Zinc coverage dependent structure of PdZn surface alloy†
Xiang
He
ab,
Yucheng
Huang
a and
Zhao-Xu
Chen
*a
aInstitute of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Key Laboratory of Mesoscopic Chemistry of MOE, Nanjing University, China. E-mail: zxchen@nju.edu.cn
bEco-Materials and Renewable Energy Research Center, Department of Physics, National Laboratory of Solid State Microstructures, Nanjing University, China
First published on 16th November 2010
Abstract
Catalytic performances of alloy and surface alloy are sensitive to the surface structures and composition. In this paper we present an overall survey of the surface structure of Pd(111) covered with different amount of Zn using Monte Carlo simulations. We demonstrate that the composition of PdZn surface alloy is Zn coverage dependent: the surface concentration of Zn increases with the increase of the deposited Zn. At one or multi-layer of zinc deposited Pd(111), a multilayer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn surface alloy will be formed. Surface alloy islands dominated by palladium are formed at submonolayer Zn coverage. At very low zinc coverage, small palladium ensembles of 3 to 5 Pd atoms exist preferentially on the Pd(111) surface. Our simulated results which are consistent with the pertinent experiments indicate that the unusual high-temperature desorption peak of formaldehyde observed experimentally has likely originated from the small surface ensembles induced by deposited Zn.
1 PdZn surface alloy will be formed. Surface alloy islands dominated by palladium are formed at submonolayer Zn coverage. At very low zinc coverage, small palladium ensembles of 3 to 5 Pd atoms exist preferentially on the Pd(111) surface. Our simulated results which are consistent with the pertinent experiments indicate that the unusual high-temperature desorption peak of formaldehyde observed experimentally has likely originated from the small surface ensembles induced by deposited Zn.
Recently alloys and surface alloys have attracted much attention because of their superior catalytic properties over pure metals.1–3 The properties of alloys and surface alloys are closely related to the composition and structure. Hence understanding them is a prerequisite for elucidating their unique properties. PdZn alloy is widely accepted to be responsible for the nice selectivity and activity of Pd/ZnO catalyts for methanol steam reforming (MSR) which is an important means to produce hydrogenin situ for on-board fuel cells.4 It was believed that 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn alloy is one of the active components.5 However, Karim et al. showed that the selectivity to CO2 does not correlate with the extent of alloy formation and that catalysts with both high and low atomic ratio of Zn to Pd exhibit high selectivity.6 Jeroro and Vohs found that the proposed intermediate of MSR, formaldehyde, can be detected only when methanol is dosed onto Pd(111) on which 0.03 to 0.06 monolayer (ML) of Zn is deposited. On the Pd(111) surface deposited with larger than 0.5 ML Zn formaldehyde can hardly be observed. On the basis of the above findings the authors suspected that the 1
1 PdZn alloy is one of the active components.5 However, Karim et al. showed that the selectivity to CO2 does not correlate with the extent of alloy formation and that catalysts with both high and low atomic ratio of Zn to Pd exhibit high selectivity.6 Jeroro and Vohs found that the proposed intermediate of MSR, formaldehyde, can be detected only when methanol is dosed onto Pd(111) on which 0.03 to 0.06 monolayer (ML) of Zn is deposited. On the Pd(111) surface deposited with larger than 0.5 ML Zn formaldehyde can hardly be observed. On the basis of the above findings the authors suspected that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn alloy might have no activity for MSR.7 It is interesting that such a small amount of Zn can produce so remarkable an influence on the surface chemistry of Pd(111). Intuitively a homogenous distribution of Zn is unlikely. Some researchers believed that the deposited Zn is distributed on the outermost surface. But very recent studies demonstrate that the subsurface of PdZn alloy is responsible for the surface catalytic behaviour of Zn/Pd(111).8,9 Thus, a detailed and comprehensive investigation of surface structure of Zn deposited Pd surfaces is needed. On the other hand, experimental techniques can hardly provide sufficient information for Pd surface deposited with very small amount of Zn. It is these Pd surfaces deposited with much less Zn that are believed to be active for the MSR reaction and a high-temperature desorption peak of formaldehyde is recorded on them. In principle, various surface structures might produce a high-temperature desorption of CH2O. For example, we found that the binding energy of CH2O on PdZn pair supported on Pd(111) is as high as 1.0 eV. Then the following questions arise: is the formation of the PdZn pair favourable? Are there other possibilities? Furthermore, what is the surface structure of Pd(111) deposited with different amounts of Zn, especially when the amount of Zn is very low? To answer these questions, we appeal to theoretical modeling. Here we report Monte Carlo simulations of deposition of Zn on a Pd(111) surface.†
