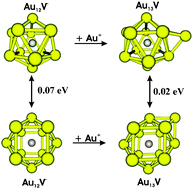
A systematic quantum chemical investigation on the electronic, geometric and energetic properties of AunV clusters with n = 1–14 in both neutral and anionic states is performed using BP86/cc-pVTZ-PP calculations. Most clusters having an even number of electrons prefer a high spin state. For odd-electron systems, a quartet state is consistently favoured as the ground state up to Au8V. The larger sized Au10V, Au12V and Au14V prefer a doublet state. The clusters prefer 2D geometries up to Au8V involving a weak charge transfer. The larger systems bear 3D conformations with a more effective electron transfer from Au to V. The lowest-energy structure of a size AunV is built upon the most stable form of Aun−1V. During the growth, V is endohedrally doped in order to maximize its coordination numbers and augment the charge transfer. Energetic properties, including the binding energies, embedding energies and second-order energy differences, show that the presence of a V atom enhances considerably the thermodynamic stability of odd-numbered gold clusters but reduces that of even-numbered systems. The atomic shape has an apparently more important effect on the clusters stability than the electronic structure. Especially, if both atomic shape and electronic condition are satisfied, the resulting cluster becomes particularly stable such as the anion Au12V−, which can thus combine with the cation Au+ to form a superatomic molecule of the type [Au12V]Au. Numerous lower-lying electronic states of these clusters are very close in energy, in such a way that DFT computations cannot clearly establish their ground electronic states. Calculated results demonstrate the existence of structural isomers with comparable energy content for several species including Au9V, Au10V, Au13V and Au14V.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...