Science as art: self-assembly of hybrid SiO2-coated nanocrystals†
Ping
Yang
*,
Zhimin
Yuan
,
Jie
Yang
,
Aiyu
Zhang
,
Yongqiang
Cao
,
Qinghui
Jiang
,
Ruixia
Shi
,
Futian
Liu
and
Xin
Cheng
School of Materials Science and Engineering, University of Jinan, 250022, Jinan, P.R. China. E-mail: mse_yangp@ujn.edu.cn; Fax: +86-531-87974453; Tel: +86-531-89736225
First published on 28th October 2010
Abstract
Hybrid SiO2-coated CdTe nanocrystals (NCs) show a drastic increase in fluorescence quantum yield with a significant red-shifted photoluminescence (PL) peak because of the hybrid shell containing CdS-like clusters which are very close to the CdTe core. With their hybrid SiO2 shell, CdTe NCs reveal self-assembly activity which creates one-dimensional nanostructured materials (fibers) with bright PL. Additionally, we experimentally observed the self-assembly of the hybrid SiO2-coated CdTe NCs into two-dimensional dendritic morphology and three-dimensional crystals through a droplet dewetting technique on a hydrophilic glass surface by using NaCl molecules as scaffolds. This phenomenon is ascribed to the domain growth of NaCl to form fractal structures through tip splitting and side branching dynamics. This is also due to a hydrodynamic mechanism through outward capiliary flow. The evaporation speed of solvent during droplet dewetting plays an important role in controlling the self-assembly of the hybrid SiO2-coated CdTe NCs. The experimental parameters such as the amount of sample on the hydrophilic glass surface and dewetting time are key for getting assemblies with tunable morphologies. The present strategy provides a new approach to study the self-assembly of a variety of NCs. This has a potential application for pattern manufacture in a natural way.
Introduction
Semiconductor nanocrystals (NCs) show strongly size-, shape-, and composition-dependent photonic and electronic properties. Novel materials based on semiconductor NCs are currently one of the most attractive research fields. An important research topic in emitting semiconductor NCs is expansion from single-component to hybrid nanostructures for extending the functionality of NCs. Novel assembly techniques are required for advanced materials with enough structural flexibility to be tuned for specific applications, and to be practical, the techniques must be implemented at relatively low cost.1 Various assembly approaches have been employed to assemble NCs in an ordered manner and to investigate the scope of potential applications. For example, it is well known that the 1–2 dimensional nanostructure of NCs can be produced on a two-phase interface (such as gas–solid, liquid–solid, gas–liquid, and liquid–liquid) where the interface acts as a template. These flexible freestanding nanostructures provide additional accessibility; they are open to multidimensional interactions, can change their shape and dimensions, are air/fluid permeable, and can be highly responsive to various external stimuli.1 Layer-by-layer (LbL) assembly is a simple, versatile, and significantly inexpensive approach. This makes LbL self-assembled methods particularly promising for optoelectronic applications.2 As nanotechnology develops, materials scientists have also been gradually shifting their past focus on the synthesis of phase-pure building block materials of low-dimensional nanostructured inorganic materials to currently creating and constructing functionally well-defined ordered superstructures or complex architectures.3 Nanocomposites of different groups can be combined to coat both macroscopically flat and non-planar (e.g. colloidal particle) surfaces.4The self-assembly of nanomaterials has attracted a great deal of attention by bridging different fields of science and engineering for the design and development of outstanding materials, methodologies, and theories. The interactions between NCs in general are complex and diverse, which offer tremendous opportunity for the design of NC assemblies with varying morphologies, structures, and functions.5 The self-assembly of NCs on the liquid–liquid or air–water interfaces has been investigated. The latter is of great significance because much of the attention on NCs has been focused on their unique optical properties, which are sensitive to interparticle distance and other factors, for example, particle size, material composition, the nature of surface stabilizing molecules, and surrounding environment.6 Kotov's group reported on the self-assembly of CdTe NCs into free-floating sheets and wires.7 Semiconductor NCs were assembled into one-dimensional (1-D) fibers in solution.8 One of the major challenges is that the relationship between NC interactions and resulting self-assembled nanostructures is not well understood.
