Highlights from the 46th EUCHEM Conference on stereochemistry, Bürgenstock, Switzerland, May 2011
Edward W.
Tate
a and
Rebecca J. M.
Goss
b
aInstitute of Chemical Biology, Department of Chemistry, Imperial College London, Exhibition Rd, London, UK SW7 2AZ. E-mail: e.tate@imperial.ac.uk
bSchool of Chemistry, University of East Anglia, Norwich Research Park, Norwich, UK NR4 7TJ. E-mail: r.goss@uea.ac.uk
First published on 26th August 2011
The sun drenched lakeside bar of the opulent Seehotel Waldstätterhof in Brunnen provided a warm welcome to participants arriving at the 46th Bürgenstock conference. The awe inspiring views of the mountains surrounding lake Lucern (Fig. 1) provided a fitting backdrop for the equally awe inspiring science that was to follow. The conference that was established in 1965, and until recently had always been hosted in the more remote location atop the neighbouring Bürgenstock mountain is steeped in history and tradition. It is not until participants arrive at the meeting that the names of the 14 world-renowned plenary speakers are revealed. This year's organising committee comprising president Jeremy Sanders (University of Cambridge), Andreas Pfaltz (University of Basel), Donald Hilvert (ETH, Zürich), Jérôme Lacour (University of Geneva), Reto Naef (Novartis Pharma AG), Phillippe Renaud (University of Bern), Jay Siegel (University of Zürich) and Helma Wennemers (University of Basel) had again compiled an outstanding programme that highlighted during the international year of chemistry the amazing contributions made by organic chemists to a global society. The Bürgenstock stereochemistry meeting is particularly renowned for the depth of discussion following the talks, and thus it was a particularly fitting tribute to Dudley Williams (University of Cambridge), the late guest of honour for this year, who was always an energetic and insightful contributor to scientific discussions at conferences. Jeremy Sanders formally welcomed the guests at dinner from a stone balcony that overlooked the splendid dining room of the hotel. | ||
| Fig. 1 View from the Seehotel Waldstätterhof terrace bar. | ||
Opening the 46th Bürgenstock Conference on Sunday evening, moderator Tom Fyles introduced Shankar Balasubramanian (University of Cambridge) who presented a thought provoking lecture on cutting edge genome sequencing, and its applications in stratified medicine and fundamental life science. He described a remarkable journey from curiosity-driven basic research through technology development to commercialisation of Illumina's sequencing platform, delivering a phenomenal increase in throughput that is accelerating the genomic revolution in biomedical sciences. A strong theme throughout the lecture was the need for in-depth understanding of both nucleotide synthesis and enzymology, and the integration of multiple technologies. The original research proposal, formulated by Balasubramanian and his colleague David Klenerman in the fertile environment of a Cambridge pub, centred around visualising incorporation of single fluorescent nucleotides by DNA polymerase at the single molecule level on a planar solid support. Subsequent development of a novel protecting group strategy was the key to enable selective release of the fluorophore and regeneration of a free 3′–OH at the incorporated nucleotide, and thus fluorescence-mediated sequencing of an entire chain of dozens of nucleotides could be realised. With the adoption of clonal PCR, which results in local amplification of the DNA chain prior to sequencing, the stochastic errors in single molecule techniques could be eliminated. Painstaking optimisation and the development of powerful algorithms for genome assembly from small fragments led to the product currently on the market that is capable of sequencing an entire human genome in a matter of days. Equally fascinating were the thought-provoking discussions following the lecture, taking in the technological and societal implications of the sub-$1000 genome that platforms such as Illumina's Solexa promise to deliver in the very near future. At the close of the lecture a vigorous debate ensued on ethics and the ownership of DNA.
