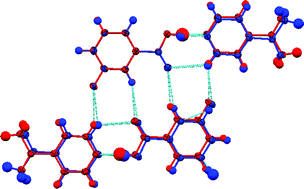
Two polymorphs of the molecular complex formed between 3-fluorobenzoic acid with 4-acetylpyridine are described and found to be based upon the same dimeric supramolecular construct. The conformational freedom around the hydrogen bond results in a 180° rotation about this intermolecular link, distinguishing the polymorphs and affecting the packing of the dimeric units. The two polymorphs are fully characterised by single crystal X-ray and neutron diffraction and quantum mechanical calculations. There is evidence of structured crystal growth defects in both polymorphic crystals via observation of diffuse scattering and a disorder model for the average structure of Form I, which can be interpreted as a mixing of the two dimer conformations. The similarity of energy of the distinct dimeric units, supporting their likely co-existence, has been verified by periodic quantum chemical calculations.