Effects of distortion of PO4 tetrahedron on the photocatalytic performances of BiPO4†
Chengsi
Pan
a,
Di
Li
a,
Xinguo
Ma
a,
Yi
Chen
b and
Yongfa
Zhu
*a
aDepartment of Chemistry, Tsinghua University, Beijing, 100084, P.R. China. E-mail: zhuyf@mail.tsinghua.edu.cn; Fax: +86-10-62787601; Tel: +86-10-62783586
bResearch Center of Nano-Science and Nano-Technology, Shanghai University, Shanghai 200444, PR China
First published on 6th September 2011
Abstract
Three kinds of crystal phase BiPO4 (HBIP, nMBIP and mMBIP) were selectively synthesized by a hydrothermal method. The governed factors for the formation of three crystal phase of BiPO4 were both on the acidity of the solution and reaction temperature. The BiPO4 with nMBIP phase structure showed higher activity for degradation of MB solution under UV irradiation than HBIP and mMBIP. The catalytic activity per surface area activity of nMBIP (3.8×105 h−1 m−2) was about ten times higher than that of P25(1.4×104 h−1 m−2). The BiPO4 with nMBIP structure exhibited the highest activity due to the most distorted PO4 tetrahedron. The induce effect of the dipole moment derived from the distorted PO4 tetrahedron promoting the separation of e−/h+ pairs and further benefited for the photocatalytic reaction. This correlation may help to design and develop other oxoacid salt photocatalysts with high activity.
1. Introduction
Photocatalysis attracts much attention these days due to the potential application of removing pollutants in water and air by the direct absorption of light. This property has been discovered mainly on metal oxide and composite metal oxide semiconductors, such as the most widely used TiO2.1 Nevertheless, the photocatalytic activity of TiO2 is not high enough to be used for industrial purposes, because of the limitation of the absorption edge and the recombination of photogenerated charges.2 Therefore, many efforts have been made to develop new semiconductor photocatalysts in order to overcome the drawbacks of TiO2 and achieve the maximum activity.Oxoacid salts like Ti3(PO4)4,3 Cu2(OH)PO4,4 Bi2SiO5,5 Ag3PO4,6,7 Bi2O2CO38 are a new kind of photocatalysts that exhibit high photocatalytic activities, due to their unique structure, such as highly crystalline, good stability, easily combining with H2O, and the high negative energy of anions to promote electron and hole separation.9 Although much attention has been paid to this new kind of photocatalysts, only a few studies on the relationships between the structure and photocatalytic activity have been made. Lee et al.4 has reported that the variety of lattice parameters influenced the band gap of Cu2(OH)PO4 and further their activities. Ye et al.6,7 reported the narrow band gap together with the (110) surface of Ag3PO4 was in response to its high activity. Besides the band gap and surface, photocatalytic activity may first be determined by the crystal phase of the photocatalysts. The crystal phase may influence the band gap, the separation of photogenerated electron-holes and the positions of the valence and conduction bands in metal oxide and composite metal oxide photocatalysts. For example, for TiO2, it was recently discovered that brookite had markedly high photocatalytic activity for H2 production as compared to those of rutile and anatase due to a high conductive band.10,11 Also, monoclinic BiVO4 has an obviously higher activity than the trigonal one due to the higher efficiency for the separation of photogenerated electron-holes derived from its distortion of V–O and Bi–O polyhedrons.12,13 However, the relationship between the phase structure and photocatalytic properties in oxoacid salt photocatalysts has not been fully studied.
