Presence and partitioning properties of the flame retardants pentabromotoluene, pentabromoethylbenzene and hexabromobenzene near suspected source zones in Norway†
Hans Peter H.
Arp
*a,
Thomas
Møskeland
b,
Patrik L.
Andersson
c and
Jenny Rattfelt
Nyholm
c
aDepartment of Environmental Engineering, Norwegian Geotechnical Institute (NGI), P.O. Box 3930 Ullevål Stadion, N-0806, Oslo, Norway. E-mail: hpa@ngi.no; Tel: +47 2202 1988
bDet Norske Veritas (DNV), Veritasveien 1, N-1363, Høvik, Norway
cDepartment of Chemistry, Umeå University, SE-901 87, Umeå, Sweden
First published on 7th December 2010
Abstract
The brominated flame retardants (BFRs), pentabromotoluene (PBT), pentabromoethylbenzene (PBEB) and hexabromobenzene (HBB), exhibit physical–chemical properties similar to other persistent organic pollutants, and have been in use as flame retardants for several decades. Data on these BFRs in diverse environmental samples can be found in studies from the 1970s and 1980s, as well as in recent years, though very little in the years in between. Due to a lack of data, the cause for the apparent re-emergence of these BFRs in recent studies is unclear, and could reflect changes in production volumes, accumulation of transformation products from BFR precursors, improved analytical techniques or simply a re-emergence in concern. Very little data are available on their environmentally relevant partitioning properties, which could help to explain the occurrence and fate of these BFRs. In this study we analysed for the presence of HBB, PBT, and PBEB in diverse environmental samples from potential Norwegian BFR source zones. Additionally, environmental partitioning properties of these BFRs as well as brominated benzenes were estimated and validated using experimental data for brominated benzenes. Of the three BFRs, HBB was identified in detectable quantities at most source zones, PBEB only near a metal recycling factory, and PBT only in a few additional locations from where PBEB was detected. Data from this study show that HBB is likely widely distributed, as verified both by chemical analysis and estimated properties. Measured HBB levels in wastewater treatment plants indicate that the treatment practices used in the study locations are not effective at lowering HBB levels, perhaps due to association with low density suspended solids (e.g. microplastics).
Environmental impactThe lesser studied brominated flame retardants (BFRs), hexabromobenzene (HBB), pentabromotoluene (PBT) and pentabromoethylbenzene (PBEB), were quantified in diverse media in suspected Norwegian source zones. HBB was present in all obtained water, sediment and air samples, though not in biota. HBB levels in wastewater did not decrease in treatment facilities, perhaps due to sorption to microplastics. PBT and PBEB were less frequently detected than HBB. Estimations of partitioning properties of HBB, PBT and PBEB indicate that environmental transport pathways should be similar. This work will assist in understanding the environmental fate of these “alternative” BFRs. Due to the levels of HBB measured here, inclusion in monitoring programs is warranted. |
Introduction
Brominated flame retardants (BFRs) have been used commercially to fire-proof plastics, textiles and electronics since the late 1960s.1 The BFR industry has grown rapidly since its introduction, with estimates of production from 2000 being at ca. 200 kilotonnes per year,1 and from 2005 at ca. 300 kilotonnes per year.2 The environmental persistence of many BFRs and the discovery of toxic effects at trace levels for some of these compounds have prompted increasing regulations on BFR usage. Notably, many polybrominated biphenyls (PBBs) and polybrominated diphenyl ethers (PBDEs) were recently added to the Stockholm Convention on Persistent Organic Pollutants.3 Increasing regulations on BFR usage and production provides incentives for the production of “new” BFRs or non-regulated BFRs, whose environmental presence or behaviour is less well established.One such “new” BFR is hexabromobenzene (HBB) (Fig. 1), which has been produced for several decades, but has not received as much attention as PBBs and PBDEs. This compound has been found recently in diverse environmental samples, including herring gull tissues and egg samples from the Great Lakes,4,5 glaucous gulls in the Norwegian arctic,6 air samples in Toronto, Canada,7 and human blood samples in Tianjin, China.8 HBB was also noticed earlier in sediment samples9 and human adipose tissue10 from Japan in the 1980s, likely related to Japanese production levels of HBBs, which were reported at 270 tonnes in 1983.10
 | ||
| Fig. 1 Structures, names and CAS numbers of the brominated flame retardants analysed for in this study. | ||
Two other commercial “new” BFRs that are structurally similar to HBB, that have been produced for just as long, and are also very rarely included in environmental monitoring studies, are pentabromotoluene (PBT) and pentabromoethylbenzene (PBEB) (Fig. 1). Like HBB, PBT and PBEB have appeared recently in herring gull eggs and glaucous gull tissues,4,6 and PBEB was detected in Chicago air samples.11 As with HBB, these compounds have also been identified in environmental samples several decades ago; PBT was spotted in Swedish wastewater treatment plants in 1975,12 and PBEB has been found in Canadian lake trout captured in 1979.13
As very few reports are available between the 1990s and 2000 on the environmental presence and industrial usage of HBB, PBT and PBEB, it cannot be stated here whether their recent reappearance in the literature is related to variations in production, release or concern. One study that is available on this topic is the aforementioned study reporting PBEB in lake trout caught in 1979,13 the same study found little variations in concentration from trout caught between 1979 and 2004,13 which would favour the explanation that environmental levels in that region were held constant over this time (though further studies would be needed to confirm this). In any case, these compounds can be considered as “re-emerging”, even if they are not “re-emerging” in the environment per se, they are “re-emerging” in interest.
Very little data can be found on what products HBB, PBT and PBEB are used for or how much is produced. From scattered reports, HBB has been used since the 1970s for fireproofing plastics, textiles and wood,14 and is released from BFR polymers.7PBT is found in unsaturated polyesters used in electrical applications15 and PBEB is used in unsaturated polyesters, polyurethane foam, textiles and adhesives.16 Current production levels are unknown and represent a knowledge gap. The EPA Inventory Update Reports list (http://www.epa.gov/iur/tools/data/2002-vol.html) levels of HBB for 1998 at 10–500 thousand pounds (4.5–227 metric tons) (somewhat less than levels in Japan in the 1980s), and 1986 levels for PBEB at 10–500 thousand pounds. More recent reports are unavailable. An additional cause for the occurrence of PBT and PBEB is that they are potential degradation products of decabromodiphenyl ethane (DBDPE) (as just one methyl bond would have to be cleaved).
