Biomonitoring 210Po and 210Pb in marine brachyuran crabs collected along the coast of Kudankulam, Gulf of Mannar (GOM), India
M.
Feroz Khan
*a,
S.
Umarajeswari
b and
S.
Godwin Wesley
b
aDepartment of Advanced Zoology and Biotechnology, Sadakathullah Appa College (Autonomous), Rahmath Nagar, Palayamkottai, 627 011, Tirunelveli District, Tamil Nadu, India. E-mail: ferozmerlin@yahoo.co.in; Tel: (+91) 9894200679
bDepartment of Zoology and Research Centre, Scott Christian College (Autonomous), Nagercoil, 629 003, Kanniyakumari District, Tamil Nadu, India
First published on 1st February 2011
Abstract
Activities of 210Po and 210Pb in whole-body and in various tissues of brachyuran crabs collected along the Kudankulam coast were studied. A non-uniform distribution of these radionuclides was observed between the various tissues. Of all the tissues, 210Po and 210Pb were found to accumulate more in the hepatopancreas and intestine. Among the crabs studied, Charybdis lucifera registered higher 210Po and 210Pb activity. Muscle tissue in all the species registered lower activity. The 210Po/210Pb activity ratio was found to be greater than unity. The biological concentration factor for organs varied between ∼104 and 106 for 210Po and ∼102 and 104 for 210Pb. A significant variation in the accumulation of 210Po and 210Pb was noted between species and between seasons (p < 0.05). The mean whole-body internal dose ranged from 1.42 to 6.86 μGy h−1 for 210Po and from 3.0 × 10−3 to 8.0 × 10−3 μGy h−1 for 210Pb. The external dose for 210Po and 210Pb was 2.41 × 10−6 to 5.76 × 10−6 μGy h−1 and 4.14 × 10−5 to 8.26 × 10−5 μGy h−1, respectively. The activity levels recorded are in agreement with values recorded in related organisms in other parts of the world. The total committed effective dose due to the intake of both radionuclides ranged from 80.3 to 871.7 μSv y−1. The median dose calculated due to 210Po and 210Pb in certain crabs in Kudankulam is less and would not pose any significant radiological impact on health or a cancer risk to the public, and the seafood is considered safe for human consumption.
Environmental impactProtecting the environment from ionizing radiation is mandatory around nuclear installations. Therefore, environmental monitoring and radioecology is becoming popular among ecologists and industrialists. It is also essential to ensure that certain limits of contamination are not exceeded, for absolute radiological protection of mankind. The present study gives a comprehensive picture of the radionuclide accumulation preference of crabs collected from the southeastern coast of India, the Gulf of Mannar, a pristine environment. The monitored radionuclides are very important in terms of radioprotection, since they deliver the maximum dose. Moreover, crabs are monitored less internationally compared to other marine biota; crabs are also an important protein source for human beings. This study will also provide better understanding of the real risks associated with nuclear practices. |
Introduction
Monitoring radioactive elements in the natural environment and the assessment of their effects on living organisms has been one of the most important issues in radioecology and radiological protection around nuclear installations in recent years. 210Po (t1/2 = 138 days) is a naturally occurring radionuclide formed by the beta decay of its grandparent 210Pb (t1/2 = 22 years) via210Bi of the 238U decay chain. The concentration of 210Po and 210Pb in marine food has received much interest from the marine scientific community because of the high radioactive dose they deliver to marine organisms compared to anthropogenic radionuclides released into coastal waters.1–3 Furthermore, these radionuclides are accumulated in the edible portions of marine organisms, and are considered to be the most important contributors of radiation received by humans via fish and shellfish consumption.3–6 The 210Po accumulated in marine organisms is generally derived from the food chain,7 and significant differences are noted in its accumulation in different species.Crabs form an important component of the marine food web as both predator and prey. They are mostly benthic with restricted mobility and are especially sensitive to habitat degradation due to pollution because they reside on the bottom where chemical contaminants accumulate. Crabs have the potential to accumulate significant amounts of pollutants including metals, which may be biomagnified in the food chain to higher trophic levels including human consumers.8 They can also serve as a potential sentinel species in coastal areas by reflecting the effects of natural and anthropogenic stressors.9
Marine crabs are of great demand, forming about 11% of the edible crustacea landing in India. Although about 700 species of brachyuran crabs have been reported from Indian waters, the family Portunidae contributes the most to the commercially important crab fishery of the country. The largest landing of crabs was reported from the east coast of Tamil Nadu (28%).10 Although many studies of 210Po have been conducted in the marine environment, sufficient data are not available for different marine species of the Kudankulam coast. The study aims (i) to understand the distribution of 210Po and 210Pb in the tissues of marine crabs, (ii) to study the internal and external dose rate in crabs, and (iii) to evaluate the effective dose to humans consuming the crab meat. This study will provide baseline data for the Kudankulam coast, where a mega nuclear power hub is being established (4 × 1000 MWe), and will also help in the development of a comprehensive marine radioecological database.