Recently, our group found that monoclinic BiPO4 (space group: P21/n), as one of oxoacid salt photocatalysts, had a superior photocatalytic performance to P25 (TiO2) but its surface area is only one-sixteenth.14 It was noted that BiPO4 had three main crystal phases: hexagon BiPO4 (space group: P3121, HBIP), monoclinic BiPO4 (space group: P21/n, nMBIP), and monoclinic BiPO4 (space group: P21/m, mMBIP). So, it is important to evaluate the photocatalytic activity in other BiPO4 crystal phases (HBIP and mMBIP) with the same oxoacid salt structure and make further understanding on relationship between this unique structure and photocatalytic activity. This requires a controlled synthesis of three crystal phases of BiPO4 in the analogous condition. Up to now, the crystal phase with a hexagon structure (HBIP) could be easily obtained by a direct deposition method15 or electrochemical deposition16 at room temperature in aqueous system. But the monoclinic structure (nMBIP and mMBIP) needed high temperature solid state reaction.15,17,18 The controlled preparation method of three phases of BiPO4 especially for mMBIP by an aqueous process has not been developed. On the other hand, it is difficult to compare the properties among the different crystal phases of BiPO4 obtained by various methods because the chemical history of the preparation determine the properties.19
In this paper, crystalline HBIP, nMBIP and mMBIP powders were selectively synthesized in aqueous media by a hydrothermal reaction. The correlation between the photocatalytic activity and PO4 tetrahedral distortion was demonstrated for the three BiPO4 crystal phases. This correlation may help to the synthesis of other non-metal oxoacid salt photocatalysts with high activity.
2. Experimental section
2.1 Synthesis
Three crystal phases of BiPO4 powders were synthesized through a hydrothermal process. All chemicals used were analytic grade reagents without further purification. 3 mmol Bi(NO3)3·6H2O and 30 mL, a given concentration of H3PO4 were put into a beaker and then magnetically stirred to form a homogeneous solution at room temperature. The resulting precursor suspension was transferred into a Teflon-lined stainless steel autoclave. The autoclave was sealed and maintained at the given temperature for 72 h, without shaking or stirring, then allowed to cool naturally to room temperature. The products were filtered off, washed several times with distilled water, and dried at 80 °C for 24 h, subsequently.2.2 Characterization
The products were characterized by powder X-ray diffraction (XRD) on Bruker D8-advance X-ray diffractometer at 40 kV and 40 mA for monochromatized Cu Kα (λ = 1.5406 Å) radiation. Morphologies and structures of the prepared samples were further examined with JSM 6301 electron scanning microscope (SEM) and transmission electron microscopy (TEM) by a JEM 1010 electron microscope operated at an accelerating voltage of 100 kV. UV–vis diffuse reflectance spectra (DRS) of the samples were measured by using Hitachi U-3010 UV–vis spectrophotometer. The Brunauer–Emmett–Teller (BET) specific surface area of the samples was characterized by nitrogen adsorption at 77 K with Micromeritics 3020. PL spectra were obtained using an Edinburgh Analytical Instruments FL/FSTCSPC920 coupled with a timecorrelated single-photo counting system.2.3 Photocatalytic evaluation
Photocatalytic activities of BiPO4 were evaluated by degradation of methylene blue (MB) under ultraviolet light irradiation of 11W low-pressure lamp with 254 nm, respectively. The average light intensity was 1.5 m W cm−2. The radiant flux was measured with a power meter from Institute of Electric Light Source (Beijing). MB solutions (200 ml, 10−5 mol L−1) containing 0.100 g of BiPO4 were put in a glass beaker. Before the light was turned on, the solution was first ultrasonicated for 10 min, and then stirred for 10 min to ensure equilibrium between the catalysts. Three millilitres of sample solution were taken at given time intervals and separated through centrifugation (4000 rpm, 10 min). The supernatants were analyzed by recording variations of the absorption band maximum (664 nm) in the UV–vis spectra of MB using a U-3010 spectrophotometer (Hitachi).Photoelectrochemical measurements were carried out in a conventional three-electrode, single-compartment glass cell, fitted with a synthesized quartz window, using a potentiostat. The quartz electrolytic cell was filled with 0.1 M Na2SO4. The ITO/BiPO4 electrodes served as the working electrode. The counter and the reference electrodes were platinum black wire and saturated calomel electrode (SCE), respectively. An 11 W germicidal lamp were used as the excitation light source for ultraviolet irradiation.