Two recently developed screening tools for identifying persistent organic pollutants (POP) also indicate that monitoring for these BFRs is warranted. One screening tool17 gave a “POP score” of 4.1 for HBB and 3.5 for PBT, which is not significantly different from the average score of POPs identified in the arctic (4.45 ± 1.12). Another similar study classified HBB as a POP of moderate concern and PBEB of low/moderate concern.18
To increase our understanding on the abundance and environmental behaviour of HBB, PBT, and PBEB, their presence was investigated in various suspected source zones in Norway, including air, water, sediment and biota samples, as well as municipal wastewater entering and exiting wastewater treatment plants (WWTPs). In addition, we validated a variety of predictive software models for their ability to estimate relevant environmental partitioning properties of these compounds, as well as brominated benzenes.
Experimental
Sampling
The sampling campaign targeted potential source zones for BFRs in three municipal areas in Norway: Drammen, Lillehammer and Tromsø (Fig. 2); background sites were not included. Source zones were chosen based on their potential exposure to waste that contains BFRs, or on available data that indicated the presence of other BFRs.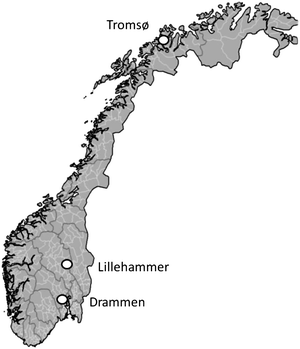 | ||
| Fig. 2 Areas of Norway in which potential BFR source zones were sampled. | ||
At each municipality, water and sludge samples were collected from the main municipal WWTP (Table 1). Sampling at WWTP was conducted as follows. 1 L water samples were obtained daily from the inlet and outlet monitoring stations within each WWTP using amber glass jars that were thoroughly rinsed with sampled water. Combined weekly samples (7 L) were stored in amber glass containers wrapped in aluminium foil. Outlet sludge samples were sampled daily using a solvent- or detergent-rinsed metal spoon, and weekly samples were combined in an amber jar, wrapped in aluminium foil and homogenized. Sampling was done over three consecutive weeks, allowing three inlet, outlet and sludge samples per WWTP. Sediment samples in diverse locations from the receiving water body of the WWTP (either a lake or harbour) were also obtained. Sediment samples were collected using a van Veen grab sampler (0.0025 m2), and sediments on the surface 0–2 cm were collected by spooning different areas from the top of the grab sample into an amber glass container, which was wrapped in aluminium foil and packed in an LDPE bag. From the receiving waters, fish livers, blue mussel and crab samples were also obtained. Marine biota samples were captured by local fisherman as instructed, with entire specimen samples stored in glass jars, wrapped in aluminium foil and placed in LDPE bags. In the field all samples were kept cool and dark in sealed containers and were immediately stored frozen upon arrival at DNV. At DNV's biology laboratory, portions of the samples were isolated and combined, with one sample consisting generally of 4 fish livers, 3 blue mussel meat samples and 4 crab meat samples. Biological samples were homogenized in Na2SO4 and stored in amber glass cans.
| Municipality | Area | Name | Dates (2009) | Matrix |
|---|---|---|---|---|
| a GPS coordinates (WGS84, with directions given as N, E, respectively) of sediment sampling stations in Drammen (Site 1 59.713361, 10.279872, Site 2 59.714308, 10.273864, Site 3 59.712827, 10.269132), fish species are cod (Gadus morhua) (59.709402, 10.275886), edible crab (Cancer pagurus) (59.707818, 10.275405) and blue mussel (59.708955, 10.273632). b GPS coordinates (WGS84, with directions given as N, E, respectively) of sediment sampling stations in Lake Mjøsa (Site 1 61.095049, 10.4482, Site 2 61.072324, 10.441814, Site 3 61.046207, 10.448962), Lake Losna (61.368346, 10.277723) fish species is trout (61.089639, 10.434022). c GPS coordinates (WGS84, with directions given as N, E, respectively) of sediment sampling stations in Sagnessundet (Site 1 69.6839, 18.884032, Site 2 69.68585, 18.896366, Site 3 69.68755, 18.899932), fish species (and GPS coordinates) are cod (69.691947, 18.899808), crab genus Hyas (spider crab and lyre crab) (69.684924, 18.899957), and blue mussel (69.688954, 18.906765). d Sed = sediment, ww = wastewater/seepage water, sl = sludge, m = moss, n = needle, a = air, bm = blue mussel, fl = fish liver, cr =crab. | ||||
| Drammen | WWTP | Solumstrand RA | July 9th to July 29th | ww, sl |
| Fjord | Drammensfjorda | June 16th to Oct 16th | sed, fl, cr, bm | |
| City centre | Aug 11th to Oct 15th | a | ||
| Waste-to-energy plant | Hurum | Sept 29th | m, n | |
| Landfill | Lindum | ww | ||
| Metal recycling factory | Hellik Teigen AS | July 8th to July 15th | ww, sed | |
| Lillehammer | WWTP | Lillehammer RA | Aug 19th to Sep 1st | ww, sl |
| Lake Mjøsa | Mjøsab | Aug 19th | sed, fl | |
| Lake Losna | Losna | Aug 18th | sed | |
| Tromsø | WWTP | Langnes RA | Sept 7th to Oct 27th | ww, sl |
| Fjord | Sagnessundetc | Oct 8th to Oct 15th | sed, fl, cr, bm |
In the area of Drammen several diverse source zones were additionally sampled including a metal recycling factory (Hellik Teigen), a municipal waste landfill (Lindum) and air samples from the city centre and a nearby waste-to-energy plant (Hurum). At the metal recycling factory, seepage water at the discharge point (to the Loselva River) was taken (1 week sample as previously for WWTPs), as well as a sediment sample ca. 10 m from the discharge point. At Lindum, seepage water and sediments were sampled (using the same method as for the WWTPs) from a drainage area where they are collected before being pumped to the Drammen WWTP. In Drammen city centre, three air particle samples were collected using a high volume air pump with a particle mass < 10 µm (PM10)-cutoff inlet (Sierra Instruments) equipped with a glass microfiber filter (Whatman EPM 2000), 291 m3, 667 m3 and 197 m3 were sampled over Aug 11–13 (average temp. 16.3 ± 3.5 °C), Sep 14–18 (12 ± 4 °C) and Oct 14th and 15th (−1 ± 4 °C), 2009, respectively. After sampling, filters were wrapped in two layers of aluminium foil (non-rinsed) and were stored frozen. Additionally, as passive air samplers, pine needles and stair step moss (Hylocomium splendens) samples were taken near the Hurum waste-to-energy plant. Samples were collected at four locations around the plant, where they were wrapped in aluminium foil, stored in a dark glass container, and placed in LDPE bags. Throughout sampling no cosmetics or electronics were worn, and nitrile gloves were used. Additional information on sampling can be found in Table 1.