The self-assembly of NCs, which was initially based on alternating electrostatic absorption, has successfully been extended to encompass different kinds of driving forces, such as hydrogen bonding, covalent bonding, charge transfer, biological recognition, hydrophobic interactions, and other weak intermolecular interactions. Recent studies indicated NCs self-assembled into a 1-D structure driven by anisotropic dipolar interparticle forces.7 Compared with assembly using an external electronic field, self-assembly based on coordinative or hydrogen bonding interactions is a useful tool for superstructure preparation because of its low cost, easy control, and simplicity.9 In recent years, interest in using biomolecules, such as crystalline S-layer proteins and ferritin protein cages, as templates to scaffold inorganic nanostructures has arisen.10 Various organic and biochemical molecules have been used to drive the self-assembly of chemically produced NCs. Alternatively, self-assembly of NCs without capping molecules has been recently developed. The method utilized charge stabilized NC colloids to spontaneously self-assemble on a water–oil interface. Although many approaches are promising, it is still a challenge for materials scientists to develop straightforward and controllable methods for self- or direct-assembly of nanostructures, especially the structures with fractal alignment.
As we know, the self-assembly of NCs is ascribed to their physical dimensions, surface chemistry, and the degree of anisotropic interaction in solution. Among them, the surface chemistry plays an important role because the surface state of NCs creates different interactions which result in the ability of the NCs to assemble. In addition, the assembly of NCs is usually driven by the interactions between the individual building blocks, and therefore, control over the surface properties is an important factor in the realization of assembly.11 In order to obtain the assembly of 1-D and more complex nanostructures, one approach is the use of molecules with special structures and several coordinating centers. Some biomacromolecules, including DNA and proteins have been used to mediate the formation of super-nanostructures based on the self-assembly of NCs. For example, Ge and co-workers reported on the fractal alignments of as-prepared CdS NCs by a droplet dewetting technique using DNA as scaffolds.12 However, this potential self-assembly technique is still in its infancy, especially in the nano-assembly field.
Recently, hybrid SiO2-coated CdTe NCs were prepared by using a simple reflux procedure including a sol–gel reaction which resulted in the formation of a hybrid SiO2 shell with CdS-like clusters on the CdTe core.13 The hybrid NCs exhibited tunable photoluminescence (PL) color from green to red, increased fluorescence quantum yields (QYs), and high stability. Because of the hybrid SiO2 shell linkers, the hybrid NCs were encapsulated in fibers by self-assembly.14 Since the hybrid NCs have a functional surface, it is possible to create special assemblies by using their surface chemistry. Therefore, we probed the self-assembly of such hybrid NCs for the purpose of getting novel nanostructures in a natural way.
We have now researched hybrid SiO2-coated CdTe NCs and fibers containing the hybrid NCs further. The hybrid NCs were incorporated into composite fibers during reflux. Additionally, we experimentally observed the self-assembly of the hybrid NCs through a droplet dewetting technique for assembling the NCs on a substrate. The hybrid NCs were assembled into two-dimensional (2-D) dendritic morphology and three-dimensional (3-D) crystals by using NaCl molecules as scaffolds. Hydrogen bonding plays an important role during assembly. The morphology of the assembly depended strongly on the preparation parameters. This method should open a new stratagem for super-nanostructure preparation and pattern fabrication by using domain growth and hydrodynamic mechanisms.