3. Results and discussion
3.1 Formation and transformation of HBIP, nMBIP and mMBIP
The three crystal phases of BiPO4 could be controlled synthesis through hydrothermal reactions. By studying these reactions in the system Bi3+-H3PO4-H2O, the formation of different BiPO4 crystal phases was found depending both on the hydrothermal temperature and phosphorus-containing reagents in the reaction mixture and on the acidity of the solution. Regulation of the acidity of the reaction system was achieved by adding H3PO4. Fig. 1 shows the ranges of concentration used for the preparation of individual crystalline phases. At lower H3PO4 concentration and temperature, the formation of HBIP as the sole product takes place. The increasing of temperature to the reaction system favors the synthesis of nMBIP. The compound mMBIP as a pure phase is formed when the acidity of the solution and temperature is higher. It also indicated that the formation of mMBIP and nMBIP costs lower temperature with the increasing of the acidity of the solution. As previous study,14,16 all the three structures had an analogous structure but a little difference due to the symmetries and the choice of coordinate systems, as shown in Supporting Information Figure S1.† Therefore, the transformation between the three BiPO4 crystal phases only requires a small rotation of the tetrahedron to adopt appropriate symmetric arrangement, with no change of the topological features. | ||
| Fig. 1 Phase diagram for various BiPO4 crystal phases of (○) HBIP, (△) nMBIP, (□) mMBIP. | ||
More detailed information on the phase purity and crystal structure of the products synthesized at 12 M H3PO4 obtained by XRD measurement is shown in Fig. 2. In this concentration, the transformation of BiPO4 from hexagon to monoclinic occurs with the temperature increasing. All the diffraction peaks of HBIP can be indexed to the pure hexagonal phase of BiPO4 (JPCDS 45–1370) with lattice constants a = 6.986, b = 6.986 and c = 6.475 Å. While at higher temperature HBIP is converted into monoclinic phase: nMBIP (below 160 °C) and then mMBIP (above 160 °C). nMBIP is consistent with JPCDS 80–0209 with lattice constants a = 6.762, b = 6.951 and c = 6.482 Å. While the lattice constants of mMBIP(JPCDS 43–0637) are a = 4.882, b = 7.068 and c = 4.704 Å. During the transformation, the peak at 14.6°, 20.1°, 29.5° belongs to (100), (101), (200) of HBIP is disappeared while the peak at 17.0°, 21.3°, 34.4° belongs to (−101), (−111), (−202) of nMBIP emerged. Finally, at above 160 °C, the crystal phase is completely transformed into mMBIP and the peak at 18.3°, 22.8°, 37.0° attributed to the (100), (011), and (200) surfaces can be recognized as the unique peak of mMBIP.
 | ||
| Fig. 2 X-ray-diffraction patterns of BiPO4 crystal phases obtained in 12 M H3PO4 for 72 h at various hydrothermal temperature: at 20 °C for HBIP; at 40 °C for HBIP and nMBIP; at 100 °C for nMBIP; at 160 °C for nMBIP and mMBIP; and at 200 °C for mMBIP. | ||
The size and morphology of the products were examined by SEM and TEM. Fig. 3 shows that the obtained HBIP and nMBIP samples exhibit 1D structure but with large difference in size, while the mMBIP has a lamellar structure. The as-prepared nMBIP rods are several micrometres long and about 1 μm wide. On the other hand, HBIP rods are 100–400 nm in length and about 50 nm in width. The mMBIP plates are larger than 10 μm2 in size and about 1 μm in thickness. It is well-known that when the particle size is smaller than 100 nm, the nano-size effect mainly influences the photocatalytic activity. In our case, the large size of the three BiPO4 structures ensures that the photocatalytic activities of three phases are mainly influenced by their crystal structures, not by the nano-size effect.