Chemicals
High purity analytical standards (12C) HBB, PBT and PBEB and 13C-labeled internal standard HBB were bought from Wellington Laboratories (Guelph, ON, Canada). The recovery standard PCB-208 was bought from Cambridge Isotope Laboratories (Andover, MA, USA). Methanol (SupraSolv), diethyl ether (SeccoSolv) and ethyl acetate (LiChrosolv) were bought from Merck, Germany. Hexane (Picograde) and acetone (Picograde) were bought from Promochem. Dichloromethane (glass distilled), cyclohexane (glass distilled) and toluene (glass distilled) were bought from Fluka, Switzerland.Extraction and clean-up
After sample collection and frozen storage at DNV, samples were shipped to Umeå University. Water, sediment, and sludge were sent frozen with road transport (Bring Frigoscandia). Biota samples, moss and needles were sent by air with DHL (frozen and stored in an insulated box). Air filters were sent with Schenker. Samples were stored frozen until analysis. Time between sampling and analysis ranged between 2 weeks and three months. All glassware was pre-combusted at 550 °C.Sediment, sludge, and moss were freeze-dried, and the sample dryness was verified by checking for weight loss after heating subsamples at 105 °C overnight. For sediment and sludge the loss of ignition was recorded after heating the subsamples to 550 °C for two hours. Freeze-dried samples were spiked with internal standards and extracted in Soxhlet apparatus with methanol for at least 15 h. Subsamples of the extract were evaporated until near dryness, redissolved in hexane and underwent clean-up using a multi-layer silica column. The silica columns were packed with glass wool, 3 g KOH–silica, 3 g neutral silica, 6 g of 40% (w/w) H2SO4–silica, and 3 g Na2SO4, rinsed with solvent, and eluted with 60 mL of a mixture of hexane and dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Activated copper (pre-soaked in hydrochloric acid, then washed with water, methanol and hexane) was added to sediment and sludge samples for the reduction of sulfur, by incremental addition until reaction with sulfur ceased. Recovery standard (13C PCB-208) was added and the samples were reduced in volume to approximately 100 µL. The recovery standard was added to calculate losses of the internal standard.
1). Activated copper (pre-soaked in hydrochloric acid, then washed with water, methanol and hexane) was added to sediment and sludge samples for the reduction of sulfur, by incremental addition until reaction with sulfur ceased. Recovery standard (13C PCB-208) was added and the samples were reduced in volume to approximately 100 µL. The recovery standard was added to calculate losses of the internal standard.
Total water (dissolved plus particle bound) and air particle extraction and workup were as follows: water samples (1.9–7.5 L) were spiked with internal standards, acidified with hydrochloric acid to pH 3, and then filtrated through glass microfibre filters (GF/B, Whatman) followed by 0.45 µm nylon membrane filters (Sartourius, Goettingen, Germany), and 90 mm SPE disks (ENVI-18 Dsk, Supelco, Bellefonte, USA). The filters and the SPE disks were air dried in a fume hood, then extracted and cleaned-up as described above. The filtrate was discarded. Air filters were spiked with internal standards and extracted and cleaned-up as described above.
Needle samples were extracted with dichloromethane in an ultrasonic bath for 10 minutes. The procedure was repeated two times and the extracts were combined. The amount of extracted waxes was determined gravimetrically. The samples were cleaned-up as described above.
Fish liver, crab, and mussel samples, homogenized in Na2SO4, were extracted on an open column with acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (5
hexane (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and hexane
2) and hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethyl ether (9
diethyl ether (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The internal standard was added and the lipid contents were determined gravimetrically. The extracted samples were dissolved in cyclohexane
1). The internal standard was added and the lipid contents were determined gravimetrically. The extracted samples were dissolved in cyclohexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and lipids were reduced with gel permeation chromatography. The gel permeation chromatography column (1.5 cm inner diameter) was wet-packed in-house to a height of 40 cm with 25 g of SX-3 Bio-beads (Bio-Rad Laboratories, Hercules, CA, USA) that had been pre-swollen in cyclohexane
1) and lipids were reduced with gel permeation chromatography. The gel permeation chromatography column (1.5 cm inner diameter) was wet-packed in-house to a height of 40 cm with 25 g of SX-3 Bio-beads (Bio-Rad Laboratories, Hercules, CA, USA) that had been pre-swollen in cyclohexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 2 h. The flow rate was set to 2 mL min−1, and the fractions containing the BFRs were collected between 20 and 50 min. The samples were additionally cleaned on a H2SO4–silica column (6 g of 40% H2SO4–silica) eluted with 60 mL hexane
1) for 2 h. The flow rate was set to 2 mL min−1, and the fractions containing the BFRs were collected between 20 and 50 min. The samples were additionally cleaned on a H2SO4–silica column (6 g of 40% H2SO4–silica) eluted with 60 mL hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (1
dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The samples were reduced in volume to approximately 100 µL in toluene and recovery standard was added.
1). The samples were reduced in volume to approximately 100 µL in toluene and recovery standard was added.