Experimental
Chemicals and materials
All chemicals were obtained from Sigma Aldrich and used as received. All chemicals were of analytical grade or of the highest purity available. The pure water was obtained from a Milli-Q synthesis system. Petri dishes with a glass bottom and a plastic cover were discharged to obtain a hydrophilic glass surface (–OH groups on the surface).Preparation of CdTe NCs
Thiolglycolic acid (TGA)-capped CdTe NCs with green emitting (d ∼2.6 nm) in aqueous solution were prepared using a procedure including the use of cadmium perchlorate and hydrogen telluride, as described in a previous paper.15Preparation of hybrid SiO2-coated CdTe NCs
Hybrid SiO2-coated CdTe NCs were prepared using a two-step synthesis process. In step 1, CdTe NCs were coated with a thin SiO2 layer by stirring them in an aqueous solution containing Cd2+, TGA, CdTe NCs, tetraethyl orthosilicate (TEOS), and NH3. Typically, CdTe colloidal solution (2 mL) was precipitated by 2-propanol and redispersed in a 2-mL aqueous solution of Cd2+ and TGA (pH ∼10, adjusted using a 1 M NaOH solution). The molar ratio of TGA/Cd in solution was 1.5. Diluted ammonia (50μL, 10 wt %) and TEOS (10 μL) were mixed with the redispersed CdTe colloidal solution in a beaker sealed to reduce the ammonia evaporation during incorporation. After being stirred for 3 h, the CdTe NCs were coated with a thin SiO2 layer containing Cd2+ and TGA. The solution then became homogenous and transparent. In step 2, a reflux process using this solution (diluted 2.5 times by H2O) caused CdS-like clusters to nucleate and grow in the SiO2-shell. The reflux was done for 2 h. The TGA concentration in the solution was 0.05 M during reflux. The resulting sample was centrifuged at 5000 rpm for 10 min to separate fibers and hybrid SiO2-coated CdTe NCs for further characterisation.Self-assembly of hybrid SiO2-coated CdTe NCs by using NaCl molecules as scaffolds
After precipitation, hybrid SiO2-coated CdTe NCs were redispersed in a 0.15 M NaCl solution. The sample was then dropped on a hydrophilic glass surface (the bottom of an as-discharged Petri dish) for a droplet dewetting process. The Petri dish has a glass bottom and a plastic cover. To control the evaporation speed of solvent, different numbers of holes were made on the plastic cover according to the amount of sample dropped. For investigating the morphological differences of NC assembly, the amount of sample on the hydrophilic glass surface was adjusted as summarized in Table 1.Characterization
The PL and optical images of samples were obtained with an Olympus IX 71 fluorescence microscope (Olympus Optical Co.). The absorption and PL spectra of samples were taken using conventional spectrometers (Hitachi U-4100 and F-4600). The fluorescence QYs of samples in an aqueous solution were estimated by comparison with standard solutions, such as a rhodamine 6G solution.16 The PL lifetime of samples was measured using a time-correlated single-photon-counting spectrofluometer system (FluoroCube-01-NL). The recorded decay curves were fitted with a multiexponential function by least squares fitting.Results and discussion
Formation of hybrid SiO2-coated CdTe NCs and luminescent fibers
Green-emitting CdTe NCs (2.7 nm in diameter) were coated with a thin SiO2 layer by adding TEOS in an alkaline CdTe colloidal solution with Cd2+ ions and TGA. The Cd2+ ions and TGA in solution remained within the SiO2 layer or adsorbed on the layer. A subsequent reflux process including a sol–gel reaction resulted in the formation of hybrid SiO2-coated CdTe NCs. Namely, CdS-like clusters nucleated and grew in the SiO2 shell. The SiO2 shell became thicker due to the deposition of SiO2 monomers. At the same time, the hybrid NCs were incorporated into composite fibers consisting of Cd–TGA complex and SiO2 monomers as depicted in our recently published paper.14Scheme 1 shows the formation process of hybrid SiO2-coated CdTe NCs and fibers with the hybrid NCs.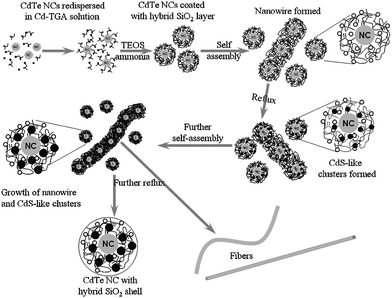 | ||
| Scheme 1 Formation process of hybrid SiO2-coated CdTe NCs and fibers with hybrid NCs. The process included CdTe NCs coated with a thin functional SiO2 layer, the formation of nanowires, the growth of CdS-like clusters, and the fabrication of hybrid SiO2-coated NCs and fibers. | ||
Luminescent properties of hybrid SiO2-coated CdTe NCs