 | ||
| Fig. 3 SEM (a, c, e) and TEM (b, d, f) images of BiPO4 crystal phases obtained in 12 M H3PO4 for 72 h at various hydrothermal temperature: (a, b) HBIP at 20 °C, (c, d) nMBIP at 100 °C, (e, f) mMBIP at 200 °C. | ||
3.2 Photo absorption properties and photocatalytic performance of HBIP, nMBIP, and mMBIP
Diffuse reflectance spectra of three BiPO4 crystal phases synthesized at 12 M H3PO4 are shown in Fig. 4. According to the plots, the absorption edges of HBIP, nMBIP, and mMBIP occur at about 360nm, 322 nm, and 296nm, respectively, due to the excitation of electrons from valence gap to conductive gap. The inset profiles show that the plots of absorption1/2 (or absorption2]) versus energy, which may helps to distinguish the direct or indirect transition of BiPO4 crystal phases. In semiconductors, the square of absorption coefficient is linear with energy for direct optical transitions in the absorption edge region; whereas the square root of absorption coefficient is linear with energy for indirect transitions.20 According to the plots inset Fig. 4, the monoclinic BiPO4 is indirect, while the hexagon BiPO4 is direct. The band-gap energies are estimated to be 4.6, 3.8 eV, and 4.2 eV for HBIP, nMBIP, and mMBIP, respectively. The difference may be due to their electronic structural difference limited by the crystal phase, as also reported in brookite and anatase TiO211 | ||
| Fig. 4 UV-DRS patterns of various BiPO4 crystal phases obtained in 12 M H3PO4 for 72 h at different hydrothermal temperatures: (a) HBIP at 20 °C, (b) nMBIP at 100 °C, (c) mMBIP at 200 °C. | ||
Photocatalytic activities for MB degradation catalyzed by HBIP, nMBIP, and mMBIP are shown in Fig. 5 and Table 1. Although the absorption bands, pore volumes and average pore diameters of HBIP, nMBIP, and mMBIP are almost the same, nMBIP shows much higher photocatalytic activity than the other two. The degradation of MB without catalysts can be ignored, and thus the activities for three crystal phases decrease in the following order: nMBIP > mMBIP > HBIP. According to the BET surface area listed in Table 1, the catalytic activity per surface area of HBIP, nMBIP, and mMBIP can be calculated as 6.1 × 102 h−1m−2, 3.8 × 105 h−1m−2, 3.0 × 104 h−1 m−2. Among the three crystal phases the the catalytic activity per surface area of nMBIP is about ten times higher than that of P25 (1.4 × 104 h−1 m−2)14 but competing to the nanosized BiPO4 (3.9 × 105 h−1 m−2) as we reported before.14 The apparent quantum efficiency was also calculated according to ref. 1, that is, 0.06%, 0.54% and 0.15% for HBIP, nMBIP and mMBIP, respectively. This is consistent with the order of photocatalytic activity. A similar photodegrading behavior was also found in the methyl orange (MO) degrading as shown in Supporting Information Figure S2.† The MO is known as a kind of cation dye molecular(Figure S2 inset), while MB is regarded as an anion dye(Figure 5 inset). Furthermore, the degradation activity of colorless 4-chlorophenol (4-CP) on three BiPO4 crystal phases has been given in Supporting Information Figure S3, which shows the same order.† As a result, the activity differences for three BiPO4 crystal phase are supposed not due to the effect of dye adsorption, but due to the change of crystal phase structures.