Experimental
1. Description of the study area
The Kudankulam coast (8°10′716′′N; 77°44′640′′E) is located on the southeastern coast of the Indian peninsular, at the distal end of the Gulf of Mannar Biosphere Reserve (GOMBRe) (Fig. 1). The total length of the coastline is about 100 km, from Kanniyakumari to Uvari. The Kudankulam landform may be divided into marine and inland planes. The marine landform occurs along the coast surrounded by many fishing villages. This place is endowed with a rich diversity of marine organisms because the biosphere includes varied ecosystems such as coral reefs, seagrass beds, rocky shores, sandy beaches and mud flats. The coast is rocky and rich in monazite. Two major fish-landing centres, namely Chinnamuttom and Idinthakarai, are located one on either side of the reactor site. Pelagic and demersal fin and shellfish of this coast are consumed largely by the local population. Kudankulam is very close to one of the major tourist destinations of south India – Kanniyakumari. | ||
| Fig. 1 Map showing the study area. | ||
2. Collection of samples
The collection of crabs is very much based on availability and seasonal abundance. Crabs which are most commonly eaten, such as Charybdis lucifera Fabr., n = 28; Charybdis natator Herb., n = 25 and Charybdis feriatus Linn., n = 30 were collected from station 1 (Kanniyakumari), station 2 (Chinnamuttom) and station 6 (Idinthakarai). Charybdis annulata Fabr., n = 28 and Portunus sanguinolentus Herb., n = 23 were collected from station 3 (Kutapuli) and station 7 (Kuthankuzhi). Portunus pelagicus L., n = 32 and Atergatis integerrimus Lamrk., n = 30 were collected from station 1, station 4 (Perumanal) and station 8 (Uvari). All these crabs were obtained from fishing nets. Species such as Matuta lunaris Forsk., n = 22 and Calappa lophos Herb., n = 26 were collected from station 8 and the species Uca annulipes H. Milne-Edwards., n = 52 were collected along the Kudankulam reactor site coastline. These species are nocturnal in behaviour and hence were collected during the night using traps. These species are consumed only during the off season for fishing. The samples were collected in three different seasons: pre-monsoon (July to September), monsoon (October to February) and post-monsoon (March to June) during July 2008 to June 2009.The fishery data pertaining to edible crabs show that the coastal waters are dominated by the cross crab, Charybdis feriatus, the spotted crab Portunus sanguinolentus and the reticulate crab Portunus pelagicus. Charybdis lucifera is slowly becoming popular as an edible crab. Charybdis annulata and Charybdis natator also contribute to the fishery, though in smaller magnitudes. Crabs are caught as bycatch and more than 80% of the total landing is by trawlers. Indigenous gears such as gill nets and traps are used to target individual species, especially Portunus pelagicus. They are usually caught from a depth of about 10 m to 60 m. It is the recent advances in fishing technology that have enabled fishermen to venture into deeper waters engaging themselves in multi-day fishing, especially for Charybdis feriatus. Catamarans and bottom-set gill nets are also used along the coast. Much of the landing was reported during post-monsoon months.10,11 All collected crabs were sexually mature adults, since all the samples had attained their maximum carapace width (Table 1). Crabs of different maturity stages were not considered in the present study due to the low availability of samples. From each species, certain crabs were randomly selected and analysed whole and the rest dissected into separate tissues. Tissues of interest were pooled from all the specimens representing each species, weighed and dried in an oven at 105 °C. The dried samples were homogenized and taken for analysis.
| Species | n | Family and common name | Habitat | Food habit | Carapace width CW (mm) (min.–max.) | Weight (g) (min.–max.) |
|---|---|---|---|---|---|---|
| Charybdis lucifera | 28 | Portunidae (Ghost crab) | Benthic, demersal, inhabit sandy-muddy substrates, coral reef flats and rocky coast, depth 30–60 m | Carnivorous feeder | 18.9–19.4 | 222.3–246.2 |
| Charybdis natator | 25 | Portunidae (Coral crab) | Benthic, demersal, inhabit rocky-sandy substrate near reefs, depth 5–40 m | Carnivorous feeder | 16.6–16.9 | 189.6–192.6 |
| Charybdis feriatus | 30 | Portunidae (Christ crab) | Benthic, demersal, inhabit sandy-muddy substrates, coral reef flats and rocky coast, depth 20–60 m | Carnivorous feeder | 17.2–18.9 | 172.6–175.9 |
| Charybdis annulata | 28 | Portunidae (Banded-legged swimming crab) | Benthic, demersal, inhabit rocky reef sand, soft bottom sands, depth 20–30 m | Carnivorous, feeds on various fishes and invertebrates | 6.4–6.9 | 92.3–96.8 |
| Portunus sanguinolentus | 23 | Portunidae (Three spot swimming crab) | Benthic, demersal, inhabit sandy-muddy substrates, coral reef flats and rocky coast, depth 30 m | Carnivorous, feeds on various fishes and invertebrates | 17.2–18.1 | 282.6–289.1 |
| Portunus pelagicus | 32 | Portunidae (Blue swimmer crab) | Pelagic, common among sandy, muddy or algal and sea grass, juveniles live in intertidal region, depth 40 m | Carnivorous, feeds on various fishes and invertebrates | 18.9–19.2 | 123.9–131.9 |
| Atergatis integerrimus | 30 | Portunidae (Red-egg crab) | Benthic, demersal, inhabit sandy-muddy substrates, coral reef flats and rocky coast, depth 60 m | Carnivorous, feeds on various fishes and invertebrates | 14.2–14.7 | 300.6–311.4 |
| Matuta lunaris | 22 | Calappidae (Moon crab) | Prefers sandy area, from the intertidal zone, depth 20 m | Carnivorous, foraging on small shellfish, worms and other animals at night | 4.2–4.6 | 42.3–53.6 |
| Calappa lophos | 26 | Calappidae (Box crab) | Dwells in sandy-muddy areas, depth 10–100 m | Carnivore, feeds on small shellfish, worms and other animals at night | 9.2–9.7 | 96.3–97.6 |
| Uca annulipes | 52 | Ocypodidae (Fiddler crab) | Semi terrestrial marine, found in salt marshes, tidal and mud flats, inhibit in burrows (60 cm) deep in the muddy substrate and retreat during high tides | Exclusively herbivore, feeds on algae, rarely decaying matter | 8.9–9.4 | 30.2–31.5 |
The 210Po activity of seawater in the study area was measured for calculating the biological concentration factor (BCF) and for estimation of dose rate. Sediment samples (2–3 kg) were also collected from the site of crab collection to estimate the external dose rate. Bed sediments (30 to 40 m depth) and intertidal sediments were collected at different sites for calculating dose for demersal and pelagic crabs. Beach sediment samples were collected for estimating dose to Uca annulipes, since it resides on the shore.
3. Radiochemical analysis
The 210Po and 210Pb in sediment and crabs were analysed by acid leaching as per Jia et al.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ammonia. The solution was stirred on a hot plate using a magnetic stirrer. A Perspex holder with a silver disk was immersed into the solution. The 210Po deposition was continued for 6 h at 85–90 °C, then the disk was removed, washed with distilled water and acetone, dried and measured in an alpha counting system for 6000 s (Nucleonix make, efficiency 35% using 241Am standard, minimum detectable limit 0.5 Bq kg−1). A second plating was also carried out to completely remove the polonium and tracer. The solution, after plating, was stored for 6 months to allow the ingrowth of 210Po from its parent, 210Pb. Subsequent determination of the ingrown 210Po was carried out as described above.13 The 210Po activity corresponds to that of its parent, 210Pb, in the sample. 208Po recovery was 98 ± 2% by this method. Quality was checked internally using the reference material, IAEA 375.
5 ammonia. The solution was stirred on a hot plate using a magnetic stirrer. A Perspex holder with a silver disk was immersed into the solution. The 210Po deposition was continued for 6 h at 85–90 °C, then the disk was removed, washed with distilled water and acetone, dried and measured in an alpha counting system for 6000 s (Nucleonix make, efficiency 35% using 241Am standard, minimum detectable limit 0.5 Bq kg−1). A second plating was also carried out to completely remove the polonium and tracer. The solution, after plating, was stored for 6 months to allow the ingrowth of 210Po from its parent, 210Pb. Subsequent determination of the ingrown 210Po was carried out as described above.13 The 210Po activity corresponds to that of its parent, 210Pb, in the sample. 208Po recovery was 98 ± 2% by this method. Quality was checked internally using the reference material, IAEA 375.