Starting the second day of the conference, Peter Kündig provided eloquent introductions for the morning speakers, Karl Gademann (University of Basel) and Mo Movassaghi (MIT), having much in common being both interested in natural products, highly successful, very young and sharing the same postdoctoral mentor, Eric Jacobsen. Prof. Gademann encouraged us to “reduce to the max”, illustrating how complex natural products could be truncated to a simple core whilst maintaining or improving biological activity. He provided the audience with an overview of three areas in which his group has identified truncated natural products that maintain their activity in technological or biological settings. Identification of a anachelin-derived catechol capable of binding tightly to a range of metal surfaces provides a novel technique for surface functionalisation, and has been adapted to the creation of fouling-proof PEGylated materials and presentation of antibiotic or quorum-quenching chemicals, which may in turn be derived from truncated natural products. He also described his work on novel inhibitors of protein trafficking, showing the striking results that can be achieved with minimised scaffolds derived from natural products that retain sufficient activity to prevent the nuclear export of specific proteins. He concluded with how compounds from the insect pathogenic fungus known in China as “Winter Insect, Summer Plant” might be utilised for inducing neurite growth. Following a coffee break in the bright May sunshine with the lake and mountains behind us, the emphasis on natural product synthesis continued with a crystal clear lecture delivered by Prof. Movassaghi encompassing a broad and deep treatment of the total synthesis of complex alkaloids. The audience was treated to a tour-de-force of clever methodologies, and total syntheses inspired by formal consideration of biosynthesis. Synthesis of all the known agelastatins and an approach enabling the tethered installation of a variety of thiane bridges into the epipolythioketopiperazine class of natural products were just two of the outstanding features of the work presented (Fig. 2).
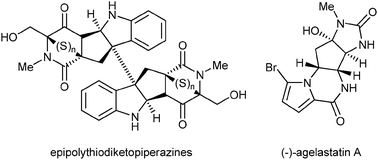 | ||
| Fig. 2 Mo Movassaghi: targets conquered by bio-inspired total synthesis. | ||
After a generous lunch break and a further gourmet meal at the hotel's dining room, 5 short talks were presented by young academics. Pressure was intense not only because of speaking alongside such well renowned speakers who had already become leaders of their respective fields but due to the strict instructions to keep to eight minutes, or have the talk terminated by Helma Wennemers' amplified Swiss cow-in-a-can! The poster session that followed until dinner was excellent with much time and space to enable the presenters to talk to the majority of the other delegates.
Following another excellent dinner the Monday sessions concluded with moderator Ehud Keinan's introduction of a lecture from Alanna Schepartz (Yale University), who provided a panoramic view of novel unnatural proteins devoid of alpha amino acids, that nonetheless exhibit cooperative folding. Introducing the grand challenge of inventing the first molecules with these properties and taking inspiration from prior modeling experiments, beta amino acids were suggested as a potentially powerful building block towards this goal. Prof. Schepartz proceeded to describe her lab's recent expedition through the terra incognita of beta peptides, analogues of natural peptides that contain only beta-amino acids. Remarkable and immediate success was achieved using logically designed beta peptide helices that were shown, through an elegant series of biophysical measurements and structural analyses, firstly to form well-defined helical structures, and furthermore to pack into 8 helix bundles (Fig. 3). These bundles possess extraordinary thermal stability thanks to a robust hydrophobic core, and cooperative melting profiles within the range of known well-folded proteins. The fascinating sequence-structure relationship of this unnatural protein molecule was then explored, and subsequent discussions with the audience ranged from the potential for genetically encoded and evolvable beta proteins, to new applications in catalysis and medicine.
