba, Y. Nakamura, M. Kanegae and S. Nagaoka, Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet, BioFactors, 2008, 33, 211–23 CrossRef CAS.
- C. Auger, P. Gérain, F. Laurent-Bichon, K. Portet, A. Bornet, B. Caporiccio, G. Cros, P. L. Teissèdre and J. M. Rouanet, Phenolics from commercialized grape extracts prevent early atherosclerotic lesions in hamsters by mechanisms other than antioxidant effect, J. Agric. Food Chem., 2004, 52, 5297–5302 CrossRef CAS.
- C. Auger, B. Caporiccio, N. Landrault, P. L. Teissedre, C. Laurent, G. Cros, P. Besançon and J. M. Rouanet, Red wine phenolic compounds reduce plasma lipids and apolipoprotein B and prevent early aortic atherosclerosis in hypercholesterolemic Golden Syrian hamsters, J. Nutr., 2002, 132, 1207–13 CAS.
- E. Andriambeloson, A. L. Kleschyov, B. Muller, A. Beretz, J. C. Stoclet and R. Andriantsitohaina, Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta, Br. J. Pharmacol., 1997, 120, 1053–8 CrossRef CAS.
- C. Auger, M. Chaabi, E. Anselm, A. Lobstein and V. B. Schini-Kerth, The red wine extract-induced activation of endothelial nitric oxide synthase is mediated by a great variety of polyphenolic compounds, Mol. Nutr. Food Res., 2010, 54, 1–13 Search PubMed.
- V. L. Singleton and J. A. Rossi Jr, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16, 144–58 CAS.
- K. Chira, G. Schmauch, C. Saucier, S. Fabre and P. L. Teissèdre, Grape variety effect on proanthocyanidin composition and sensory perception of skin and seed tannin extracts from bordeaux wine grapes (Cabernet Sauvignon and Merlot) for two consecutive vintages (2006 and 2007), J. Agric. Food Chem., 2009, 57, 545–53 CrossRef CAS.
- K. Décordé, E. Ventura, D. Lacan, J. Ramos, J. P. Cristol and J. M. Rouanet, An SOD rich melon extract Extramel prevents aortic lipids and liver steatosis in diet-induced model of atherosclerosis, Nutr., Metab. Cardiovasc. Dis., 2010, 20, 301–7 CrossRef.
- L. Jaouad, C. de Guise, H. Berrougui, M. Cloutier, M. Isabelle, T. Fulop, H. Payette and A. Khalil, Age-related decrease in high-density lipoprotein antioxidant activity is due to an alteration on the PON1's free sulfhydryl groups, Atherosclerosis, 2006, 185, 191–200 CrossRef CAS.
- A. Fort, M. Cordaillat, C. Thollon, G. Salazar, I. Mechaly, N. Villeneuve, J. P. Vilaine, S. Richard and A. Virsolvy, New insights in the contribution of voltage-gated Na(v) channels to rat aorta contraction, PLoS One, 2009, 4, e7360 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