4. Statistical analysis
The normality of the data set was checked using the Shapiro–Wilk W-test (n ≤ 50) and potential outliers were tested using Rosner's test.15 These tests were performed using ProUCL v 4.0. An analysis of variance (ANOVA) was used to test for group mean differences for each element by tissue type and collection site. Tukey's studentized range (HSD) test was the multiple comparison procedure used in conjunction with ANOVA to determine which mean values were significantly different from one another. These tests were applied using Primer 6.0 demo version.Results and discussion
The average 210Po and 210Pb activities in seawater were found to range between 1.0 ± 0.2 and 2.5 ± 0.5 mBq L−1. The data set followed a normal distribution when tested using the Shapiro–Wilk W-test (p > 0.05). No outlier was found when it was tested using Rosner's test (p < 0.05). No significant mean difference was noted between stations (p < 0.05). The 210Po activity in sediments ranged from 9.5 ± 2.1 to 26.5 ± 3.6 Bq kg−1 dry weight (d.w.). Meanwhile, 210Pb activity varied between 13.5 ± 1.6 and 27.9 ± 2.5 Bq kg−1 d.w. The results are shown in Table 2. Both the radionuclides were found to be higher in the offshore sediments, which are silty in nature, and lower in the beach sediments. Since silt and clay sediments have more organic matter, these radionuclides get adsorbed and associated with it and thus display higher activity when compared to beach shore sediments. A significant difference in activity was noted between stations (p < 0.05).| Station | Coordinates | Seawater (mBq L−1) | Sediment (Bq kg−1) | ||
|---|---|---|---|---|---|
| 210Po | 210Pb | 210Po | 210Pb | ||
| Kanniyakumari | N 08°05′283′′ | 1.5 ± 0.06 | 2.9 ± 0.1 | 12.6 ± 1.9 | 13.5 ± 1.6 |
| E 77°29′232′′ | |||||
| Chinnamuttom | N 08°06′498′′ | 1.2 ± 0.03 | 2.9 ± 0.06 | 16.9 ± 2.5 | 18.7 ± 2.2 |
| E 77°34′353′′ | |||||
| Kutapuli | N 08°08′752′′ | 1.3 ± 0.05 | 3.1 ± 0.05 | 9.9 ± 2.1 | 15.6 ± 3.2 |
| E 77°36′060′′ | |||||
| Perumanal | N 08°09′360′′ | 0.9 ± 0.02 | 2.1 ± 0.11 | 9.5 ± 1.5 | 16.7 ± 4.4 |
| E 77°38′492′′ | |||||
| Kudankulam | N 08°10′716′′ | 1.9 ± 0.01 | 2.6 ± 0.06 | 12.4 ± 2.5 | 22.3 ± 3.6 |
| E 77°44′640′′ | |||||
| Idinthakarai | N 08°11′993′′ | 1.6 ± 0.03 | 2.5 ± 0.07 | 24.5 ± 3.1 | 26.5 ± 5.6 |
| E 77°45′611′′ | |||||
| Kuthankuzhi | N 08°12′997′′ | 1.5 ± 0.02 | 4.9 ± 0.15 | 26.5 ± 3.6 | 27.9 ± 2.5 |
| E 77°46′905′′ | |||||
| Uvari | N 08°16′456′′ | 1.4 ± 0.02 | 5.4 ± 0.16 | 22.1 ± 4.1 | 23.5 ± 4.3 |
| E 77°53′340′′ | |||||
The activities of 210Po and 210Pb in crabs are reported in wet-weight basis and presented in Table 3. The activity concentrations of the two radionuclides were found to be higher in the hepatopancreas of crabs. The activity concentrations of 210Po in various organs were in the order: hepatopancreas > gills > viscera (intestine) > soft tissue > exoskeleton. The 210Po and 210Pb activities in whole body of crabs ranged from 45.9 ± 2.6 to 221.2 ± 7.1 Bq kg−1 and from 12.4 ± 2.4 to 53.6 ± 3.8 Bq kg−1, respectively. Higher activity was noted in the species Charybdis lucifera and lower activity in the semi-terrestrial crab Uca annulipes. This could be due to the fact that the species Charybdis lucifera is a carnivore which feeds on small fishes and invertebrates whereas Uca annulipes is an algal feeder and also sometimes feeds on decaying matter.9 The former resides in water all the time and so may also uptake radionuclides from the seawater and silt. On the other hand, the latter species is immersed in water for less time, leading to less uptake of the radio-elements. A significant variation in 210Po between seasons and between species for the whole body was noted (p < 0.05). Average activities of 210Po and 210Pb were found to be higher during pre- and post-monsoon seasons (Fig. 2 and 3). Crabs breed throughout the year; breeding and spawning rates are higher during the post-monsoon season and lower during pre-monsoon. During monsoon the breeding will be much lower and may even stop for some species, due to the changing environmental conditions. Crabs are very sensitive to changing environmental conditions, especially to pH, temperature and food availability.10 Hence lower activity was observed during monsoon months. Also, due to the effect of dilution, the availability of 210Po and 210Pb would be less in the seawater since most of the radionuclides get adsorbed to suspended particles arising due to the monsoon. The activities of 210Po and 210Pb between seasons were also found to be significantly different (p < 0.05).