 | ||
| Fig. 5 Photocatalytic degradation curves of MB for various BiPO4 crystal phases obtained in 12 M H3PO4 for 72 h at different hydrothermal temperatures: (a) HBIP at 20 °C, (b) nMBIP at 100 °C, (c) mMBIP at 200 °C. Photocatalyst, 0.5 g L−1; MB concentration, 10−5 mol/L. Inset is the structure of MB. | ||
| Sample | kMB/h−1 | BET surface area/m2 g−1 | Pore Volume/cm3 g−1 | Average Pore Diameter/nm |
|---|---|---|---|---|
| HBIP | 0.05 | 0.82 | 0.008 | 5.6 |
| nMBIP | 1.13 | 0.03 | 0.002 | 4.7 |
| mMBIP | 0.18 | 0.06 | 0.003 | 8.7 |
To further confirm this conclusion, the transient photocurrent responses were measured via three on-off cycles of irradiation as shown in Fig. 6. As previous studies, the photocatalytic reactions could be regarded as an electrochemical process and thus, photocurrent was regarded as equivalent to photocatalytic activity.21,22 Furthermore, this magnitude of the photocurrent directly reflected the number of photogenerated electrons and holes but with less regards to the degrading substrate difference. From Figure 6 a prompt generation of photocurrents is observed and with good reproducibility when samples are irradiated by UV light. This indicates that our electrode is stable and photoresponsive phenomenon is entirely reversible. The photocurrent of HBIP, nMBIP, and mMBIP is 0.6, 2.1, and 0.2 μA, respectively. This order is consistent with their photocatalytic activities. Therefore, the decrease in photocatalytic activity is mainly due to the concentration reduction in photogenerated carriers, which is probably caused by e−/h+ recombination in three BiPO4 crystal phases discussed in the next paragraph.
 | ||
| Fig. 6 Transient photocurrent responses for various crystal phases BiPO4 electrodes. Electrolyte: 0.1 M Na2SO4. | ||
3.3 The influence of the crystal phases on the photocatalytic activity
The photocatalytic activity is governed by various factors such as surface area, photo absorption, the oxidation potential of photogenerated holes, and the separation efficiency of photoinduced electrons and holes. In our case, it is noted that activity decreasing order is just opposite to BET surface area of the three structures, while the photo absorption is all around 300 nm, as shown above. Therefore, it may be due to other structure factors that influence the photocatalytic activity, such as the oxidation potential of photogenerated holes, and the transfer and separation of the photogenerated electron-hole pairs.In general, the high potential of photogenerated holes in the valence band benefits for the production of active ·OH radicals, as reported in In(OH)3 (Evb = 4.2eV)23 and CaSb2O5(OH)2 (Evb = 4.1eV).24 To indentify the influence of the oxidation potential of holes, the position of the valence band is estimated by the position of the conductive band and the band gap. The former one can be reflected by the flat band in n-typed semiconductors.25,26 As shown in Fig. 7, the flat band potential for HBIP, nMBIP, mMBIP, is −0.60 V, −0.70 V, −0.68 V vs. SCE, respectively. In addition, the differences (ΔE = ECB − Efb) between the bottom of conduction band and the flat band potential for three BiPO4 crystal phases were assumed to be −0.3 V due to the similar composition and insulating n-type semiconducting properties.26,27 As a result, the potential of conductive bands of HBIP, nMBIP and mMBIP is the −0.9 V, −1.0 V, −0.98 V vs. SCE, respectively. Combining with the band gap, the oxidation potential in the valence band for HBIP, nMBIP and mMBIP is estimated to be 0.37 V, 0. 28 V, 0.32 V vs. SCE. It can be seen that the HBIP may have the highest the oxidation potential of the holes in the valence band, while nMBIP has lowest oxidation potential. However, the photocatalytic activity of HBIP is much less than the nMBIP as discussed above. This implies that the photocatalytic activity difference among HBIP, nMBIP, and mMBIP is not due to the oxidation potential of photogenerated holes.