1 PdZn alloy might have no activity for MSR.7 It is interesting that such a small amount of Zn can produce so remarkable an influence on the surface chemistry of Pd(111). Intuitively a homogenous distribution of Zn is unlikely. Some researchers believed that the deposited Zn is distributed on the outermost surface. But very recent studies demonstrate that the subsurface of PdZn alloy is responsible for the surface catalytic behaviour of Zn/Pd(111).8,9 Thus, a detailed and comprehensive investigation of surface structure of Zn deposited Pd surfaces is needed. On the other hand, experimental techniques can hardly provide sufficient information for Pd surface deposited with very small amount of Zn. It is these Pd surfaces deposited with much less Zn that are believed to be active for the MSR reaction and a high-temperature desorption peak of formaldehyde is recorded on them. In principle, various surface structures might produce a high-temperature desorption of CH2O. For example, we found that the binding energy of CH2O on PdZn pair supported on Pd(111) is as high as 1.0 eV. Then the following questions arise: is the formation of the PdZn pair favourable? Are there other possibilities? Furthermore, what is the surface structure of Pd(111) deposited with different amounts of Zn, especially when the amount of Zn is very low? To answer these questions, we appeal to theoretical modeling. Here we report Monte Carlo simulations of deposition of Zn on a Pd(111) surface.†
We started from surfaces covered with 1 to 3 ML of Zn for which experimental results are available. Fig. 1 shows the simulated surface structures of Pd(111) covered with 1 ML Zn from 300 K to 500 K. Fig. 1(a) and (b) clearly show that both the original adlayer and top layer of the substrate become a well-defined PdZn alloy of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. This result agrees with the scanning tunneling microscopy (STM) results where two layers of surface alloy were observed after depositing Zn on the Pd(111) surface at 300 K.10,11 Simulations were also performed on the Pd(111) surface covered with 2 and 3 ML of Zn. In both cases, 1
1 stoichiometry. This result agrees with the scanning tunneling microscopy (STM) results where two layers of surface alloy were observed after depositing Zn on the Pd(111) surface at 300 K.10,11 Simulations were also performed on the Pd(111) surface covered with 2 and 3 ML of Zn. In both cases, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn surface alloy is obtained (see Fig. S1 in the ESI†). Previously it was argued that beyond 2 ML the deposited Zn grows in a layer by layer mode on the Pd(111) surface.10 However, more than four layers of 1
1 PdZn surface alloy is obtained (see Fig. S1 in the ESI†). Previously it was argued that beyond 2 ML the deposited Zn grows in a layer by layer mode on the Pd(111) surface.10 However, more than four layers of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn surface alloy were observed by depositing Zn on Pd(111),8 which is consistent with our results. Furthermore, it was also observed that at higher temperatures there are two or three peaks of CO polarization-modulated infrared reflection absorption spectroscopy (PM-IRAS) on PdZn 1
1 PdZn surface alloy were observed by depositing Zn on Pd(111),8 which is consistent with our results. Furthermore, it was also observed that at higher temperatures there are two or three peaks of CO polarization-modulated infrared reflection absorption spectroscopy (PM-IRAS) on PdZn 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 alloy surface whereas at lower temperatures only a single peak exists; it was suggested that this phenomenon is due to the corrugation of the surface layer.8 Our simulated surface structures at 400 K and 500 K, Fig. 1(c) and (d), indicate another explanation for this phenomenon. According to our simulation, with increase of the temperature, the surface alloy structure becomes more and more disordered. At 500 K, different types of adsorption sites appear, especially there are a lot of hollow sites consisting of three Pd atoms (as shown by the inset of Fig. 1(d)) whereas at a lower temperature of 300 K, only top and bridge sites comprising Pd atom(s) exist. (Though CO adsorption on Zn was detected on PdZn alloy, possibly due to CO adsorbing at step edges,12 at 100 K CO does not adsorb on pure metallic zinc13 and theoretical study shows that CO prefers Pd rather than Zn on the 1
1 alloy surface whereas at lower temperatures only a single peak exists; it was suggested that this phenomenon is due to the corrugation of the surface layer.8 Our simulated surface structures at 400 K and 500 K, Fig. 1(c) and (d), indicate another explanation for this phenomenon. According to our simulation, with increase of the temperature, the surface alloy structure becomes more and more disordered. At 500 K, different types of adsorption sites appear, especially there are a lot of hollow sites consisting of three Pd atoms (as shown by the inset of Fig. 1(d)) whereas at a lower temperature of 300 K, only top and bridge sites comprising Pd atom(s) exist. (Though CO adsorption on Zn was detected on PdZn alloy, possibly due to CO adsorbing at step edges,12 at 100 K CO does not adsorb on pure metallic zinc13 and theoretical study shows that CO prefers Pd rather than Zn on the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn alloy surface.14 Thus only sites with Pd atoms are considered.) Although the binding energy of CO on the bridge position is the same as that at the top site,14 the single peak at the low temperature originates from atop bonded CO on Pd atom.8 At higher temperatures more adsorption peaks were detected because there are more different adsorption sites for CO. It is worth mentioning that since the “newly appeared” sites on the higher temperature disordered surfaces are composed of more Pd atoms, these “new peaks” should show up at the lower frequencies because CO interacts more strongly with these sites containing more Pd atoms, which is just the experimentally observed result.