| Tissues | C. lucifera | C. natator | C. feriatus | C. annulata | P. sanguinolentus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | |
| Whole body | 221.2 ± 7.1 | 21.2 ± 1.9 | 99.6 ± 3.5 | 23.6 ± 1.8 | 101.2 ± 2.9 | 18.9 ± 2.2 | 196.4 ± 7.5 | 12.5 ± 1.8 | 145.9 ± 3.6 | 32.4 ± 2.5 |
| Soft tissue (muscle) | 18.9 ± 4.2 | 8.9 ± 2.4 | 21.6 ± 8.1 | 5.7 ± 1.4 | 16.5 ± 5.2 | 4.5 ± 1.9 | 14.2 ± 2.9 | 6.2 ± 1.8 | 17.1 ± 4.7 | 4.9 ± 2.4 |
| Exoskeleton | 10.5 ± 4.9 | 3.6 ± 1.9 | 30.6 ± 9.2 | 2.3 ± 1.2 | 24.6 ± 3.7 | 2.5 ± 0.5 | 24.2 ± 11.4 | 3.4 ± 0.7 | 29.9 ± 8.4 | 2.7 ± 1.2 |
| Hepatopancreas | 1877 ± 12.3 | 59.6 ± 4.4 | 896.2 ± 10.4 | 38.5 ± 3.6 | 754.5 ± 15.9 | 47.8 ± 2.4 | 982 ± 9.6 | 41.2 ± 8.4 | 1045.5 ± 16.4 | 57.2 ± 3.9 |
| Gills | 74.15 ± 6.1 | 14.4 ± 3.9 | 99.8 ± 9.6 | 14.7 ± 2.9 | 66.9 ± 5.5 | 5.9 ± 1.1 | 75.6 ± 20.1 | 9.2 ± 3.1 | 92.86 ± 6.4 | 3.4 ± 1.7 |
| Intestine | 189.4 ± 3.2 | 7.4 ± 1.5 | 172.4 ± 6.4 | 24.2 ± 5.1 | 175.8 ± 6.5 | 6.9 ± 1.5 | 131.8 ± 7.7 | 4.9 ± 1.4 | 177.4 ± 3.2 | 29.4 ± 2.1 |
| Tissues | P. pelagicus | A. integerrimus | M. lunaris | C. lophos | U. annulipes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | |
| Whole body | 154.2 ± 7.1 | 31.2 ± 1.9 | 125.6 ± 4.5 | 53.6 ± 3.8 | 77.2 ± 4.9 | 12.9 ± 3.2 | 96.4 ± 5.5 | 13.5 ± 1.6 | 45.9 ± 2.6 | 12.4 ± 2.4 |
| Soft tissue (muscle) | 13.5 ± 5.2 | 12.9 ± 3.4 | 12.6 ± 4.1 | 9.7 ± 1.9 | 10.2 ± 6.2 | 7.5 ± 1.5 | 9.9 ± 1.9 | 8.2 ± 1.4 | 7.5 ± 1.7 | 2.9 ± 0.9 |
| Exoskeleton | 18.1 ± 2.9 | 6.6 ± 2.9 | 20.5 ± 3.2 | 3.3 ± 1.1 | 14.6 ± 1.7 | 3.6 ± 1.5 | 14.2 ± 1.4 | 7.4 ± 1.7 | 9.9 ± 2.4 | 2.6 ± 1.2 |
| Hepatopancreas | 926.5 ± 16.3 | 49.6 ± 5.4 | 596.2 ± 20.4 | 58.5 ± 6.6 | 325.5 ± 10.9 | 37.8 ± 2.9 | 82 ± 6.6 | 21.2 ± 2.4 | 96.2 ± 6.4 | 27.2 ± 2.9 |
| Gills | 81.1 ± 7.1 | 17.4 ± 4.9 | 79.8 ± 6.6 | 19.7 ± 3.9 | 72.9 ± 5.9 | 15.9 ± 2.1 | 65.6 ± 10.1 | 12.2 ± 3.4 | 22.8 ± 3.4 | 4.4 ± 1.2 |
| Intestine | 178.4 ± 4.2 | 8.4 ± 2.5 | 192.4 ± 3.4 | 29.2 ± 5.5 | 112.8 ± 4.5 | 7.8 ± 1.2 | 98.8 ± 6.7 | 13.9 ± 1.4 | 41.4 ± 3.9 | 19.4 ± 2.5 |
 | ||
| Fig. 2 Seasonal whole-body accumulation of 210Po in brachyuran crabs. | ||
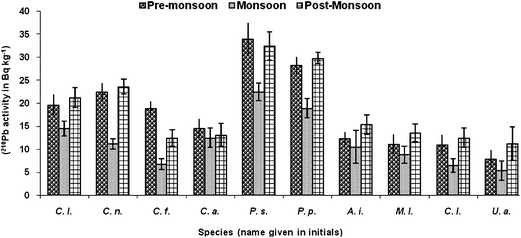 | ||
| Fig. 3 Seasonal whole-body accumulation of 210Pb in brachyuran crabs. | ||
Generally, the concentration of 210Po was higher in females compared to males (Fig. 4). A significant variation in the concentration between males and females (p < 0.05) was noted. Meanwhile, not much variation was found in the concentration of 210Pb between gender (p > 0.05). Female crabs considered in the present study were in an ovigerous condition. The metabolic need and physiological specificities may be higher in the case of females during gametogenesis. In some cases, the females grow faster than males and attain early maturity. These phenomena lead to a chance of increased uptake of food along with radionuclides and other metals. It has been reported that the gonadosomatic index (GSI) and hepatosomatic index (HSI) play an important role in female crabs during maturity. The concentration of the particular element also depends on the detoxification process, and the binding of 210Po to metallothioneins has been observed in marine invertebrates.16 Unlike 210Po, 210Pb does not have much specific binding capacity to metalloproteins which in turn needs further investigation. Thus, no variation in accumulation was observed for 210Pb.
