Electronic state selectivity in dication-molecule single electron transfer reactions: NO2+ + NO†
Abstract
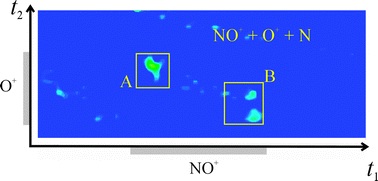
The single-electron transfer reaction between NO2+ and NO, which initially forms a pair of NO+ ions, has been studied using a position-sensitive coincidence technique. The reactivity in this class of collision system, which involves the interaction of a dication with its neutral precursor, provides a sensitive test of recent ideas concerning electronic state selectivity in dicationic single-electron transfer reactions. In stark contrast to the recently observed single-
- This article is part of the themed collection: Multiply charged ions (MCIs) in the gas-phase

 Please wait while we load your content...
Please wait while we load your content...