Weights of extracted samples (with dw = dry weight and ww = wet weight) were for sediment 4–15 g dw, sludge 1.5–4 g dw, moss 1.5–2.5 g dw, needles 17–30 g ww, fish liver 0.5–2 g ww, crab 1.3–6 g ww, and mussel 6–12 g ww.
Quantification
Quantification was done using gas chromatography-mass spectrometry (GC-MS) in electron capture negative ionization (ECNI) mode with an MSD 5975 quadrupole system (Agilent). Samples (1 µL) were injected with a programmable temperature vapourising injector operated in pulsed splitless mode with the following temperature program: 80 °C for 0.10 min followed by heating at 720 °C min−1 to 300 °C (held for 2 min). The pressure pulse was set to 15.8 psi for 1.80 min. For separation a 15 m Rxi-5sil MS column (0.25 mm i.d. × 0.10 µm film thickness; Restec, USA) was used with helium as the mobile phase (flow rate 1.5 mL min−1), and the following oven temperature program: 80 °C (held for 3 min) rising to 240 °C at 25 °C min−1, then to 315 °C at 10 °C min−1 (held for 5.5 min). The ion source temperature was set to 230 °C and methane was employed as the reagent gas. Ions were recorded in SIM mode and the retention time (min) and monitored ions (m/z) were as follows: PBT—7.94 min, ions 485.6 and 487.6; PBEB—8.17 min, ions 499.6 and 501.6; HBB—8.87 min, ions 549.5 and 547.5.Quality assurance
BFRs can be present in sampling or analysis materials, thus blank determinations are particularly important. Laboratory blanks were analysed in parallel to the samples. A BFR was considered detected if its signal-to-noise ratio was >3. The limit of detection (LOD) was based on the signal-to-noise in the quantification standard. The limit of quantification (LOQ) was set to ten times the signal-to-noise ratio. If a BFR was present in a laboratory blank the LOQ was set to three times the level detected in the blank. LOD and LOQ values are listed with results in Table 2.| Sample | Municipality | Location | PBT 87-83-2 | PBEB 85-22-3 | HBB 87-82-1 |
|---|---|---|---|---|---|
| a LOD = limit of detection, LOQ = limit of quantification, data are averages of three replicates, concentrations without a reported standard deviation were just found in one replicate. b Detected but below LOQ. | |||||
| Sediment and seepage sediments (ng g−1 dw) | Tromsø | Sagnessundet 1 | b | <LOD | b |
| Sagnessundet 2 | <LOD | <LOD | b | ||
| Sagnessundet 3 | <LOD | <LOD | <LOD | ||
| Lillehammer | Mjøsa 1 | <LOD | <LOD | <LOD | |
| Mjøsa 2 | <LOD | <LOD | <LOD | ||
| Mjøsa 3 | <LOD | <LOD | 0.0237 | ||
| Losna 1 | <LOD | <LOD | <LOD | ||
| Drammen | Drammensfjord 1 | <LOD | <LOD | <LOD | |
| Drammensfjord 2 | <LOD | <LOD | <LOD | ||
| Drammensfjord 3 | <LOD | <LOD | <LOD | ||
| Metal recycling area | 0.032 ± 0.012 | 0.028 ± 0.036 | 0.077 ± 0.021 | ||
| Near landfill | 0.22 ± 0.10 | <LOD | 1.33 ± 0.78 | ||
| LOD | 0.006 | 0.004 | 0.007 | ||
| LOQ | 0.019 | 0.01 | 0.023 | ||
| Wastewater and seepage water (ng L−1) | Tromsø | In | <LOD | <LOD | 1.82 ± 1.40 |
| Out | <LOD | <LOD | 0.93 ± 0.72 | ||
| Lillehammer | In | b | <LOD | 0.40 ± 0.20 | |
| Out | <LOD | <LOD | 0.58 ± 0.46 | ||
| Drammen | In | <LOD | <LOD | 1.24 ± 0.70 | |
| Out | b | <LOD | 0.69 ± 0.23 | ||
| Drammen | Metal recycling area | 5.63 ± 1.66 | 0.94 ± 0.32 | 15.37 ± 3.85 | |
| Landfill | 0.43 | <LOD | 1.74 ± 1.11 | ||
| LOD | 0.06 | 0.04 | 0.03 | ||
| LOQ | 0.22 | 0.12 | 0.12 | ||
| Wastewater sludge (ng g−1 dw) | Tromsø | <LOD | <LOD | 0.34 ± 0.08 | |
| Lillehammer | <LOD | <LOD | b | ||
| Drammen | <LOD | <LOD | 0.39 ± 0.17 | ||
| LOD | 0.09 | 0.07 | 0.11 | ||
| LOQ | 0.30 | 0.25 | 0.36 | ||
| Atmospheric particles (ng m−3), pine needles (ng g−1 ww), moss (ng g−1 dw) | Drammen | Air – city centre | <LOD | <LOD | 0.0043 ± 0.0051 |
| LOD | 0.001 | 0.0006 | 0.0005 | ||
| LOQ | 0.003 | 0.002 | 0.002 | ||
| Pine needles – Hurum | <LOD | <LOD | 0.05 | ||
| LOD | 0.013 | 0.009 | 0.01 | ||
| LOQ | 0.044 | 0.029 | 0.04 | ||
| Moss–Hurum | <LOD | <LOD | b | ||
| LOD | 0.044 | 0.026 | 0.060 | ||
| LOQ | 0.148 | 0.089 | 0.203 |
Before the extraction and clean-up of samples the recovery of the analytes was tested. This was done by adding known amounts of the BFRs of interest to three different matrices: dry artificial OECD soil, cod liver oil, and river water from Umeå (Umeälven). Duplicates of each matrix were analysed. The recoveries were high in all matrices being >78% in dry soil, >80% in fish oil and >58% in spiked water.
The average recoveries (±one standard deviation) of the internal standard 13C-labeled HBB, accounting for sample losses during clean-up, were calculated after GC-MS analysis and were 72 (±18)% in sediment, 56 (±16)% in water, 68 (±10)% in sludge, 49 (±19)% in air, 74 (±25)% in fish and 74 (±11)% in moss and needle samples.