 | ||
| Fig. 4 Whole-body accumulations of 210Po and 210Pb in male and female brachyuran crabs. | ||
The species C. natator registered higher 210Po activity in the soft tissue, exoskeleton and gills. C. lucifera accumulated more 210Po in the hepatopancreas and intestine compared to other organs. Except the intestine, no significant variation in 210Po concentration was found in tissues between species (p > 0.05). A significant difference in 210Pb concentration was observed in hepatopancreas and exoskeleton between species (p < 0.05). The 210Pb activity concentration was higher in hepatopancreas and lower in soft tissues. The 210Po/210Pb ratio was found to be higher than unity in all the species. The BCF ranged from ∼104 to 106 for 210Po and from ∼102 to 104 for 210Pb. The BCF values calculated in the present study were found to be higher than the values reported for 210Po and lower than the values for 210Pb reported by IAEA.17
In this study, the high 210Po levels recorded in the hepatopancreas of crabs may be due to the fact that this organ exhibits intracellular digestion by absorbing food from the stomach. The assimilation of radionuclides depends upon the digestive physiology of individual organisms.12 The 210Po and 210Pb activity in the intestine was found to be second only to the hepatopancreas in crabs. Studies on the food and feeding habits of crabs show that they generally feed on small crustaceans, fishes and molluscs, detritus, bits of plants and other organic materials, as noticed in the stomach contents. 210Po concentration in the intestine of crabs reported from the Baltic Sea environment18 was 353.6 Bq kg−1, which is higher compared with the values of the present investigation. The reason for the difference in the activity may be due to the difference in the metabolic rates and absorption efficiencies of the organisms. The higher 210Po concentration in gills reveals that it is the main entry point for polonium; the gills play a role in the direct absorption of radionuclides from water and they also filter radionuclide-rich suspended solids.19
In the present study, the 210Po activity in the soft tissue of crabs (36.8 ± 9.9 to 68.6 ± 13.1 Bq kg−1) was higher than values reported in similar organisms from the Bombay harbour bay (29.5 Bq kg−1)20 and lower than values reported from the Palk Strait (149.6 Bq kg−1),4 Gulf of Mannar (195.0–219.8 Bq kg−1)21 and Kalpakkam (107.5 Bq kg−1).22 On the other hand, the value for 210Po in the soft tissue of crabs was less when compared to the value reported from the Syrian coast (120 Bq kg−1)23 and higher than those in the crabs of the Baltic Sea environment (20 to 29.5 Bq kg−1).18,24 The organ values were also comparable with those reported for different organs of the crustacean Saduria entomon from the Gulf of Gdansk, Baltic Sea (0.6 to 543.5 Bq kg−1)25 and of crabs reported from Cienfuegos Bay, Cuba (50 to 151 Bq kg−1).26
The exoskeleton of crabs tends to concentrate less 210Po when compared with other organs and this could be due to the fact that endogenous and exogenous factors do not have any effect on the shell.27 The shell is just a protective layer and has no specific function other than this. The principal component of the crustacean exoskeleton is chitin linked with proteins, which may carry some 210Po and also 210Pb which replaces calcium.28 The 210Pb content in the exoskeleton of crabs was found to vary between 2.3 ± 1.2 and 7.4 ± 1.7 Bq kg−1. These values were lower when compared to the values reported for the exoskeleton of crabs of the Palk Strait (19.9–46.4 Bq kg−1).29
The organs involved in digestion and metabolism are characterized by higher concentrations of 210Po relative to the muscle and external organs, which supports the view of Skwarzec30 and Swift et al.31 Elemental turnover rates are, in general, higher in digestive organs than in muscle. Hence, elemental levels in the muscle reflect the elemental intake over a longer time frame than the other tissues. Since demersal crabs have a tendency to migrate long distances, it could be suggested that the crab muscle impregnation is the result of the accumulation of radionuclides from prey living in environments other than where the crabs were caught and which contained higher 210Po and 210Pb levels than those consumed by crabs in the present environment. This noticeable difference indicates the prominent role of the digestive organs in controlling the absorption and elimination of 210Po as well as 210Pb. The deposition of 210Po in digestive organs leads to higher radiation dose equivalent to these organs.32
The weighted internal dose rate received chronically by crabs was calculated using a simplified approach based on the equation and dose conversion coefficients (DCCs) recommended by the FASSET programme33,34 and Ulanovsky and Prohl.35 External DCCs have been derived for a uniform isotropic infinite absorbing medium. The results are shown in Table 4. All the crabs except Portunus pelagicus and Uca annulipes considered for this study are demersal species. The former is pelagic and the latter is a semi-terrestrial marine crab. Except Uca, all the species reside above the sandy-muddy bottom and over rocks and hardy substrates and are immersed in water all the time. Therefore, they live in a sediment–water interface and an occupancy factor of 0.5 is applied to both water and sediment. Thus, the external dose rate was calculated using the equation:
| Djext = ∑i DCCjext,i × [0.5 × Cwater,i + 0.5 × Csed,i] |
| Species | External dose rate [μGy h−1] | Internal dose rate [μGy h−1] | Summed dose rate (external + internal) [μGy h−1] | Total dose rate [210Po + 210Pb] [μGy h−1] | Screening value | Risk quotient [RQ] | |||
|---|---|---|---|---|---|---|---|---|---|
| 210Po | 210Pb | 210Po | 210Pb | 210Po | 210Pb | ||||
| C. lucifera | 4.28 × 10−6 | 5.87 × 10−5 | 6.86 | 0.005 | 6.86 | 5.15 × 10−3 | 6.86 | 10 | 0.69 |
| C. natator | 4.60 × 10−6 | 6.41 × 10−5 | 3.09 | 0.006 | 3.09 | 5.73 × 10−3 | 3.09 | 10 | 0.31 |
| C. feriatus | 4.45 × 10−6 | 5.40 × 10−5 | 3.14 | 0.005 | 3.14 | 4.59 × 10−3 | 3.14 | 10 | 0.31 |
| C. annulata | 5.76 × 10−6 | 6.86 × 10−5 | 6.09 | 0.003 | 6.09 | 3.07 × 10−3 | 6.09 | 10 | 0.61 |
| P. sanguinolentus | 5.59 × 10−6 | 6.53 × 10−5 | 4.52 | 0.008 | 4.52 | 7.84 × 10−3 | 4.53 | 10 | 0.45 |
| P. pelagicus | 3.76 × 10−6 | 4.14 × 10−5 | 4.78 | 0.007 | 4.78 | 7.53 × 10−3 | 4.79 | 10 | 0.48 |
| A. integerrimus | 2.24 × 10−6 | 4.23 × 10−5 | 3.89 | 0.013 | 3.89 | 1.29 × 10−2 | 3.91 | 10 | 0.39 |
| M. lunaris | 2.41 × 10−6 | 4.21 × 10−5 | 2.39 | 0.003 | 2.39 | 3.14 × 10−3 | 2.40 | 10 | 0.24 |
| C. lophos | 5.61 × 10−6 | 6.53 × 10−5 | 2.99 | 0.003 | 2.99 | 3.31 × 10−3 | 2.99 | 10 | 0.30 |
| U. annulipes | 4.43 × 10−6 | 8.26 × 10−5 | 1.42 | 0.003 | 1.42 | 3.06 × 10−3 | 1.43 | 10 | 0.14 |
The semi-terrestrial species reside most of the time buried in shore sediment, comes to water only during the night and gets immersed by water only for some of the time. An assumed occupancy factor of 0.2 in water and 0.8 in sediment was applied. Therefore, the external dose rate was calculated using the equation:
| Djext = ∑i DCCjext,i × [0.2 × Cwater,i + 0.8 × Csed,i] |
The internal dose rate for crabs was derived from the activity concentration using the following equation:
| Djint = ∑iCji × DCCjint,i |
Committed effective dose (CED) values derived from ingestion of 210Po and 210Pb through consumption of crabs were estimated for adults. The daily intake of both the radionuclides was calculated by multiplying the respective elemental concentration in edible tissue by the average daily consumption, according to the values reported for the Kudankulam population.39 The effective dose was calculated by applying ICRP dose conversion factors of 1.2 and 0.69 μSv Bq−1 for 210Po and 210Pb, respectively.40 Therefore, the dose calculation was completed using the following formula:
| Ding = DCF × CR × IT |
The average daily intake of 210Po and 210Pb through consumption of crabs ranged from 0.2 to 1.7 and 0.1 to 0.9 Bq d−1, respectively. The dominant contribution to the radiological accumulation received by the population is derived from 210Po due to fish and shellfish consumption.41 In Japan, the intake levels of 210Po and 210Pb by marine food consumption were estimated to be 0.48–0.69 and 0.022–0.042 Bq d−1 per person, respectively,42 less than those obtained here. Recently, Yamamoto et al.43 reviewed information on the intake of 210Po and 210Pb in various countries. In comparison to those data, the dietary intake of 210Po is more or less comparable while 210Pb is within the reported range (0.04–0.88 Bq d−1).