Fig. 1 shows the absorption and PL spectra of green-emitting CdTe NCs before and after coating with a hybrid SiO2 shell. The hybrid SiO2-coated CdTe NCs exhibited a red-shifted PL peak wavelength (559 nm for initial CdTe NCs, 621 nm for the hybrid NCs) and a high fluorescence QY (20% for initial CdTe NCs, 55% for the hybrid NCs) compared with initial CdTe NCs. This phenomenon is ascribed to the CdS-like clusters in the SiO2 shell formed very close to the CdTe core. The molar extinction coefficient (ε) of CdTe NCs at the first absorption peak wavelength remains unchanged during reflux. This phenomenon is different from conventional growth of NCs where ε at the first absorption peak wavelength increases in the manner of the square or cube of the size.17 Very recently, we reported on the preparation condition dependence of PL properties including PL peak wavelength, the full width at half maximum (FWHM) of PL spectra, lifetimes, and fluorescence QYs of the hybrid SiO2-coated CdTe NCs in detail.13a | ||
| Fig. 1 Absorption and PL spectra of CdTe NCs before and after coating with hybrid SiO2 shell. The CdTe NCs revealed a red–shifted PL peak wavelength (before: 559 nm; after: 621 nm) and a high fluorescence QY (before: 20%; after: 55%) after coating with the hybrid SiO2 shell. | ||
To confirm the different surface state of CdTe NCs and hybrid SiO2-coated CdTe NCs, Fig. 2 shows the PL decay curves (measured at the maximum PL peak, λex = 374 nm) of CdTe NCs and hybrid SiO2-coated CdTe NCs. Reproduced curves for data shown in Table 1 are plotted as thin blue lines. The decay curves can be well fitted to a biexponential model described by F(t) = A + B1exp(–t/τ1) + B2exp(–t/τ2), where τ1 and τ2 represent the time constants, and B1 and B2 represent the amplitudes of the fast and slow components, respectively. Average lifetime τ is calculated using τ = (B1τ12 + B2τ22)/(B1τ1 + B2τ2).18 The average lifetime τ and PL properties of green-emitting CdTe NCs before and after coating with a hybrid SiO2 shell are summarized in Table 2. The fast component of the PL decay in the initial CdTe NCs is associated with exciton recombination. The slow component is considered to originate from the surface-related emission of the CdTe NCs. The fast component (B1) of PL decay for the hybrid NCs decreased compared with that of CdTe NCs while the slow component (B2) increased.
 | ||
| Fig. 2 PL decay curves (measured at maximum emission peak, λex = 374 nm) of CdTe NCs and hybrid SiO2-coated CdTe NCs. Reproduced curves for data are plotted as thin blue lines. | ||
| τ/ns | Fluorescence QYs (%) | FWHM/nm | PL peak wavelength/nm | |
|---|---|---|---|---|
| CdTe NCs | 24.89 | 20 | 48 | 559 |
| Hybrid NCs | 35.02 | 55 | 49 | 621 |
Self-assembly of hybrid SiO2-coated CdTe NCs in fibers
During reflux, brightly luminescent fibers were fabricated. These fibers revealed the same PL properties (such as PL peak wavelength and the FWHM of PL spectrum) as hybrid SiO2-coated CdTe NCs. Therefore, similar hybrid nanostructures were created in both the hybrid SiO2-coated NCs and fibers. For comparison, we prepared fibers without the addition of CdTe NCs during preparation, keeping other parameters the same. The results demonstrate fibers without CdTe NCs just revealed a very weak yellow emission while fibers with CdTe NCs showed bright red emission. The morphology of fibers without CdTe NCs was different from those fibers with CdTe NCs. Fig. 3 shows the optical and color images of fibers with ((a), (b)) and without (c) hybrid SiO2-coated CdTe NCs: (a), (c), color images under 365 nm UV light; (b), optical image under white light. Namely, when CdTe NCs were incorporated in the fibers, the CdS-like clusters can be formed near the CdTe NCs because the Cd2+ ions in solution and S2− ions generated by decomposition of TGA can be moved from the solution into the fiber matrix. In addition, transmission electron microscopy observation shows CdTe NCs were homogeneously dispersed in luminescent fibers (See Supporting Information, Fig. S1†). | ||
| Fig. 3 Images of fibers with (a, b) and without (c) hybrid SiO2-coated CdTe NCs: (a), (c), color images under 365 nm UV light; (b) optical image under white light. | ||
Various cadmium complex structures, such as clusters, 1-D materials, and bulk materials, have been created using functional thiolates such as TGA.19 The Cd2+ ions link with the mercapto group in the TGA to form a complex. The single Cd–TGA polymeric chains quite possibly present anisotropic aggregation behaviors, which result in the formation of fibers.