1 PdZn alloy surface.14 Thus only sites with Pd atoms are considered.) Although the binding energy of CO on the bridge position is the same as that at the top site,14 the single peak at the low temperature originates from atop bonded CO on Pd atom.8 At higher temperatures more adsorption peaks were detected because there are more different adsorption sites for CO. It is worth mentioning that since the “newly appeared” sites on the higher temperature disordered surfaces are composed of more Pd atoms, these “new peaks” should show up at the lower frequencies because CO interacts more strongly with these sites containing more Pd atoms, which is just the experimentally observed result.
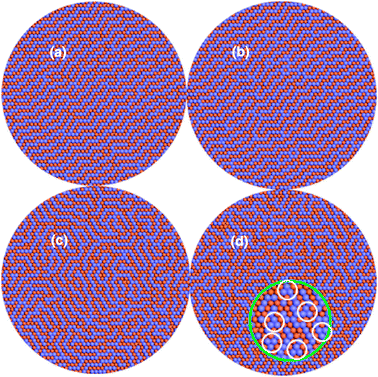 | ||
| Fig. 1 Simulated PdZn alloy patterns of 1 ML Zn coverage. (a) The adlayer at 300 K, (b) the top layer of the substrate at 300 K, (c) the adlayer at 400 K, (d) the adlayer at 500 K. Inset: The three-fold hollow sites of Pd are circled. The light blue and orange balls denote the Pd and Zn atoms respectively. | ||
The above results show that when the Pd(111) surface is fully covered with Zn, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn surface alloy will be formed at certain temperatures. We are more interested in the surface structures of Pd(111) partially covered with Zn for which only a little experimental information is available and it is practically impossible to locate the most favourable configurations from first principles calculations since the possible surface configurations are numerous. Fig. 2 shows the composition, Pd and Zn coverage of the resulting adlayer, as a function of the total amount (in terms of coverage) of deposited Zn simulated at 300 K. It can be seen that when the deposited Zn is less than 1 ML, most of the Zn atoms are exchanged with the substrate Pd atoms, leading to an adlayer dominated by Pd. With the increase of the deposited Zn, the percentage of Zn in the adlayer increases. For example, at the total coverage of 0.12 ML only 25% of the deposited Zn atoms is retained in the adlayer whereas 40% of the deposited Zn atoms remains in the adlayer when totally 0.5 ML Zn is deposited on Pd(111). Our simulations reveal that at submonolayer coverage of Zn, surface islands are formed, which is consistent with the formation of the surface PdZn alloy bilayer islands detected in the STM experiment upon deposition of 0.5 ML Zn on the Pd(111) at 300 K.10 Furthermore, after annealing to 500 K (which should accelerate the swapping process), the bilayer islands decompose and more Pd atoms segregate on the adlayer.10 The above experimental finding is also in accordance with our simulations (the inset in Fig. 2), which features the long-time behaviours which show that the composition of the adlayer is dominated by Pd atoms.