The annual dose was estimated to range between 65.7 to 756.9 μSv y−1 due to 210Po and between 14.6 and 227.4 μSv y−1derived from 210Pb ingestion (Table 5). The annual dose from joint intake of 210Po and 210Pb ranged from 80.3 to 871.7 μSv y−1 when consuming both the species. The calculated dose values were compared with those published elsewhere. The present values are lower than values reported for marine organisms from the Kalpakkam coast (1302.3 μSv y−1)44 and from the Gulf of Mannar (2287.9 μSv y−1).45 Aarkrog et al.3 estimated the individual dose to be between 5.1 and 9.1 μSv y−1, with a value of 160 μSv y−1 for a critical group consuming large quantities of seafood products. Connan et al.5 reported a range of 78–129 μSv y−1 for the adult population of the western coast of the English Channel, France. Pollard et al.46 calculated a dose of 19 μSv y−1, Nielson et al.47 estimated a dose of 700 μSv y−1 and Jia et al.48 gave exposure values of between 50 and 200 μSv y−1. 210Po contributed 60% to the total effective dose. The calculated dose values for 210Po and 210Pb in crabs were more or less comparable with the global levels.
| Species | Daily consumption (kg d−1) | Activity intake (Bq d−1) | CED (μSv y−1) | Total | ||
|---|---|---|---|---|---|---|
| 210Po | 210Pb | 210Po | 210Pb | |||
| C. lucifera | 0.07 | 1.3 | 0.6 | 579.5 | 156.9 | 736.4 |
| C. natator | 0.08 | 1.7 | 0.5 | 756.9 | 114.8 | 871.7 |
| C. feriatus | 0.09 | 1.5 | 0.4 | 650.4 | 102.0 | 752.4 |
| C. annulata | 0.05 | 0.7 | 0.3 | 311.0 | 78.1 | 389.1 |
| P. sanguinolentus | 0.08 | 1.4 | 0.4 | 599.2 | 98.7 | 697.9 |
| P. pelagicus | 0.07 | 0.9 | 0.9 | 413.9 | 227.4 | 641.3 |
| A. integerrimus | 0.05 | 0.6 | 0.5 | 275.9 | 122.1 | 398.1 |
| M. lunaris | 0.04 | 0.4 | 0.3 | 178.7 | 75.6 | 254.3 |
| C. lophos | 0.03 | 0.3 | 0.2 | 130.1 | 62.0 | 192.0 |
| U. annulipes | 0.02 | 0.2 | 0.1 | 65.7 | 14.6 | 80.3 |
The lifetime cancer risk is calculated based on the activity per unit intake as per USEPA49 and Mishra et al.6 using the formula
| RK = AI × FI × ED × RC |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000).55 Therefore, the median dose calculated due to 210Po and 210Pb in certain crabs in Kudankulam is lower and would not pose any significant radiological impact on health or an increased cancer risk to the public, and the seafood are considered safe for human consumption. The calculated lifetime risk due to 210Po in the present study was higher than values reported from Mumbai (9.74 × 10−6 to 2.12 × 10−4)6 and lower than those reported from Pakistan (4.5 × 10−4).56
000).55 Therefore, the median dose calculated due to 210Po and 210Pb in certain crabs in Kudankulam is lower and would not pose any significant radiological impact on health or an increased cancer risk to the public, and the seafood are considered safe for human consumption. The calculated lifetime risk due to 210Po in the present study was higher than values reported from Mumbai (9.74 × 10−6 to 2.12 × 10−4)6 and lower than those reported from Pakistan (4.5 × 10−4).56
| Species | 210Po | 210Pb | Total life time risk (a + b) | ||||
|---|---|---|---|---|---|---|---|
| Annual intake (Bq y−1) | Risk (y) | Life time risk (a) | Annual intake (Bq y−1) | Risk (y) | Life time risk (b) | ||
| C. lucifera | 482.9 | 2.9 × 10−5 | 1.8 × 10−3 | 227.4 | 2.1 × 10−5 | 1.3 × 10−3 | 3.1 × 10−3 |
| C. natator | 630.7 | 3.8 × 10−5 | 2.4 × 10−3 | 166.4 | 1.5 × 10−5 | 9.6 × 10−4 | 3.3 × 10−3 |
| C. feriatus | 542.0 | 3.3 × 10−5 | 2.0 × 10−3 | 147.8 | 1.4 × 10−5 | 8.5 × 10−4 | 2.9 × 10−3 |
| C. annulata | 259.2 | 1.6 × 10−5 | 9.8 × 10−4 | 113.2 | 1.0 × 10−5 | 6.5 × 10−4 | 1.6 × 10−3 |
| P. sanguinolentus | 499.3 | 3.0 × 10−5 | 1.9 × 10−3 | 143.1 | 1.3 × 10−5 | 8.2 × 10−4 | 2.7 × 10−3 |
| P. pelagicus | 344.9 | 2.1 × 10−5 | 1.3 × 10−3 | 329.6 | 3.1 × 10−5 | 1.9 × 10−3 | 3.2 × 10−3 |
| A. integerrimus | 230.0 | 1.4 × 10−5 | 8.7 × 10−4 | 177.0 | 1.6 × 10−5 | 1.0 × 10−3 | 1.9 × 10−3 |
| M. lunaris | 148.9 | 9.1 × 10−5 | 5.6 × 10−4 | 109.5 | 1.0 × 10−5 | 6.3 × 10−4 | 1.2 × 10−3 |
| C. lophos | 108.4 | 6.6 × 10−6 | 4.1 × 10−4 | 89.8 | 8.3 × 10−6 | 5.2 × 10−4 | 9.3 × 10−4 |
| U. annulipes | 54.8 | 3.3 × 10−6 | 2.1 × 10−4 | 21.2 | 2.0 × 10−6 | 1.2 × 10−4 | 3.3 × 10−4 |
Conclusions
The results of this study indicate a significant difference in 210Po and 210Pb concentrations between brachyuran crabs. The radionuclides accumulated by these organisms lead to a higher internal dose rate. In the present study, the hepatopancreas was found to be the indicator tissue and it can be considered as a critical organ for 210Po accumulation. The assessment of 210Po and 210Pb concentrations in environmental samples is mandatory for determining its contribution to the background radiation as well as for estimating the intake levels of these radionuclides by consuming seafood. The reduced concentrations of 210Po and 210Pb in the soft tissue of crabs is important to humans, who consume mostly the meat, leading to less internal dosage. The whole-body exposures to the crabs were lower than the screening dose rate and the risk quotient was found to be less than one. The total lifetime cancer risk estimated indicated no significant health hazard and the crabs are safe and within international standards. The findings of the present study revealed that the natural radiation level is higher, whereas the fallout radiation level is very much lower than values reported internationally. The data obtained in the present study would be a baseline pre-operational input, filling the radioecological database and will also be useful in the future impact assessment of the Kudankulam Nuclear Power Project after it becomes operational.Acknowledgements
The authors would like to express their thanks to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Government of India, for providing financial assistance for this study (Sanction No. 2004/36/16-BRNS).References
- I. L. Cunha, L. Bueno, D. I. T. Fávaro, V. A. Maihara and S. Cozzollino, J. Radioanal. Nucl. Chem., 2001, 247, 447 CrossRef CAS.