 | ||
| Fig. 3 Alanna Shepartz's 8-helix beta peptide bundle (image courtesy of Doug Daniels). | ||
The morning session of the third day of lectures, moderated by Gebhard Haberhauer (University of Duisburg-Essen), commenced with an impressive talk by Jason Chin (MRC, Cambridge). Decoupling of a distinct form of the ribosome from routine protein synthesis has permitted, amongst many other remarkable innovations, the design and engineering of a 4 base code operational in parallel with a three base code and tRNAs designed to incorporate unnatural amino acids. Jason's presentation highlighted how this approach is on the verge of revolutionising our understanding of multiple aspects of fundamental cell biology. For example, an encoded photocaged lysine permits light-activated control of protein localisation, and direct activation of the MEK1/ERK activation pathway; this permits the determination of precise kinetics for these processes in living cells. Acetylated lysine may also be encoded in proteins, providing a powerful tool to decode the ‘histone code’ and the role of lysine acetylation in regulation of metabolism. Needless to say, the talk stimulated energetic discussion from the other participants. Following the coffee break John Robinson (University of Zurich) delivered a compelling lecture on peptide-based protein epitope mimetics with utility across an eclectic range of biological contexts, from HIV vaccine development to novel antibacterial agents. He started with a retrospective of the D-Pro-L-Pro cyclic peptide backbone constraint, a scaffold upon which a network of inter-strand hydrogen bonds is built up, resulting in a protein fragment analogous to a two-stranded beta sheet. Prof. Robinson proceeded to demonstrate the potential applications of these motifs in mimicking the V3 loop of the viral spike protein complex in HIV (a potential vaccine target) and in binding viral RNAs with exceptional potency. Finally, the audience was treated to the fascinating story of the characterisation of the first lipopolysaccaride export inhibitors, and their progress towards the clinic as antibiotics with an unprecedented mode of action. Initial testing of peptide loop libraries based on naturally occurring peptide antibiotics revealed antibiotic selectivity against Pseudomonas aeruginosa, with a strong dependence on sequence, topology and stereochemistry. Recognising a potentially interesting mode of action distinct from classical membrane disruption, the Robinson lab embarked on a focused and tenacious campaign, facilitated by fruitful industrial and academic collaborations, to identify the target of these molecules. Through a combination of sophisticated chemical microbiology and genetic manipulation, the outer membrane LPS transporter LptD was shown by a wide range of approaches to bind to these compounds in a highly selective manner in vivo, resulting in dramatic aberrations in the bacterial membrane microstructure and potent antibiotic activity. Serendipity vs. design was a common theme both throughout the lecture and in the subsequent discussion with the audience. A convincing case was made for the value of carefully designed scaffolds coupled with iterative random library design as a powerful approach in inhibiting protein and nucleic acid interactions. Dinner on the third day was slightly unusual, after an excellent starter and main course the lights were dimmed as a cake acting as a platform for a further firework display was brought into the dining room. “Happy Birthday” was sung to Jeremy Sanders who was presented with a large scimitar with which to cut the desert.
The after dinner speaker Scott Miller (Yale University) was, introduced by Helma Wennemers (University of Basel), who gave a highly inspiring talk. He reminded the audience of an insightful paper of Jeremy Knowles entitled “Enzymatic Catalysis: Not Different, Just Better” and demonstrated this as his reference point for his group's elegant and ground breaking work on the use of short peptide sequences in the organocatalysis of an impressive catalogue of reactions. He discussed that natural products are frequently shunned by the pharmaceutical industry as the libraries of analogues required for medicinal chemistry approaches are often at times intractable. He proceeded to demonstrate the development of catalysts that enabled precise site selective modification of natural products such as erythromycin without requirement for protecting groups. During the lively discussion that followed, the “sanctity of the catalyst” was questioned and whether in a parallel lab an entirely unrelated peptide could be found that would catalyse the same reaction, and it was concluded that this was highly likely.