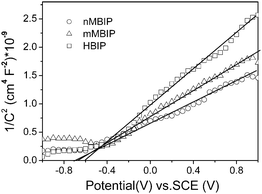 | ||
| Fig. 7 Mott-Schottky plots for various BiPO4 crystal phases obtained in 12 M H3PO4 for 72 h at different hydrothermal temperatures: (a) HBIP at 20 °C, (b) nMBIP at 100 °C, (c) mMBIP at 200 °C. Counter electrode: Pt. Electrolyte: 0.1 M Na2SO4. Frequency: 1 kHz. | ||
On the other hand, the electrochemical impedance spectroscopy was performed in order to evaluate the differences of transfer and separation of the photogenerated electron-hole pairs between the photocatalysts. (Fig. 8) The diameters of the EIS Nyquist plots under UV illumination are supposed to indicate the charge separation and transfer process in the electrode-electrolyte interface region.28–30 Under illumination, the diameters tend to decrease from HBIP to mMBIP, and nMBIP, significantly. This indicates that the activity difference in HBIP, mMBIP, nMBIP is due to variation of the charge transfer and the recombination of e−/h+ pairs. The recombination of e−/h+ pairs is also characterized by the lifetime of the carriers. It can be reflected by the decays of PL transition centered on 550 nm excited at 254 nm as shown in Fig. 9. Moreover, the PL lifetime of three samples calculated by the exponential analysis was also shown in Fig. 9 inset. The PL lifetime decreases in the following order: nMBIP > mMBIP > HBIP. A longer PL lifetime means lower recombination rate of the electron–hole pairs, and thus higher photocatalytic activity. This order is quit consistent with EIS results.
 | ||
| Fig. 8 EIS spectra for various BiPO4 crystal phases obtained in 12 M H3PO4 for 72 h at different hydrothermal temperatures: (a) HBIP at 20 °C, (b) nMBIP at 100 °C, (c) mMBIP at 200 °C. | ||
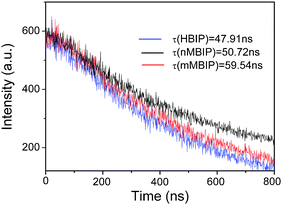 | ||
| Fig. 9 PL decay curves measured at λex = 254 nm and λem = 550 nm for various BiPO4 crystal phases. Inset is the lifetime of carriers for three BiPO4 crystal phases. | ||
In a word, the nMBIP phase favors the generation and separation of the photo-excited electron-hole pairs and thus enhances the photocatalytic activity.
3.4 Relationship between structure and photocatalytic activity
According to the above discussion, the photocatalytic activity difference between three BiPO4 structures is mainly due to the difference of their separation efficiency of the photogenerated electron-hole pairs. This difference in reactivity among the BiPO4 polymorphs might be related to their geometric and electronic structures.Using the crystallographic data regarding the atom positions, three structures of BiPO4 are shown in Fig. 10. The data of the crystal is obtained from ref. 17 (HBIP and nMBIP) and ICSD No. 060522 (mMBIP), and further optimized by the CASTEP. In these calculations, the energy cutoff was chosen at 380 eV.31,32 In all three crystals, one bismuth atom is surrounded by eight oxygen atoms and one phosphorus atom is surrounded by four oxygen atoms. However, the distortion of Bi–O polyhedron and P–O tetrahedron are quit different due to the limitation by the symmetry of the crystal phase. The Bi–O and P–O bond length in nMBIP are dispersed mostly broadly while the slightest distortion occurs in HBIP. As discussed before,14 the high photocatalytic activity of BiPO4 was mainly derived from the induced effect of phosphate, compared with the Bi2O3 structure.33 The PO43− was assumed to motivate the separation of photogenerated electron-hole pairs and then improve the photocatalytic activity of the catalysts. It was also reported that not only the PO43− itself but also the dipole moment derived from the distorted PO4 tetrahedron would yield the induce effect.34 Therefore, the distortion of it would change the distribution of the electronic cloud between P–O, which may further change the electron-hole separation.
 | ||
| Fig. 10 Crystal structures of (a) HBIP, (b) nMBIP, (c) mMBIP. | ||
To understand this feature deeply, we calculated the dipole moment of the three BiPO4 structures as shown in Fig. 11. The dipole moment of PO4 tetrahedron was calculated according to the center of gravity of oxygen ions in the tetrahedron. According to Fig. 11, the dipole moment in HBIP is just 0.05D, while in nMBIP it is 10.6D. At the same time the photocatalytic activities for the above two structures are 0.05 h−1 and 1.13 h−1, respectively. This obviously shows that BiPO4 with large dipole moments are photocatalytically active, whereas the slight distortion BiPO4 exhibited negligible activity, demonstrating that a correlation exists between the photocatalytic activity and the dipole moment. The one who has the most distortion P–O tetrahedron has the highest activity.