Partitioning models
The. EPA's EPI Suite (US EPA. 2010. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.00. United States Environmental Protection Agency, Washington, DC, accessed April 2010) was used here to predict the subcooled liquid vapour pressure pL*, the octanol–water partitioning constant KOW, air–water partitioning constant KAW (i.e. dimensionless Henry's Law constant), and atmospheric half-lives. EPI Suite estimates these parameters from molecular structure by using calibrated quantitative-structure–activity relationships (QSARs).ACD/log P (included in the freeware version of ACD/ChemSketch, v12.01, http://www.acdlabs.com/download/, accessed April 2010) determines KOW values using a QSAR approach similar to EPI Suite, though calibrated somewhat differently.
The SPARC online calculator (v4.5, http://sparc.chem.uga.edu/sparc/, accessed April 2010) determines sorbate–sorbent interactions using various empirical molecular descriptors for the sorbate and sorbent,19 and can be used to predict pL*, water solubility as well as partitioning between water, air and any combination of organic phases.
COSMOtherm (version C2.1 release 01.08 in combination with Turbomole 5.10) is a commercially available software that performs solvation calculations using statistical thermodynamic approaches based on density functional quantum chemical calculations.20 Like SPARC, it can be used to predict partitioning constants for organic molecules between almost any organic sorbent–solvent pair, as well as pL* and water solubility.
Results and discussion
Presence in suspected Norwegian source zones
As is evident in Table 2, HBB was the most dominant analyte detected, being found in all other media. All analytes could be found in the seepage water and sediments from the metal recycling facility, which was the only location in which PBEB was detected. PBT was additionally found in sediment and seepage effluents from the landfill at Lindum and occasionally in WWTP and sediment samples.
HBB was not detected in marine biota analysed in this study. As mentioned in the Introduction, previous studies reported HBB in birds and bird eggs.4,6 Its presence in sediment samples but not in marine biota from the same water body may be attributable to strong sorption to sediments and solid phases, low uptake efficiencies, or rapid metabolisation rates. Sorption will be discussed in more detail below. Low uptake rates were observed for HBB in Zebrafish,22 and reported metabolization rates for HBB were found to be quicker than for hexachlorobenzenes in rats (phase 1)23 as well as in aerobic and anaerobic soil.24 Based on this, HBBs (and also PBT and PBEB) are expected to exhibit less bioaccumulation potential than their chlorinated equivalents.
The only known study reporting PBEB in sediments is for the Llobregat River basin, Spain,21 where levels ranged from not detectable to up to 3.1–9.6 ng g−1 dw. This is substantially higher than near the metal recycling facility (0.028 ng g−1 dw). PBEB has been reported in air samples in Chicago 2003, with concentrations reported at 520 pg m−3 in the gas phase and 29 pg m−3 in the particle phase11 (the same study also indicated that PBEB was not present in nearby sediment cores from Lake Michigan). From the time series on PBEB in lake trout captured in 1979–2004 as mentioned earlier,13 levels of 17–320 ng g−1lipid were reported for whole fish samples, possibly indicating that PBEB exposure to fish in the Great Lakes is higher than in Norwegian waters. Whole fish samples are not directly comparable to fish liver samples, especially for compounds exhibiting low uptake rates, as whole fish samples include particles in the digestive tract and exterior lipid phase; whereas, fish liver samples target the fraction of compounds that enter the cardiovascular system.
PBT was quantified in the effluents of the metal recycling factory and landfill in Drammen, and was detected in one sediment sample from Sagnessundet, Tromsø. The highest quantified sediment concentration, from the landfill, was 0.22 ng g−1 dw. The only other known study to measure PBT in sediments was for the Elbe river in Germany,25 in which the three samples, where PBT was quantifiable (2–25 ng g−1 dw), exhibited higher concentrations than the landfill effluent sediment. Table 2 presents the first known occurrence of PBT in WWTPs since a study on WWTPs in Sweden from 1975.12 However, the concentrations reported here, consisting of just trace amounts in the wastewater (0.09–0.17 ng L−1), indicate far less abundance than the 1975 study, which reported levels of 8–180 µg g−1 in sludge. The non-detection of PBT in marine organisms compared to the surrounding environment may be due to similar reasons as described above for HBB.
An additional explanation for the presence of these compounds in the studied source zones, other than use as BFR additives, is the transformation of larger BFR precursors, such as DBDPE producing PBT or PBEB, or polymeric BFR leaking HBB.7 Regarding the presence of HBB in pine needles and moss close to Hurum incineration plant, it should be noted that HBB is a known thermal reaction product of PBDEs.26 Though transformation of larger BFRs may be a contributor to measured levels, its total contribution cannot be quantified. Modelling results based on the aforementioned study on HBB in air in Toronto also indicated that leakage of directly added HBB better accounted for observed atmospheric levels than release from polymer BFRs.7
As is evident from the data in Table 2, HBB concentrations in the input and output water of WWTP are not significantly different and seem unaffected by the treatment processes used. The three WWTPs use different cleaning technologies. Drammen uses grid, sand and fat trap, flocculation and then sedimentation followed by sludge removal by centrifugation. Lillehammer uses grid and sand trap, primary, secondary and final sedimentation, gravitation thickening of sludge, drainer and centrifugation. Tromsø uses four Maskozoll coarse screens with 1 mm openings and then 2 Hydrotech screens with 0.12 mm openings. Estimated partitioning constants (see below) indicate that binding of HBB to organic matter should be quite substantial, which would indicate that flocculation, sedimentation, filtration and centrifugation should be good techniques to remove HBB. One hypothesis we present to account for why these techniques were ineffective is that HBB may be associated with small, low density particles, such as low-density microplastic particles, which are insusceptible to the applied treatements.
The levels of HBB in atmospheric particle samples in Drammen are the highest atmospheric concentrations yet reported for this compound. This, along with the presence of HBB in most other samples, warrants follow-up studies that account for the long range transport of this compound from Norwegian source zones. Particularly recommended are sediment core studies to see if levels have changed over time, and particle analysis of WWTP effluent waters (e.g. the presence of microplastics). PBT and PBEB were generally found in trace quantities, especially compared to reported data elsewhere. However, out of diligence, we recommend that PBT and PBEB are monitored along with HBB, especially considering that these three compounds can be readily analysed together using the presented analytical methods.