- A. Ugur, G. Yener and A. Bassari, Appl. Radiat. Isot., 2002, 57, 565 CrossRef CAS.
- A. Aarkrog, M. S. Baxter, A. O. Bettencourt, R. Bojanowski, A. Bologa, S. Charmasson, I. Cunha, R. Delfanti, E. Duran, E. Holm, R. Jeffree, H. D. Livingston, S. Mahapanyawong, H. Nies, I. Osvath, L. Pingyu, P. P. Povinec, A. Sanchez, J. N. Smith and D. Swift, J. Environ. Radioact., 1997, 34(1), 69 CrossRef CAS.
- S. Suriyanarayanan, G. M. Brahmanandhan, J. Malathi, S. Ravikumar, V. Masilamani, S. P. Hameed and S. Selvasekarapandian, J. Environ. Radioact., 2007, 99(4), 766.
- O. Connan, P. Germain, L. Solier and G. Gouret, J. Environ. Radioact., 2007, 97(2–3), 168 CrossRef CAS.
- S. Mishra, S. Bhalke, G. G. Pandit and V. D. Puranik, Chemosphere, 2009, 76, 402 CrossRef CAS.
- F. P. Carvalho and S. W. Fowler, Mar. Ecol.: Prog. Ser., 1994, 103, 251 CrossRef CAS.
- N. K. Karouna-Renier, R. A. Snyder, J. G. Allison, M. G. Wagner and K. R. Rao, Environ. Pollut., 2007, 145(2), 474 CrossRef CAS.
- S. D. Gerhart and T. M. Bert, J. Crustacean Biol., 2009, 28(2), 252 Search PubMed.
- E. V. Radhakrishnan, M. K. Manisseri and G. Nandakumar, Status of research on crustacean resources, in Status and Perspectives in Marine Fisheries Research in India, ed. Mohan Joseph Modayil and N. G. K Pillai, Central Marine Fisheries Research Institute (CMFRI), Kochi, India, 2007, 135–172 Search PubMed.
- N. Ramjee and V. Naganathan, Glimpses of the Gulf. The First Field Discovery book to the Living Marine Resources in the Gulf of Mannar. Centre for Environment Education (CEE), Publication No. 15, Gulf of Mannar Biosphere Reserve Trust (GoMBRT), Tamil Nadu, India, 2008, 88–91 Search PubMed.
- G. Jia, M. Belli, M. Blasi, A. Marchetti, S. Rosamilia and U. Sansone, J. Radioanal. Nucl. Chem., 2001, 247(3), 491 CrossRef CAS.
- P. Vesterbacka and T. K. Ikaheimonen, Anal. Chim. Acta, 2005, 545(2), 252 CrossRef CAS.
- M. A. R. Iyengar, PhD thesis, University of Bombay, India, 1983.
- USEPA, United States Environmental Protection Agency, Guidance for data quality assessment. Practical methods for data analysis. EPA QA/G-9, 2000 Search PubMed.
- A. Z. Mason and K. D. Jenkins, Metal detoxification in aquatic organisms. Metal Specification and bioavailability in Aquatic Systems, in IUPAC Series on Analytical and Physical Chemistry of Environmental Systems, John Wiley and Sons, Chichester, England, 1995, vol. 3479–607 Search PubMed.
- IAEA, International Atomic Energy Agency. Sediment distribution coefficients and concentration factors for biota in the marine organisms. Tech. Report Ser. No. 422, Vienna, 2004 Search PubMed.
- P. Stepnowski and B. Skwarzec, J. Environ. Radioact., 2000, 49, 201 CrossRef CAS.
- P. Bustamante, P. Germain, G. Leclerc and P. Miramand, Mar. Pollut. Bull., 2002, 44, 997 CrossRef CAS.
- V. S. Bangera and K. Rudran, Bull. Radiat. Prot., 1995, 18(1–2), 192 Search PubMed.
- S. M. M. Hameed, T. Karuppuchamy, V. Masilamani, S. Ravikumar and S. P. Hameed, Study on the distribution of alpha-emitting radionuclide, polonium-210 in the ecosystem of Kattumavadi Coast (Palk Strait), in Proceedings of the National Seminar on Atomic Energy, Ecology and Environment, Thiruchirapalli, India, 2001, 143–150 Search PubMed.
- M. P. Rajan, V. Kannan, S. Ganapathy and M. A. R. Iyengar, Bull. Radiat. Prot., 1980, 3(4), 69 Search PubMed.
- I. Othman, T. Yassine and I. Bhat, Sci. Total Environ., 1994, 153, 57 CrossRef CAS.
- E. Holm, Polonium-210 and radio caesium in muscle tissue of fish from different Nordic marine areas, ed. H. Dahlgaard, in Nordic radioecology: The transfer of radionuclide through Nordic ecosystems to man, Elsevier, Amsterdam, 1994, 119–126 Search PubMed.
- P. Stepnowski and B. Skwarzec, J. Environ. Radioact., 2000, 49, 195 CrossRef CAS.
- C. Alonso-Hernandez, M. D. Asencio, A. M. Caravaca, E. S. Morell and R. A. Moreno, J. Environ. Radioact., 2002, 61, 203 CrossRef CAS.