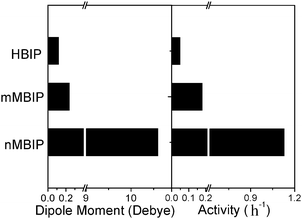 | ||
| Fig. 11 Correlation between dipole moments in various BiPO4 crystal phases and photocatalytic activities. | ||
In previous studies, a good correlation between the photocatalytic activity and the dipole moment has been demonstrated.35–37 It is known that the dipole moment induces the formation of local fields in the distorted polyhedron. The fields are considered to promote the separation of photogenerated e−/h+. Such as in a simple metal oxide Ga2O3, the distorted tetrahedral and octahedral in β-Ga2O3 with dipole moment showed high photocatalytic activity.35 While in composite metal oxides, it has been reported that the induce effect of alkaline earth metal will influence the dipole moment in distorted SbO6 polyhedron and further make the catalysts photocalytically active for water decomposition.37 It has also reported that the dipole moment in V–O tetrahedron in BiVO4 will facilitates the electron excitation.13 In our case, the Bi–O bonds contain not only valence bonds but also ionic bonds, like in BiVO4, so dipole moment in Bi–O polyhedron can't describe the influence of the electron separation exactly. On the contrary the dipole moment derived from the distorted PO4 tetrahedron will induce a dipole moment in Bi–O polyhedron, which is further useful for electron-hole separation. In a word, it should be noted that the correlation between the photocatalytic activity and PO4 tetrahedron distortion is true for both three BiPO4 crystal phases and other inorganic salt photocatalysts of non-metal oxoacid.
4. Conclusion
Crystalline HBIP, nMBIP and mMBIP powders in aqueous media by a hydrothermal reaction have been synthesized through the control of the acidity of the solution and temperature in the reaction. The nMBIP who has the most distortion PO4 tetrahedron exhibits the highest activity for degradation of MB solution among three structures. The correlation between the photocatalytic activity and P–O tetrahedron distortion is demonstrated for the three BiPO4 crystal phases. We also assume that this correlation may be useful in other oxoacid salt photocatalysts. In this regard, it is an important discovery for the design and synthesis of other oxoacid salt photocatalysts with a high activity.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (20925725 and 50972070) and National Basic Research Program of China (2007CB613303) and Key Subject of Shanghai Municipal Education Commission (J50102).Notes and references
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- M. P. Kapoor, S. Inagaki and H. Yoshida, J. Phys. Chem. B, 2005, 109, 9231 CrossRef CAS.
- I. Cho, D. W. Kim, S. Lee, C. H. Kwak, S. T. Bae, J. H. Noh, S. H. Yoon, S. H. Jung, D. W. Kim and K. S. Hong, Adv. Funct. Mater., 2008, 18, 2154 CrossRef CAS.
- R. Chen, J. Bi, L. Wu, W. Wang, Z. Li and X. Fu, Inorg. Chem., 2009, 48, 9072 CrossRef CAS.
- Z. Yi, J. Ye, N. Kikugawa, T. Kato, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559 CrossRef CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS.
- Y. Zheng, F. Duan, M. Chen and Y. Xie, J. Mol. Catal. A: Chem., 2010, 317, 34 CrossRef CAS.
- D. Zhao, C. Chen, Y. Wang, H. Ji, W. Ma, L. Zang and J. Zhao, J. Phys. Chem. C, 2008, 112, 5993 CAS.
- B. Zhao, F. Chen, Q. Huang and J. Zhang, Chem. Commun., 2009, 5115 RSC.
- T. A. Kandiel, A. Feldhoff, L. Robben, R. Dillert and D. W. Bahnemann, Chem. Mater., 2010, 22, 2050 CrossRef CAS.
- S. Tokunaga, H. Kato and K. A. Kudo, Chem. Mater., 2001, 13, 4624 CrossRef CAS.
- J. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163 CrossRef CAS.