Environmental partitioning properties
| CAS-# | log pL* (Pa) | Lit.27 | Lit.28 | COSMOtherm | SPARC | EPI Suite | Max–Minb | |
|---|---|---|---|---|---|---|---|---|
| 106-37-6 | 1,4-DBB | 1.4 | 1.4 | 1.4 | 1.5 | 0.1 | ||
| 615-54-3 | 1,2,4-TriBB | 0.0 | −0.1 | −0.2 | 0.0 | 0.2 | ||
| 636-28-2 | 1,2,4,5-TetraBB | 0.1 | −1.3 | −1.5 | −0.7 | 0.8 | ||
| 87-82-1 | HBB | −3.5 | −3.1 | −3.1 | −4.7 | −2.2 | 2.4 | |
| 87-83-2 | PBT | −7.4 | −9.0 | −6.8 | 2.2 | |||
| 85-22-3 | PBEB | −7.6 | −9.2 | −7.1 | 2.2 |
| CAS-# | log KAW (–) | COSMOtherm | SPARC | EPI Suite bondb | EPI Suite groupb | |||
|---|---|---|---|---|---|---|---|---|
| 106-37-6 | 1,4-DBB | −1.4 | −1.7 | −1.6 | −1.5 | −1.4 | 0.3 | |
| 615-54-3 | 1,2,4-TriBB | −1.9 | −1.9 | −2.0 | −1.9 | −1.8 | 0.2 | |
| 636-28-2 | 1,2,4,5-TetraBB | −0.8 | −2.0 | −2.3 | −2.8 | −2.2 | 0.8 | |
| 87-82-1 | HBB | −2.4 | −4.2 | −2.2 | −2.8 | −3.1 | −2.9 | 0.8 |
| 87-83-2 | PBT | −2.0 | −2.8 | −2.6 | −2.5 | 0.8 | ||
| 85-22-3 | PBEB | −1.9 | −2.8 | −2.5 | −2.3 | 0.9 |
| CAS-# | log KOW (–) | COSMOtherm | SPARC | EPI Suite | ACD | |||
|---|---|---|---|---|---|---|---|---|
| a The difference between the largest estimated value and the smallest estimated value. b EPI Suite uses two different QSARS for predicting log KAW: the bond contribution approach and the group contribution approach, see EPI Suite documentation (http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm). | ||||||||
| 106-37-6 | 1,4-DBB | 3.6 | 3.3 | 3.4 | 3.8 | 3.8 | 0.5 | |
| 615-54-3 | 1,2,4-TriBB | 4.3 | 4.0 | 4.2 | 4.7 | 4.4 | 0.7 | |
| 636-28-2 | 1,2,4,5-TetraBB | 5.0 | 4.6 | 5.0 | 5.6 | 5.0 | 0.9 | |
| 87-82-1 | HBB | 6.1 | 5.6 | 6.8 | 7.3 | 5.9 | 1.8 | |
| 87-83-2 | PBT | 5.5 | 6.4 | 7.0 | 5.9 | 1.5 | ||
| 85-22-3 | PBEB | 5.9 | 6.8 | 7.5 | 6.4 | 1.6 |
For estimations of log pL*, agreement within 0.2 log units between all models and literature values resulted for 1,4-DBB and 1,2,4-TriBB. For other compounds, model predictions deviate by 0.8 to 2.4 log units from each other, with SPARC predicting the highest values, EPI Suite the lowest and COSMOtherm in between. The COSMOtherm prediction for HBB is in good agreement with the two literature values (deviating by <0.3 log units), whereas SPARC and EPI Suite deviate from the literature value by Kuramochi et al.27 by −1.2 and +1.2 log units, respectively. The experimental pL* value for 1,2,4,5-tetraBB is suspect as it is smaller than 1,2,4-TriBB and thus does not fit with the general trend that pL* decreases with increasing bromination (a general QSAR assumption), and it was considered dubious by the authors reporting the value.27 COSMOtherm is thus recommended as the best approach to estimate pL* values for these compounds, and is considered to be accurate within 0.5 log units of actual values.
Deviations in model predictions with log KAW are generally analogous to pL*. Model estimations and literature values are in agreement for 1,4-DBB and 1,2,4-TriBB within 0.3 log units. The literature 1,2,4,5-TetraBB value is suspect, because it was derived with the aforementioned pL* value. COSMOtherm agrees with the Karamochi et al.27 value of HBB within 0.3 log units, though not with the value of Tittlemier et al.,28 which is not favoured here as it is off by two orders of magnitude from the more consistent value by Karamuchi et al.27SPARC and EPI Suite predictions give decent agreement throughout, generally less than one order of magnitude from each other and Karamuchi et al.27 It appears that biases observed in the estimation of pL* of SPARC and EPI Suite are compensated by opposite and nearly equal biases in the subcooled solubility estimations (not shown). Overall, COSMOtherm is recommended as the best predictor of log KAW approach for these compounds, and is expected to be accurate within 0.5 log units.
For log KOW, COSMOtherm, SPARC and ACD/Labs predictions were in good agreement with literature values, with largest deviations being for HBB of 0.5, 0.7 and 0.2 log units, respectively. EPI Suite predicted a value that deviated by 1.2 log units. Though ACD/Labs predictions agree with experimental values (within 0.2 log units) better than COSMOtherm predictions (0.5 log units), COSMOtherm predicted log KOW values are favoured when used in combination with the other COSMOtherm predicted partitioning constants.
