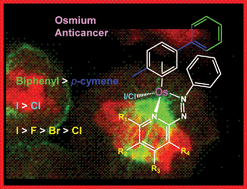
We report the synthesis and characterisation of 32 half sandwich phenylazopyridine OsII arene complexes [Os(η6-arene)(phenylazopyridine)X]+ in which X is chloride or iodide, the arene is p-cymene or biphenyl and the pyridine and phenyl rings contain a variety of substituents (F, Cl, Br, I, CF3, OH or NO2). Ten X-ray crystal structures have been determined. Cytotoxicity towards A2780 human ovarian cancer cells ranges from high potency at nanomolar concentrations to inactivity. In general the introduction of an electron-withdrawing group (e.g. F, Cl, Br or I) at specific positions on the pyridine ring significantly increases cytotoxic activity and aqueous solubility. Changing the arene from p-cymene to biphenyl and the monodentate ligand X from chloride to iodide also increases the activity significantly. Activation by hydrolysis and DNA binding appears not to be the major mechanism of action since both the highly active complex [Os(η6-bip)(2-F-azpy)I]PF6 (9) and the moderately active complex [Os(η6-bip)(3-Cl-azpy)I]PF6 (23) are very stable and inert towards aquation. Studies of octanol–water partition coefficients (log P) and subcellular distributions of osmium in A2780 human ovarian cancer cells suggested that cell uptake and targeting to cellular organelles play important roles in determining activity. Although complex 9 induced the production of reactive oxygen species (ROS) in A2780 cells, the ROS level did not appear to play a role in the mechanism of anticancer activity. This class of organometallic osmium complexes has new and unusual features worthy of further exploration for the design of novel anticancer drugs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...