In the current experiment, the molar ratio of TGA to Cd2+ in solution was 1.5. Therefore, the Cd–TGA complex exhibited 1 or 2 ligands. In this case, the carboxyl group can link to another Cd–TGA chain-like structure through hydrogen bonds. The overlap of Cd–TGA complexes resulted in the formation of longer Cd–TGA clusters. These clusters grew into nanowires which were used as seeds to grow fibers. In addition, the hybrid SiO2-coated NCs can easily attached to the nanowire because of the hybrid SiO2 shell with Cd2+ and TGA. This attachment was created either via the connection of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH⋯+Cd–TGA or via Cd–TGA complex within the hybrid SiO2 shell. Subsequently, the nanowire was grown into a 2-D sheet-like morphology. The flat sheet has a tendency to roll in order to reduce the surface energy in water. The rolling of the flat sheet into a curled one is favorable because this can reduce both the surface energy and the tension brought by the asymmetry of the sheet. Finally, the curled sheet will seam into a fiber by ring-closure of the curled chains through the formation of new hydrogen bonding between the Cd–TGA chain-like structures at the two edges. A similar mechanism has been used to explain the formation of microtubes by using small organic molecules.20 The morphology of the final fibers depended strongly on the concentration of starting materials as described in our recently published paper in detail.14 The details of the self-assembly of hybrid SiO2-coated CdTe NCs in fibers are shown in Scheme 1.
Si–OH⋯+Cd–TGA or via Cd–TGA complex within the hybrid SiO2 shell. Subsequently, the nanowire was grown into a 2-D sheet-like morphology. The flat sheet has a tendency to roll in order to reduce the surface energy in water. The rolling of the flat sheet into a curled one is favorable because this can reduce both the surface energy and the tension brought by the asymmetry of the sheet. Finally, the curled sheet will seam into a fiber by ring-closure of the curled chains through the formation of new hydrogen bonding between the Cd–TGA chain-like structures at the two edges. A similar mechanism has been used to explain the formation of microtubes by using small organic molecules.20 The morphology of the final fibers depended strongly on the concentration of starting materials as described in our recently published paper in detail.14 The details of the self-assembly of hybrid SiO2-coated CdTe NCs in fibers are shown in Scheme 1.
Self-assembly of hybrid SiO2-coated CdTe NCs using NaCl molecules as scaffolds
Sodium chloride forms crystals with face-centered cubic symmetry. In these, the larger chloride ions are arranged in a cubic close-packing fashion, while the smaller sodium ions fill all the cubic gaps between them. Each ion is surrounded by six ions of the other kind; the surrounding ions are located at the vertices of a regular octahedron. The reasons for selecting NaCl molecules as scaffolds to assemble hybrid SiO2-coated CdTe NCs into fractal alignment are the following: (1) NaCl crystals grow quickly in an aqueous solution and without any crystalline H2O molecules in their structure; (2) the carboxyl group on the surface of the hybrid NCs links with Na+ ions through hydrogen bonding.Because the evaporation speed of solvent drastically affects the growth of NaCl crystals, the evaporation of H2O during droplet dewetting was smartly controlled by making holes in the plastic cover of a Petri dish. The effect of experimental conditions on the assembly morphology of hybrid SiO2-coated CdTe NCs was investigated. Fig. 4 shows the color images (under 365 UV light) of the hybrid SiO2-coated CdTe NCs (Sample 1 shown in Table 2) assembled into fractal morphology on a hydrophilic glass surface by using NaCl molecules as scaffolds. The result indicates the hybrid NCs were assembled into pretty and brightly luminescent fractal dendritic morphology. This is ascribed to the domain growth of NaCl to form fractal structures through tip splitting and side branching dynamics. To investigate the role of NaCl molecules, a hybrid SiO2-coated CdTe NC solution without Na+ and Cl− ions was used instead of the CdTe colloidal solution containing NaCl. The result indicates that no well-ordered alignment was observed. Therefore, NaCl molecules act as scaffolds in the self-assembly of the hybrid NCs. We also experimentally observed the morphology and emitting color of the assembly at a natural solvent evaporation speed (without a cover on a Petri dish during droplet dewetting). The droplet dewetting process was carried out after 3 min. Crystalline NaCl still exhibited dendritic morphologies on a hydrophilic glass surface. However, hybrid SiO2-coated CdTe NCs were not incorporated into the fractal NaCl crystallite because of no PL observed from the NaCl crystals. Therefore, the growth speed of NaCl crystals, in other words the solvent evaporation speed during droplet dewetting has to be optimized for the hybrid NCs to be assembled into fractal alignment.