COSMOtherm and SPARC have recently been validated for the direct prediction of typical sorption coefficients over an ambient temperature range to several environmental phases, including terrestrial aerosols,29 humic acids30 and impacted sediments.31 In Table 4, outputs of these methods for HBB, PBT and PBEB are presented for the aerosol–air partition constant, Kp, humic-acid total organic carbon (TOC)–air partition constant, KHA-TOC,air, and the anthropogenic impacted sediment TOC–porewater partition constant, KCSed-TOC,water. Model predictions were similar for KHA-TOC,air, for both models (within 0.5 log units), though for Kp and KCSed-TOC,water, SPARC predicted values were higher by an order of magnitude. Based on the better performance of COSMOtherm for predicting the pure phase partitioning constants in Table 3, COSMOtherm predictions in Table 4 are favoured.
| Aerosol/air29 | Humic acid/air30 | Impacted sediment/water31 | |||||
|---|---|---|---|---|---|---|---|
| log Kp/mair3 g−1PM10 | log KHA-TOC,air/Lair kgTOC−1 | log KCSed-TOC,water/Lwater kgTOC−1 | |||||
| 25 °C | 15 °C | 25 °C | 15 °C | 25 °C | 15 °C | ||
| a Modelling to aerosols and humic acid is done using surrogate structures to represent the sorbing phase, in which the sorbing phases is assumed to make up 10% of the PM10,29 100% of humic-acid TOC.30 Estimations of partitioning to impacted sediments is done based on a validated Raoult's law model for PAHs and chlorinated aromatic hydrocarbons:31 log KCSed-TOC,water/Lwater kgTOC−1 = −log CLsat − log 0.248, where CLsat is the estimated subcooled liquid solubility. | |||||||
| SPARC | |||||||
| 87-82-1 | HBB | 2.3 | 2.8 | 8.5 | 8.9 | 8.8 | 9.4 |
| 87-83-2 | PBT | 2.0 | 2.5 | 8.2 | 8.6 | 8.1 | 8.6 |
| 85-22-3 | PBEB | 2.3 | 2.8 | 8.3 | 8.7 | 8.5 | 9.0 |
| COSMOtherm | |||||||
| 87-82-1 | HBB | 1.8 | 2.0 | 8.5 | 8.7 | 7.9 | 8.1 |
| 87-83-2 | PBT | 1.3 | 1.4 | 7.9 | 8.1 | 7.3 | 7.5 |
| 85-22-3 | PBEB | 1.5 | 1.6 | 8.0 | 8.2 | 7.7 | 7.9 |
For aerosol–air partitioning, the predicted log KP (mair3 g−1PM10) values ranged from 1.4–2.8 across the various compounds, models and temperature range. This range of KP values can be related to the expected fraction of the compound sorbed to particles in a parcel of air (φip) using eqn (1):
| φip = Kp × PM10/(1 + Kp × PM10) | (1) |
From eqn (1), a compound with a log Kp of 2 would have a φip value of 1% in an extremely smoggy atmosphere (PM10 = 100 µg m−3). Thus, in the atmosphere, only a small amount of HBB, PBEB and PBT is expected to be particle bound, with the majority in the gas phase. This accounts for the separate gas and particle analysis of PBEB in Chicago mentioned earlier,11 in which gas concentrations (520 pg m−3) were approximately a factor of 20 higher than particle-bound concentrations (29 pg m−3). This would imply that the concentrations of HBB measured in the vapour phase in Drammen are substantially higher than the measured particle concentrations. Predicted atmospheric half-lives using EPI Suite are 934, 58 and 9.3 days for HBB, PBT and PBEB, respectively. Based on these half-lives, the estimated Kp and also the log KAW of −2 (which indicates a low affinity for atmospheric water droplets), HBB, PBT and PBEB are all expected to be readily disseminated in the atmosphere as a vapour, particularly HBB. Thus, further monitoring studies that separate gas and particle phases are recommended. Note that for separating gas and particle phase concentrations, compounds like these BFRs are prone to particle filter adsorption artifacts,32 and this may cause a positive bias in resulting particle concentrations (such as that presented in Table 2). Further, polyurethane filters commonly used to collect vapour phase concentrations may contain these BFRs, which should be checked during method development.
For humic acids and impacted sediments, these compounds are predicted to be readily affiliated with TOC phases, as indicated by their very high KHA-TOC,air values (>8.0) and log KCSed-TOC,water values (>7.3). Note that a log KTOC value of 8 here implies that 1 g of soil/sediment TOC at equilibrium would have the same mass of contaminant as 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 litres of surrounding water. Thus, measured total water, sludge, sediment and soil samples in Table 2 likely represent the particle-bound phase. If HBB, PBT and PBEB are associated with microplastic residues in addition, this would increase the fraction of the measured concentrations associated with particles. Generally, the stronger the association with the particle phase, the lower the bioavailability.
000 litres of surrounding water. Thus, measured total water, sludge, sediment and soil samples in Table 2 likely represent the particle-bound phase. If HBB, PBT and PBEB are associated with microplastic residues in addition, this would increase the fraction of the measured concentrations associated with particles. Generally, the stronger the association with the particle phase, the lower the bioavailability.
Conclusions and recommended follow-up studies
Of the three BFRs screened for, HBB was present at detectable levels in all non-biotic media and in most locations. The measured air concentrations along with the predicted and measured partitioning properties indicate that HBB exhibits potential to be distributed in the atmosphere. Further atmospheric monitoring of HBB is recommended, with separate sampling of gas and particle phases. Atmospheric monitoring of PBT and PBEB is also recommended, as these compounds have similar properties to HBB, and may be in greater use in areas outside of Norway, as indicated by their increasing appearance in reports from other areas.These results alone cannot isolate whether HBB, PBEB and PBT are being reported in environmental samples in recent years due to an increase in production levels, transformation products or interest by researchers. More information on this could be obtained via dated sediment or ice core studies in areas where they are found at levels ≫ detection limits (e.g. Tromsø), or by collecting industrial reports on production levels.
Low levels of these compounds were observed in the monitored biota. This may be accountable to strong sorption to sediments, and also lower uptake rates and quicker metabolization rates than many chlorinated POPs. Nevertheless, monitoring in higher trophic levels is still warranted, as these compounds may still be prone to trophic biomagnification, and they were discovered in several bird species in other studies.
An overlooked aspect worthy of follow-up studies is the contribution of microplastic particles on BFR aerosol, water, soil, sediment and biota concentrations, as BFRs are added to many commercial plastics, and the environmental impact of BFR residuals in microplastic particles is largely unknown. We hypothesize that wastewater concentrations of HBB not diminishing by the investigated WWTP treatments is due to their presence in low density microplastic particles.