1 PdZn surface alloy will be formed at certain temperatures. We are more interested in the surface structures of Pd(111) partially covered with Zn for which only a little experimental information is available and it is practically impossible to locate the most favourable configurations from first principles calculations since the possible surface configurations are numerous. Fig. 2 shows the composition, Pd and Zn coverage of the resulting adlayer, as a function of the total amount (in terms of coverage) of deposited Zn simulated at 300 K. It can be seen that when the deposited Zn is less than 1 ML, most of the Zn atoms are exchanged with the substrate Pd atoms, leading to an adlayer dominated by Pd. With the increase of the deposited Zn, the percentage of Zn in the adlayer increases. For example, at the total coverage of 0.12 ML only 25% of the deposited Zn atoms is retained in the adlayer whereas 40% of the deposited Zn atoms remains in the adlayer when totally 0.5 ML Zn is deposited on Pd(111). Our simulations reveal that at submonolayer coverage of Zn, surface islands are formed, which is consistent with the formation of the surface PdZn alloy bilayer islands detected in the STM experiment upon deposition of 0.5 ML Zn on the Pd(111) at 300 K.10 Furthermore, after annealing to 500 K (which should accelerate the swapping process), the bilayer islands decompose and more Pd atoms segregate on the adlayer.10 The above experimental finding is also in accordance with our simulations (the inset in Fig. 2), which features the long-time behaviours which show that the composition of the adlayer is dominated by Pd atoms.
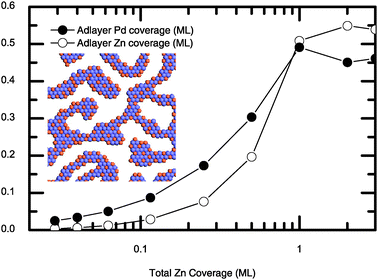 | ||
| Fig. 2 The coverage and the residual proportion of the adlayer Znversus the total Zn coverage at 300 K. The inset shows the adlayer at the coverage of 0.5 ML. The light blue and orange balls denote the Pd and Zn atoms respectively. | ||
Having analyzed the composition of the adlayer at submonolayer Zn coverage, we further identified the island size. Fig. 3 displays the percentage of small islands which contain 3–5 atoms as a function of the total coverage of Zn. It clearly shows that with the increase of Zn coverage, the percentage of the small islands decreases. When the total amount of deposited Zn is larger than 0.25 ML, the small islands can hardly be formed. On the other hand, when the total coverage of Zn is less than 0.05 ML the small islands dominate. The LEED (low energy electron diffraction) patterns distinct from the substrate could not be obtained for Zn coverage less than 0.25 ML, making the surface structure for lower Zn coverage unknown.15 Our simulation reveals that at a Zn coverage as low as 0.04 ML, small islands (dominated by about 85% Pd atoms) are scattered on the surface. In other words, by modifying the Pd(111) surface with very small amount of Zn the adlayer is mainly composed of Pd3, Pd4 and Pd5 ensembles. It should be pointed out that neither monomers nor paired atoms were observed in our simulations.
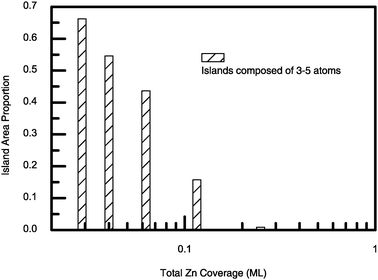 | ||
| Fig. 3 The island area proportion versus the total Zn coverage. The area proportion is defined as the ratio of the total area of the islands to the total Zn coverage (in terms of area). | ||
As mentioned before, dosing methanol onto Zn deposited Pd(111) substrate produces a temperature programmed desorption (TPD) peak of formaldehyde at 360 K when the Zn coverage is about 0.05 ML.7 However, the surface structure causing this desorption peak is unclear. A binding energy of 1.0 eV is estimated for this desorption peak using Redhead formula.16 Normally the binding energies of CH2O on Pd(111) and PdZn alloy surface are less than 0.5 eV.9,17 According to our simulation, at about 0.05 ML Zn coverage, the Pd(111) surface is most likely populated with Pd trimers, tetramers and pentamers. Therefore, the 360 K peak is most likely derived from adsorption of CH2O on these small ensembles. To verify this, we calculated the binding energy of CH2O on a Pd3 island at Pd(111) surface and found the binding energy to be 0.96 eV which is in good agreement with the estimated binding energy for the 360 K TPD peak. To take the influence of zinc on CH2O adsorption into account, a zinc atom is introduced to the bottom of the Pd3 island because we have shown that Zn atoms beneath the Pd atoms bound to the adsorbate exhibit a more significant effect.9 The binding energy of CH2O on such model is calculated to be 1.06 eV which is also close to the observed 360 K TPD peak (Fig. S2 in the ESI† provides the stable adsorption geometry). It needs to be noted that strong adsorbate–substrate interaction might induce surface segregation. Hence small ensembles composed of Zn atoms might be formed on the Pd(111) surface upon adsorption of species like methoxy.