 | ||
| Fig. 4 Color images (under 365 nm UV light) of hybrid SiO2-coated CdTe NCs (Sample 1 shown in Table 2) assembled into fractal morphology on hydrophilic glass surface by using NaCl molecules as scaffolds: (a) to (d), same sample in different area. | ||
In order to elucidate the alignment mechanism of hybrid SiO2-coated CdTe NCs, different preparation parameters were adopted for assembling the hybrid NCs. Fig. 5 shows the color (a, c and e, under 365 nm UV light) and optical (b, d and f, under white light) images of the hybrid NCs (Sample 2 shown in Table 2) assembled into fractal morphology on a hydrophilic glass surface by using NaCl molecules as scaffolds. Although the stretching direction of the assembly was always perpendicular to the air–liquid interface, thick fractal alignment (dendrites) of the hybrid NCs was observed. This is still ascribed to side-branching dynamics of NaCl crystals in domain growth. With increasing amount of sample, the number of the main branches of the fractal alignment was reduced to four. This is associated with the face-centered cubic symmetry of NaCl crystals.
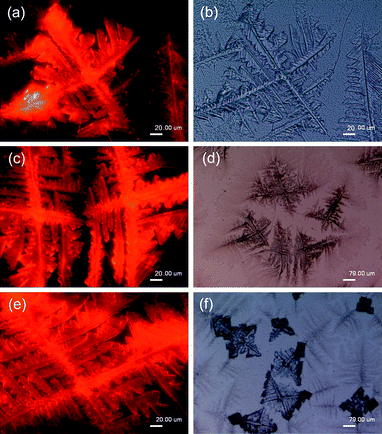 | ||
| Fig. 5 Color (a, c and e, same sample in different area, under 365 nm UV) and optical (b, d and f, same sample in different area, under white light) images of hybrid SiO2–coated CdTe NCs (Sample 2 shown in Table 2) assembled into fractal morphology on hydrophilic glass surface by using NaCl molecules as scaffolds. | ||
Fig. 6 shows the color (a and c, under 365 nm UV light) and optical (b and d, under white light) images of hybrid SiO2-coated CdTe NCs (Sample 3 shown in Table 2) assembled into fractal morphology on a hydrophilic glass surface by using NaCl molecules as scaffolds. Although the number of main branches of fractal alignment was still four, the thickness of the assembly increased compared with Sample 2 shown in Fig. 5. The side branches of the assembly were connected to each other because of deposition of Na+, Cl− and the hybrid NCs. Therefore, an increased amount of starting materials in the solution led to the 2-D fractal alignment having a tendency to a 3-D structure. Furthermore, Fig. 7 shows the color (a and c, under 365 nm UV light) and optical (b and d, under white light) images of hybrid SiO2-coated CdTe NCs (Sample 4 shown in Table 2) assembled into 3-D crystals on a hydrophilic glass surface by using NaCl molecules as scaffolds. Brightly luminescent NaCl crystals were observed because of the self-assembly of hybrid SiO2-coated CdTe NCs. The results shown in Fig. 6 and 7 demonstrate the morphology transfer of the assembly from 2-D to 3-D. The self-assembly of the hybrid NCs in NaCl crystals was assigned to a nucleation and growth mechanism. Similarly, pure NaCl crystals without the hybrid NCs were observed when a quick solvent evaporation speed was used instead of a slow one during droplet dewetting. A slow solvent evaporation speed is crucial for the hybrid NCs assembled in a NaCl crystal because the hydrogen bonding between Na+ ions and the hybrid NCs was broken in the case of a quick growth speed.
 | ||
| Fig. 6 Color (a and c, same sample in different area, under 365 nm UV light) and optical (b and d, under white light) images of hybrid SiO2-coated CdTe NCs (Sample 3 shown in Table 2) assembled into fractal morphology on a hydrophilic glass surface by using NaCl molecules as scaffolds. | ||
 | ||
| Fig. 7 Color (a and c, same sample in different area, under 365 nm UV light) and optical (b and d, under white light) images of hybrid SiO2-coated CdTe NCs (Sample 4 shown in Table 2) assembled into 3-D crystals on a hydrophilic glass surface by using NaCl molecules as scaffolds. | ||
Scheme 2 shows the self-assembly procedure of hybrid SiO2-coated CdTe NCs into different fractal alignments by using NaCl molecules as scaffolds. Briefly, hybrid SiO2-coated CdTe NCs were covered with a layer of Na+ ions when they were redispersed in a NaCl solution because of the hydrogen bonding between the Na+ ions in solution and the –COO− (or –OH) group on the surface of the hybrid NCs. The deposition of Cl− and Na+ ions, in other words, the growth of NaCl crystal results in the self-assembly of the hybrid SiO2-coated CdTe NCs. In the evolution of the assembled morphology of the hybrid NCs just described, the NaCl monolayer prefers to grow at a small amount of sample through tip-splitting dynamics forming fractal dendritic alignment, as amount of sample increases, to grow through side branching dynamics forming thick dendrites, and to grow at a large amount of sample through seed growth mechanism forming 3-D crystals.