- M. E. Havlik and L. L. Marking, U.S. Fish Wildl. Serv. Resour. Publ., 1987, 164, 1 Search PubMed.
- S. A. Bukhari, PhD thesis, Bharadhidasan University, Tiruchirapalli, India, 2004.
- S. M. M. Hameed, S. A. Bukhari and S. P. Hameed, Study on the bioaccumulation of polonium-210 and lead-210 in the abiotic and biotic components of Palk Strait, Southeast Coast of India, in 11th International Congress of the International Radiological protection Association, Madrid, Spain, 2004, 111–115 Search PubMed.
- B. Skwarzec, J. Environ. Radioact., 1988, 8, 111 CrossRef CAS.
- D. J. Swift, D. L. Smith, D. J. Allington and M. J. Ives, J. Environ. Radioact., 1994, 23, 213 CrossRef CAS.
- B. Skwarzec and L. Falkowski, J. Environ. Radioact., 1988, 8, 99 CrossRef CAS.
- Ed. G. Prohl, Dosimetric Models and Data for Assessing Radiation Exposures to Biota. Deliverable 3 to the Project FASSET (Framework for the Assessment of Environmental Impact). Contract No. FIGE-CT-2000–00102; Norwegian Radiation Protection Authority, Osteras, 2003 Search PubMed.
- Ed. J. E. Brown, P. Strand, A. Hosseini and P. Borretzen, Handbook for Assessment of the Exposure of Biota Ionising Radiation from Radionuclides in the Environment. Deliverable 5 to the Project FASSET (Framework for the Assessment of Environmental Impact), Contract No. FIGE-CT-2000–00102; Norwegian Radiation Protection Authority, Osteras, 2003 Search PubMed.
- A. Ulanovsky and G. Prohl, Radiat. Environ. Biophys., 2008, 47(2), 195 CrossRef CAS.
- J. E. Brown, S. R. Jones, R. Saxen, H. Thørring and J. Vives i Batlle, J. Radiol. Prot., 2004, 24, A63 CrossRef CAS.
- Ed. N. Beresford, J. Brown, D. Copplestone, J. Garnier-Laplace, B. Howard, C. Larsson, D. Oughton, G. Prohl and I. Ginger, D-ERICA: An integrated approach to the assessment and management of environmental risks from ionizing radiation,European Commission, ERICA (Contract No. FI6R-CT-2004–508847), 2007 Search PubMed.
- UNSCEAR, United Nations Scientific Committee on the Effects of Atomic Radiation. 5th session (10–18 July) General Assembly of Official Records, 63rd session, Supplement 46, United Nation, New York, 2008 Search PubMed.
- N. Sukumaran, G. Karunanithi and A. G. Murugesan, Studies on Demographic Pattern, Aquatic Resources and its Physical, Chemical, Biological, Pathological and Radiological Quality around Kudankulam Site Covering 30 km Radius. Technical Report Submitted to Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Mumbai (Sanc. No. 2004/36/14-BRNS), 2008 Search PubMed.
- ICRP, International Commission on Radiological Protection, Age-dependent doses to members of the public from intake of radionuclides: Part 5 Compilation of ingestion and inhalation dose coefficients, Ann. ICRP, 1996, 26(1), 1 CrossRef.
- F. Boisson, N. Yorulmaz, E. Kam, G. Karahan and A. E. Osmanlioglu, Radiat. Meas., 2001, 42, 1387.
- M. Yamamoto, T. Abe, J. Kuwabara, K. Komura, K. Ueno and Y. Takizawa, J. Radioanal. Nucl. Chem., 1994, 178, 81 CAS.
- M. Yamamoto, A. Sakaguchi, J. Tomita, T. Imanaka and K. Shiraishi, J. Radioanal. Nucl. Chem., 2009, 279, 93 CrossRef CAS.
- V. Kannan, Internal dose estimates due to ingestion of natural and man-made radionuclides via dietary and drinking water sources to public at Kalpakkam, in Proceedings of the National Seminar on Atomic Energy, Environment and Human Welfare, Tiruchirapalli, India, 2005, 47–53 Search PubMed.
- V. Masilamani, PhD thesis, Bharathidasan University, Tiruchirappalli, India, 2001.
- D. Pollard, T. P. Ryan and A. Dowdall, Radiat. Prot. Dosim., 1998, 75(1–4), 139 CAS.
- S. P. Nielson, P. Bengston, R. Bojanowsky, P. Hagel, J. Herrmann, E. Ilus, E. Jakobson, S. Motiejunas, Y. Panteleev, A. Skujina and M. Suplinska, Sci. Total Environ., 1999, 237, 133 CrossRef.
- G. Jia, M. Belli, U. Sansone, S. Rosamilia and M. Blasi, J. Radioanal. Nucl. Chem., 2003, 256(3), 513 CAS.
- USEPA, United States Environmental Protection Agency. Estimating Radiogenic Cancer Risks. Federal Guidance Report. EPA-402-R-93-076, Washington, DC, 1994 Search PubMed.
- M. D. Lagrega, P. L. Buckingham and J. C. Evans, Hazardous Waste Management. McGraw-Hill, New York, 1994 Search PubMed.
- USEPA, United States Environmental Protection Agency. Cancer risk Coefficients for Environmental Exposure to Radionuclides. Federal Guidance Report No. 13, EPA-402-R-99-001, Washington, DC, 1999 Search PubMed.
- D. W. Connell, C. N. Fung, T. B. Minh, S. Tanabe, P. K. S. Lam, B. S. F. Wong, M. H. W. Lam, L. C. Wong, R. S. S. Wu and B. J. Richardson, Water Res., 2003, 37, 459 CrossRef CAS.
- C. L. H. Hung, M. K. So, D. W. Connel, C. N. Fung, M. H. W. Lam, S. Nicholson, B. J. Richardson and P. K. S. Lam, Chemosphere, 2004, 56, 643 CrossRef CAS.
- USDOE, United States Department of Energy. Uncertainities in Cancer Risks Coefficient for Environmental Exposure to Radionuclides. An Uncertainity Analysis for Risk Coefficients Reported in Federal Guidance Report No. 13, Oak Ridge National Laboratory, ORNL/TM-2006/583, 2007 Search PubMed.
- ICRP, International Commission on Radiological Protection. Recommendation of International Commission of radiological Protection. ICRP Publication 60, Pergamon Press, Oxford, UK, 1991 Search PubMed.
- P. Akhter, K. Rahman, S. D. Orfi and N. Ahmad, Food Chem. Toxicol., 2007, 45, 272 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