Acknowledgements
Thanks are extended to the Norwegian Climate and Pollution Agency (KLIF) for funding this work (from the “Statlig program for forurensningsovervåkning” program) and to Bård Nordbø, Ingunn Correll Myhre and Jon L. Fuglestad (KLIF) for continuous feedback. Kai-Uwe Goss (UFZ Leipzig) performed COSMOtherm calculations and provided critical comments. Several colleagues assisted with logistics, sampling and quality control, including Tormod Glette, Amund Ulfsnes, Anders Bergsli, Christian Volan, Marte Braathen, Gjermund Gravir and Sam Arne Nøland (DNV), Roman Grabic (Umeå), Arne Pettersen (NGI), Marco Skibnes and Venzi (Molab AS). We are also grateful for permission and assistances of site managers at Lillehammer RA, Solumstrand RA, Langnes RA, Hurum Energigjenvinning KS, Hellik Teigen AS and Lindum Ressurs og Gjenvinning AS with sampling.References
- M. Alaee, P. Arias, A. Sjodin and A. Bergman, Environ. Int., 2003, 29, 683–689 CrossRef CAS.
- U. Fink, F. Hajduk and Y. Ishikawa, Flame Retardants, SRI Consulting, 2005 Search PubMed.
- UNEP, Stockholm Convention on Persistent Organic Pollutants, 2009.
- L. T. Gauthier, C. E. Hebert, D. V. C. Weseloh and R. J. Letcher, Environ. Sci. Technol., 2007, 41, 4561–4567 CrossRef CAS.
- L. T. Gauthier, D. Potter, C. E. Hebert and R. J. Letcher, Environ. Sci. Technol., 2009, 43, 312–317 CrossRef CAS.
- J. Verreault, W. A. Gebbink, L. T. Gauthier, G. W. Gabrielsen and R. J. Letcher, Environ. Sci. Technol., 2007, 41, 4925–4931 CrossRef CAS.
- B. Gouteux, M. Alaee, S. A. Mabury, G. Pacepavicius and D. C. G. Muir, Environ. Sci. Technol., 2008, 42, 9039–9044 CrossRef CAS.
- L. Y. Zhu, B. L. Ma and R. A. Hites, Environ. Sci. Technol., 2009, 43, 6963–6968 CrossRef CAS.
- I. Watanabe, T. Kashimoto and R. Tatsukawa, Bull. Environ. Contam. Toxicol., 1986, 36, 778–784 CrossRef CAS.
- Y. Yamaguchi, M. Kawano, R. Tatsukawa and S. Moriwaki, Chemosphere, 1988, 17, 703–707 CrossRef CAS.
- E. Hoh, L. Y. Zhu and R. A. Hites, Environ. Sci. Technol., 2005, 39, 2472–2477 CrossRef CAS.
- P. E. Mattsson, A. Norstrom and C. Rappe, J. Chromatogr., 1975, 111, 209–213 CrossRef CAS.
- N. Ismail, S. B. Gewurtz, K. Pleskach, D. M. Whittle, P. A. Helm, C. H. Marvin and G. T. Tomy, Environ. Toxicol. Chem., 2009, 28, 910–920 CrossRef CAS.
- C. E. Mendoza, B. T. Collins, J. B. Shields and G. W. Laver, J. Agric. Food Chem., 1978, 26, 941–945 CrossRef CAS.
- OECD, Selected Brominated Flame Retardants, Background and National Experience with Reducing Risk, Organisation for Economic Co-operation and Development, Paris, 1994 Search PubMed.
- WHO, Flame Retardants: a General Introduction Environmental Health Criteria 92, World Health Organisation, Geneva, 1997 Search PubMed.
- T. N. Brown and F. Wania, Environ. Sci. Technol., 2008, 42, 5202–5209 CrossRef CAS.
- P. H. Howard and D. C. G. Muir, Environ. Sci. Technol., 2010, 44, 2277–2285 CrossRef CAS.
- S. H. Hilal, S. W. Karickhoff and L. A. Carreira, QSAR Comb. Sci., 2004, 23, 709–720 CrossRef CAS.
- F. Eckert and A. Klamt, AIChE J., 2002, 48, 369 CrossRef CAS.
- P. Guerra, E. Eljarrat and D. Barcelo, J. Hydrol., 2010, 383, 39–43 CrossRef CAS.
- J. R. Nyholm, A. Norman, L. Norrgren, P. Haglund and P. L. Andersson, Environ. Toxicol. Chem., 2009, 28, 1035–1042 CrossRef CAS.
- Y. Yamaguchi, M. Kawano and R. Tatsukawa, Chemosphere, 1986, 15, 453–459 CrossRef CAS.
- J. R. Nyholm, C. Lundberg and P. L. Andersson, Environ. Pollut., 2010, 158, 2235–2240 CrossRef CAS.
- J. Schwarzbauer, M. Ricking, S. Franke and W. Francke, Environ. Sci. Technol., 2001, 35, 4015–4025 CrossRef CAS.
- H. R. Buser, Environ. Sci. Technol., 1986, 20, 404–408 CrossRef.
- H. Kuramochi, K. Maeda and K. Kawamoto, J. Chem. Eng. Data, 2004, 49, 720–724 CrossRef CAS.
- S. A. Tittlemier, T. Halldorson, G. A. Stern and G. T. Tomy, Environ. Toxicol. Chem., 2002, 21, 1804–1810 CrossRef CAS.
- H. P. H. Arp and K. U. Goss, Environ. Sci. Technol., 2009, 43, 1923–1929 CrossRef CAS.
- C. Niederer and K. U. Goss, Environ. Sci. Technol., 2007, 41, 3646–3652 CrossRef CAS.
- H. P. H. Arp, G. D. Breedveld and G. Cornelissen, Environ. Sci. Technol., 2009, 43, 5576–5585 CrossRef CAS.
- H. P. H. Arp, R. P. Schwarzenbach and K. U. Goss, Atmos. Environ., 2007, 41, 8241–8252 CrossRef CAS.
Footnote |
| † Published as part of a special issue dedicated to Emerging Investigators. |
| This journal is © The Royal Society of Chemistry 2011 |