 | ||
| Scheme 2 Self-assembly of hybrid SiO2-coated CdTe NCs into fractal alignment by using NaCl molecules as scaffolds. | ||
Two models were proposed to explain the genesis of the fractal alignment of hybrid SiO2-coated CdTe NCs on a hydrophilic glass surface using NaCl molecules as scaffolds. In the first one, domain growth has occurred in a Langmuir monolayer to form a fractal structure due to a hydrodynamic mechanism where concentration gradients produced by supersaturation generate a hydrodynamic flow through the Marangoni effect.21 This mechanism can be use to explain the self-assembly of hybrid SiO2-coated CdTe NCs by a droplet dewetting technique using NaCl as a scaffold. Tip-splitting growth gives rise to dense branched morphologies. There is a morphology transition from tip-splitting to side branching, that is, structures with pronounced dendrites. On the other hand, the capillary dewetting is an origin of these fractal morphologies. On a hydrophilic slide glass surface, after exterior liquid evaporation the contact line of the drying drop was pinned on the substrate, which contained almost all the solute. The liquid evaporation from the edge was replenished by liquid from the interior, producing the outward capillary flow of the solvent and leading to highly selective deposition. Because the capillary direction of flow on a hydrophilic surface depends strongly on the amount of solvent sample, the fractal alignments of hybrid SiO2-coated CdTe NCs can be adjusted. A similar mechanism has been used to explain the self-assembly of CdS NCs.12
Conclusions
Hybrid SiO2-coated CdTe NCs were fabricated using a controlled sol–gel reaction. The hybrid NCs revealed a red-shifted PL peak wavelength, long PL lifetime, and increased PL quantum yield (20% for initial CdTe NCs, 55% for the hybrid NCs), compared with initial CdTe NCs. The improved properties mean the hybrid NCs could have unique applications in biological and light-emitting devices. Due to their special core–shell nanostructures and surface properties, the hybrid NCs were found to have extraordinary properties: the self-assembly of the hybrid NCs generated not only in the fibers which were obtained during reflux, but also in the case of using NaCl molecules as scaffolds. Especially in the latter case, highly luminescent 2-D fractal alignment and 3-D crystals were created through controlling the crystalline process of NaCl during the self-assembly of the hybrid NCs. The morphology of the assembly depended strongly on the amount of sample on a hydrophilic glass surface, the solvent evaporation speed during assembly, and the molar ratio of NaCl to the hybrid NCs in the solution. The strategy described here can serve as a guide for designing and fabricating novel materials with superstructure and multifunctional properties. Because of bright PL of the assemblies, the strategy could be utilized for the fabrication of functional bulk crystal materials and chemical sensors.Acknowledgements
This work was supported in part by the Program for Taishan Scholars.Notes and references
- S. Srivastava and N. A. Kovtov, Acc. Chem. Res., 2008, 41, 1831 CrossRef CAS.
- A. A. Mamedov, A. Belov, M. Giersig, N. N. Mamedova and N. A. Kotov, J. Am. Chem. Soc., 2001, 123, 7738 CrossRef CAS; H. Zhang, Z. Zhou, K. Liu, R. Wang and B. Yang, J. Mater. Chem., 2003, 13, 1356 RSC.
- Z. T. Zhang, A. J. Rondinone, J. X. Ma, J. Shen and S. Dai, Adv. Mater., 2005, 17, 1415 CrossRef CAS; S. L. Li and A. P. Alivisatos, Adv. Mater., 2003, 15, 408 CrossRef CAS; J. G. Fan, D. Dyer, G. Zhang and Y. P. Zhao, Nano Lett., 2004, 4, 2133 CrossRef CAS; K. Takahashi, S. J. Limmer, Y. Wang and G. Z. Gao, J. Phys. Chem. B, 2004, 108, 9795 CrossRef CAS.