In summary, we presented an overall survey of surface structure of Zn deposited Pd(111) using Monte Carlo simulations. We demonstrated that when Pd(111) surface is covered by 1–3 ML zinc, a multilayer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PdZn surface alloy will be formed. Furthermore, with a temperature increase, the PdZn surface alloy becomes less ordered. For submonolayer Zn coverage, the surface alloy islands will be formed which are dominated by palladium. At very low zinc coverage, small palladium ensembles of 3 to 5 atoms exist preferentially on the Pd(111) surface. These results indicate that the composition and structure of PdZn surface alloy are sensitive to the coverage of zinc. Our simulated results are not only consistent with the pertinent experiments, but also suggest that the high-temperature desorption peak of formaldehyde observed in the experiments may be derived from small ensembles on the surface. All these results will be informative for exploring the MSR reaction mechanism, and understanding surface alloys in particular.
1 PdZn surface alloy will be formed. Furthermore, with a temperature increase, the PdZn surface alloy becomes less ordered. For submonolayer Zn coverage, the surface alloy islands will be formed which are dominated by palladium. At very low zinc coverage, small palladium ensembles of 3 to 5 atoms exist preferentially on the Pd(111) surface. These results indicate that the composition and structure of PdZn surface alloy are sensitive to the coverage of zinc. Our simulated results are not only consistent with the pertinent experiments, but also suggest that the high-temperature desorption peak of formaldehyde observed in the experiments may be derived from small ensembles on the surface. All these results will be informative for exploring the MSR reaction mechanism, and understanding surface alloys in particular.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 20973090, 973 Program 2009CB623504).References
- F. Besenbacher, I. Chorkendorff, B. S. Clausen, B. Hammer, A. M. Molenbroek, J. K. Nørskov and I. Stensgaard, Science, 1998, 279, 1913–1915 CrossRef CAS.
- J. Greeley and M. Mavrikakis, Nat. Mater., 2004, 3, 810–815 CrossRef CAS.
- S. Kandoi, P. Ferrin and M. Mavrikakis, Top. Catal., 2010, 53, 384–392 CrossRef CAS.
- J. D. Holladay, Y. Wang and E. Jones, Chem. Rev., 2004, 104, 4767–4790 CrossRef CAS.
- N. Iwasa, T. Mayanagi, S. Masuda and N. Takezawa, React. Kinet. Catal. Lett., 2000, 69, 355–360 CrossRef CAS.
- A. Karim, T. Conant and A. Datye, J. Catal., 2006, 243, 420–427 CrossRef CAS.
- E. Jeroro and J. M. Vohs, J. Am. Chem. Soc., 2008, 130, 10199–10207 CrossRef CAS.
- C. Rameshan, W. Stadlmayr, C. Weilach, S. Penner, H. Lorenz, M. Hävecker, R. Blume, T. Rocha, D. Teschner, A. Knop-Gericke, R. Schlögl, N. Memmel, D. Zemlyanov, G. Rupprechter and B. Klötzer, Angew. Chem., Int. Ed., 2010, 49, 3224–3227 CrossRef CAS.
- Y. Huang and Z. Chen, Langmuir, 2010, 26, 10796–10802 CrossRef CAS.
- G. Weirum, M. Kratzer, H. P. Koch, A. Tamtö, J. Killmann, I. Bako, A. Winkler, S. Surnev, F. P. Netzer and R. Schennach, J. Phys. Chem. C, 2009, 113, 9788–9796 CrossRef CAS.
- H. Koch, I. Bako, G. Weirum, M. Kratzer and R. Schennach, Surf. Sci., 2010, 604, 926–931 CrossRef CAS.
- A. Tamtögl, M. Kratzer, J. Killman and A. Winkler, J. Chem. Phys., 2008, 129, 224706 CrossRef.
- J. A. Rodriguez, J. Phys. Chem., 1994, 98, 5758–5764 CrossRef CAS.
- Z. Chen, K. M. Neyman, K. H. Lim and N. Rösch, Langmuir, 2004, 20, 8068–8077 CrossRef CAS.
- E. Jeroro, V. Lebarbier, A. Datye, Y. Wang and J. Vohs, Surf. Sci., 2007, 601, 5546–5554 CrossRef CAS.
- P. Redhead, Vacuum, 1962, 12, 203–211 CrossRef CAS.
- Z. Chen, K. Lim, K. Neyman and N. Rösch, J. Phys. Chem. B, 2005, 109, 4568–4574 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional simulation results, details of used Monte Carlo method and simulation parameters. See DOI: 10.1039/c0cp01344g |
| This journal is © the Owner Societies 2011 |