- C. Pan and Y. Zhu, Environ. Sci. Technol., 2010, 44, 5570 CrossRef CAS.
- R. C. L. Mooney-Slater, Z. Kristallogr., 1962, 117, 371 CrossRef CAS.
- M. Yang, N. K. Shrestha, R. Hahn and P. Schmuki, Electrochem. Solid-State Lett., 2010, 13, 5 CrossRef.
- B. Romero, S. Bruque, M. A. G. Aranda and J. E. Iglesias, Inorg. Chem., 1994, 33, 1869 CrossRef CAS.
- K. V. Terebilenko, I. V. Zatovsky, N. S. Slobodyanik, V. N. Baumer and V. G. Zatovsky, Inorg. Mater., 2007, 43, 1336 CrossRef CAS.
- N. Serpone and E. Pelizzetti, Photocatalysis Fundamentals and Applications;John Wiley & Sons: New York, 1989 Search PubMed.
- S. C. Zhang, C. A. Zhang, Y. Man and Y. Zhu, J. Solid State Chem., 2006, 179, 62 CrossRef CAS.
- H. J. Yun, H. Lee, J. B. Joo, W. Kim and J. Yi, J. Phys. Chem. C, 2009, 113, 3050 CAS.
- J. Yu, G. Dai and B. Huang, J. Phys. Chem. C, 2009, 113, 16394 CAS.
- T. Yan, J. Long, X. Shi, D. Wang, Z. Li and X. Wang, Environ. Sci. Technol., 2010, 44, 1380 CrossRef CAS.
- M. Sun, D. Li, Y. Zheng, W. Zhang, Y. Shao, Y. Chen, W. Li and X. Fu, Environ. Sci. Technol., 2009, 43, 7877 CrossRef CAS.
- A. Ishikawa, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, J. Am. Chem. Soc., 2002, 124, 13547 CrossRef CAS.
- K. Ogisu, A. Ishikawa, Y. Shimodaira, T. Takata, H. Kobayashi and K. Domen, J. Phys. Chem. C, 2008, 112, 11978 CAS.
- D. E. Scaife, Sol. Energy, 1980, 25, 41 CrossRef CAS.
- H. Liu, Sh. Cheng, M. Wu, H. Wu, J. Zhang, W. Li and Ch. Cao, J. Phys. Chem. A, 2000, 104, 7016 CrossRef CAS.
- W. H. Leng, Z. Zhang, J. Q. Zhang and C. N. Cao, J. Phys. Chem. B, 2005, 109, 15008 CrossRef CAS.
- Z. Hosseini, N. Taghavinia, N. Sharifi, M. Chavoshi and M. Rahman, J. Phys. Chem. C, 2008, 112, 18686 CAS.
- L. Zhang, L. Wang and Y. Zhu, Adv. Funct. Mater., 2007, 17, 3781 CrossRef CAS.
- D. Y. He, L. J. Qiao, A. A. Volinsky, Y. Bai1, M. Wu and W. Y. Chu, Appl. Phys. Lett., 2011, 98, 062905 CrossRef.
- D. Music, S. Konstantinidis and J. M. Schneider, J. Phys.: Condens. Matter, 2009, 21, 175403 CrossRef 175403.1.
- K. Zaghiba and C. M. Julien, J. Power Sources, 2005, 142, 279 CrossRef.
- Y. Hou, L. Wu, X. Wang, Z. Ding, Z. Li and X. Fu, J. Catal., 2007, 250, 12 CrossRef CAS.
- J. Sato, S. Saito, H. Nishiyama and Y. Inoue, J. Photochem. Photobiol., A, 2002, 148, 85 CrossRef CAS.
- J. Sato, H. Kabayashi, K. Ikarashi, N. Saito, H. Nishiyama and Y. Inoue, J. Phys. Chem. B, 2004, 108, 4369 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures of crystals and catalysis data. See DOI: 10.1039/c1cy00261a |
| This journal is © The Royal Society of Chemistry 2011 |