- P. Yang, C. L. Li and N. Murase, Langmuir, 2005, 21, 8913 CrossRef CAS; J. Ge, Y. Hu, T. Zhang and Y. Yin, J. Am. Chem. Soc., 2007, 129, 8974 CrossRef CAS.
- Z. L. Zhang and S. C. Glotzer, Nano Lett., 2004, 4, 1407 CrossRef CAS; Z. L. Zhang, M. A. Horsch, M. H. Lamm and S. C. Glotzer, Nano Lett., 2003, 3, 1341 CrossRef CAS.
- K. M. Gatta's–Asfura, C. A. Constantine, M. J. Lynn, D. A. Thimann, X. Ji and R. M. Leblanc, J. Am. Chem. Soc., 2005, 127, 14640 CrossRef CAS.
- Z. Zhang, Z. Tang, N. A. Kotov and S. C. Glotzer, Nano Lett., 2007, 7, 1670 CrossRef CAS; Z. Tang, Z. Zhang, Y. Wang, S. C. Glotzer and N. A. Kotov, Science, 2006, 314, 274 CrossRef CAS.
- H. Niu and M. Gao, Angew. Chem., Int. Ed., 2006, 45, 6462 CrossRef CAS; J. A. Goebl, R. W. Black, J. Puthussery, J. Giblin, T. H. Kosel and M. Kuno, J. Am. Chem. Soc., 2008, 130, 14822 CrossRef CAS.
- B. Kim, S. W. Park and P. T. Hammond, ACS Nano, 2008, 2, 386 CrossRef CAS; J. F. Quinn and F. Caruso, Langmuir, 2004, 20, 20 CrossRef CAS.
- N. Oh, J. H. Kim and C. S. Yoon, Adv. Mater., 2008, 20, 3404 CrossRef CAS.
- F. Gao, Q. Lu, X. Meng and S. Komarneni, J. Phys. Chem. C, 2008, 112, 13359 CrossRef CAS.
- C. Ge, M. Xu, J. Fang, J. Lei and H. Ju, J. Phys. Chem. C, 2008, 112, 10602 CrossRef CAS.
- (a) P. Yang and N. Murase, Adv. Funct. Mater., 2010, 20, 1258 CrossRef CAS; (b) N. Murase and P. Yang, Small, 2009, 5, 800 CrossRef CAS.
- P. Yang, M. Ando and N. Murase, Adv. Mater., 2009, 21, 4016 CrossRef CAS.
- N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmüller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177 CrossRef CAS; P. Yang, A. Zhang, H. Sun, F. Liu, Q. Jiang and X. Cheng, J. Colloid Interface Sci., 2010, 345, 222 CrossRef CAS.
- T. Karstens and K. Kobs, J. Phys. Chem., 1980, 84, 1871 CrossRef CAS; M. Grabolle, M. Spieles, V. Lesnyak, N. Gaponik, A. Eychmüller and U. Resch–Genger, Anal. Chem., 2009, 81, 6285 CrossRef CAS.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS.
- Q. Zeng, X. Kong, Y. Sun, Y. Zhang, L. Tu, J. Zhao and H. Zhang, J. Phys. Chem. C, 2008, 112, 8587 CrossRef CAS.
- M. G. S. Kirstein and H. Möhwald, J. Phys. Chem. B, 1998, 102, 8360 CrossRef CAS; N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmüller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177 CrossRef CAS; J. Guo, W. Yang and C. Wang, J. Phys. Chem. B, 2005, 109, 17467 CrossRef CAS; J. C. Bayón, M. C. Brianso, J. I. Briansó, P. González and P. González Duarte, Inorg. Chem., 1979, 18, 3478 CrossRef CAS; A. Shavel, N. Gaponik and A. Eychmüller, J. Phys. Chem. B, 2006, 110, 19280 CrossRef CAS; I. G. Dance, M. L. Scudder and R. Secomb, Inorg. Chem., 1983, 22, 1794 CrossRef CAS.
- Y. S. Zhao, W. Yang, D. Xiao, X. Sheng, X. Yang, Z. Shuai, Y. Luo and J. Yao, Chem. Mater., 2005, 17, 6430 CrossRef CAS.
- A. Gutierrez–Campos, G. Diaz–Leines and R. Castillo, J. Phys. Chem. B, 2010, 114, 5034 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figure. See DOI: 10.1039/c0ce00350f |
| This journal is © The Royal Society of Chemistry 2011 |
