A series of zinc complexes of the general formula {[ZnCl(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr)]+}2[Zn2Cl6]2− (where Ar = 2-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl 2a, 2-(1-(1-phenylethyl)-1H-1,2,3-triazol-4-yl)phenyl 2b, 2-(1-phenyl-1H-1,2,3-triazol-4-yl)phenyl 2c; An = acenaphthene backbone) were prepared by the condensation of acenaphthenequinone with the corresponding o-triazolyl-substituted anilines (2-(1-benzyl-1H-1,2,3-triazol-4-yl)aniline 1a, 2-(1-(1-phenylethyl)-1H-1,2,3-triazol-4-yl)aniline 1b, 2-(1-phenyl-1H-1,2,3-triazol-4-yl)aniline 1c) which were formed by the copper(I)-catalyzed Huisgen[3+2] dipolar cycloaddition between 2-ethynylaniline and the corresponding azides in high yields, using anhydrous ZnCl2 as the metal template, in boiling glacial acetic acid. Zinc complexes of the type [ZnCl(ArN
NAr)]+}2[Zn2Cl6]2− (where Ar = 2-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl 2a, 2-(1-(1-phenylethyl)-1H-1,2,3-triazol-4-yl)phenyl 2b, 2-(1-phenyl-1H-1,2,3-triazol-4-yl)phenyl 2c; An = acenaphthene backbone) were prepared by the condensation of acenaphthenequinone with the corresponding o-triazolyl-substituted anilines (2-(1-benzyl-1H-1,2,3-triazol-4-yl)aniline 1a, 2-(1-(1-phenylethyl)-1H-1,2,3-triazol-4-yl)aniline 1b, 2-(1-phenyl-1H-1,2,3-triazol-4-yl)aniline 1c) which were formed by the copper(I)-catalyzed Huisgen[3+2] dipolar cycloaddition between 2-ethynylaniline and the corresponding azides in high yields, using anhydrous ZnCl2 as the metal template, in boiling glacial acetic acid. Zinc complexes of the type [ZnCl(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr)]+[ZnCl3(NCCH3)]− (4a–c) were synthesized by crystallisation of the corresponding complexes 2a–c in acetonitrile, at −20 °C. After removal of zinc dichloride from complexes 2a–c by the addition of potassium oxalate, in dichloromethane, the tetradentate N,N,N,N-chelating α-diimine ligands of the type ArN
NAr)]+[ZnCl3(NCCH3)]− (4a–c) were synthesized by crystallisation of the corresponding complexes 2a–c in acetonitrile, at −20 °C. After removal of zinc dichloride from complexes 2a–c by the addition of potassium oxalate, in dichloromethane, the tetradentate N,N,N,N-chelating α-diimine ligands of the type ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr (5a–c) were obtained. The new ligand precursors and zinc complexes were characterised by elemental analysis, 1H and 13C{1H} NMR spectroscopy, two-dimensional NMR spectroscopy, and X-ray diffraction. Reaction of the ligand precursors 5a–c with [NiBr2(DME)], in dichloromethane, gave nickel complexes of the type [NiBr2(ArN
NAr (5a–c) were obtained. The new ligand precursors and zinc complexes were characterised by elemental analysis, 1H and 13C{1H} NMR spectroscopy, two-dimensional NMR spectroscopy, and X-ray diffraction. Reaction of the ligand precursors 5a–c with [NiBr2(DME)], in dichloromethane, gave nickel complexes of the type [NiBr2(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr)] (6a–c). The results of single crystal X-ray diffraction characterisation and magnetic susceptibility measurements demonstrated that nickel complexes 6a–c possess octahedral geometries around the nickel atoms with variable configurations, the Br atoms of which can be ionized when dissolved in methanol. In preliminary catalytic tests, complexes 6a–c revealed to be active as catalysts for the polymerisation of norbornene and styrene, when activated by cocatalyst MAO. The characterisation of the polymers by 1H and 13C{1H} NMR spectroscopy, gel permeation chromatography/size-exclusion chromatography (GPC/SEC) revealed that these polymers were formed by a coordination addition mechanism.
NAr)] (6a–c). The results of single crystal X-ray diffraction characterisation and magnetic susceptibility measurements demonstrated that nickel complexes 6a–c possess octahedral geometries around the nickel atoms with variable configurations, the Br atoms of which can be ionized when dissolved in methanol. In preliminary catalytic tests, complexes 6a–c revealed to be active as catalysts for the polymerisation of norbornene and styrene, when activated by cocatalyst MAO. The characterisation of the polymers by 1H and 13C{1H} NMR spectroscopy, gel permeation chromatography/size-exclusion chromatography (GPC/SEC) revealed that these polymers were formed by a coordination addition mechanism.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr)]+}2[Zn2Cl6]2− (where Ar = 2-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl 2a, 2-(1-(1-phenylethyl)-1H-1,2,3-triazol-4-yl)phenyl 2b, 2-(1-phenyl-1H-1,2,3-triazol-4-yl)phenyl 2c; An = acenaphthene backbone) were prepared by the condensation of
NAr)]+}2[Zn2Cl6]2− (where Ar = 2-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl 2a, 2-(1-(1-phenylethyl)-1H-1,2,3-triazol-4-yl)phenyl 2b, 2-(1-phenyl-1H-1,2,3-triazol-4-yl)phenyl 2c; An = acenaphthene backbone) were prepared by the condensation of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr)]+[ZnCl3(NCCH3)]− (4a–c) were synthesized by
NAr)]+[ZnCl3(NCCH3)]− (4a–c) were synthesized by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr (5a–c) were obtained. The new
NAr (5a–c) were obtained. The new ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(An)–C(An)
C(An)–C(An)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr)] (6a–c). The results of single crystal X-ray diffraction characterisation and magnetic susceptibility measurements demonstrated that nickel complexes 6a–c possess octahedral geometries around the nickel atoms with variable configurations, the Br atoms of which can be ionized when dissolved in
NAr)] (6a–c). The results of single crystal X-ray diffraction characterisation and magnetic susceptibility measurements demonstrated that nickel complexes 6a–c possess octahedral geometries around the nickel atoms with variable configurations, the Br atoms of which can be ionized when dissolved in 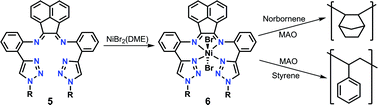

 Please wait while we load your content...
Please wait while we load your content...