The morning session of the fourth day of lectures focused on C–H activation and, following an introduction by Andreas Pfaltz (University of Basel), commenced with a characteristically high energy talk by Melanie Sandford (University of Michigan). In keeping with the founding principles of the conference, Melanie chose to present her ground breaking but unpublished work on oxidants and ligands for the Pd(II)–Pd(IV) manifold in C–H bond activation. A diverse range of novel transformations providing access to new scaffolds were presented, and a convincing case was made for the less widely-explored Pd(IV) oxidation state as a means to alternate reactivities. The second lecture of day four was given by Jin-Quan Yu (Scripps), who opened his lecture with a tribute to the vast literature on palladium catalysed reactions, whilst reinforcing the idea from the previous lecture that new approaches to C–H activation are required to advance natural product total synthesis and medicinal chemistry. With his characteristic style and infectious enthusiasm, Prof. Yu treated his audience to a kaleidoscopic overview of the exceptional contributions of his lab to Pd C–H activation over the past few years, focusing on the Pd(II)–Pd(0) manifold. At the heart of his strategy is the concept of ligand acceleration, and how it may result in enhanced enantio- and regioselectivity in remote Pd C–H bond insertion and subsequent derivitisation; by exploiting weaker ligands, the depth of the energy well for the inserted complex is reduced, enabling exquisite tolerance of functional groups during subsequent reductive elimination or cross-coupling chemistry. Some highlights of a talk overflowing with exciting ideas included the use of NMR as a tool for fine-tuning dihedral angles in Pd(II) complexes to favour conformers that reinforce the site selectivity of C–H bond activation, along with remarkable and very promising stereocontrol and conversion afforded by amino acid-derived ligands. The extensive discussions following both lectures focused on mechanistic questions, and the ability of the field to move towards more predictive models for C–H bond activation.
The afternoon of the 4th day was free for mountain climbing, hiking and less strenuous activities. After dinner entertainment was provided by a chamber music concert, for which conference president Jeremy Sanders selected a program of music for the string quartet that featured aspects of local colour and chemical significance. After opening with Mozart, the musicians turned to the twentieth century repertoire with Paysage, a series of short vignettes by the Swiss composer Ernest Bloch, drawing inspiration from the beautiful Swiss alpine landscape. The concert closed with the second string quartet of Alexander Borodin, a Russian composer who was also among the foremost organic chemists of the 19th century, a passionate campaigner for women's education, and co-discoverer of the aldol condensation.
The morning of the fifth day of the conference was moderated by Don Hilvert (ETH, Zürich) who introduced the first speaker Yamuna Krishnan (National Centre for Biological Sciences, Bangalore) as the speaker with the youngest academic career. Yamuna impressively combined her three passions of chemistry, biology and architecture using rigid strands of DNA as her building blocks. The design and utilisation of a switch that utilised Watson Crick base pairing to close in response to pH provides a quantitative colorimetric readout that can be used as a sensor within whole cells and even within the model organism C. elegans. Yamuna also developed her presentation demonstrating the use of DNA building blocks to designer microarchitectures that could be used for encapsulation was detailed and the retrosynthesis of DNA constructed architectures including icosahedra (Fig. 4).
 | ||
| Fig. 4 Yamuna Krishnan: retrosynthesis of a DNA icosahedron. | ||
Hanadi Sleiman (McGill University), further developed the DNA self-assembly theme with the generation of new structure and function through the use of small molecule elements integrated into the DNA backbone. Prof. Sleiman showed that by leveraging highly efficient solid phase oligonucleotide synthesis and the sequence rules of DNA hybridisation a plethora of useful moieties may be introduced at will into precise positions in nanoscale DNA architectures. For example, such motifs include ‘corners’ (phenanthrene) and ligands that can distinguish between a number of metals and their oxidations states (e.g. Cu(I) vs. Cu(II), Fig. 5), to permit the generation of relatively rigid cyclic loops and metalised DNA frameworks. Strategic introduction of single strand regions into these supramolecular assemblies provide a handle for subsequent functionalisation by hybridisation to short oligos that carry cargo such as gold nanoparticles, biocompatible coatings, and potentially in future also proteins. Adding more approaches to the mix, such as single strand overhangs to mediate directed polymerisation or dissociation, extends isolated polygons into polyhedral, wires and meshes, offering tools for encapsulation of gold nanoparticles. Perhaps even more exciting were the new options presented for dynamic control over shape and rigidity: by careful design of these single strand modifiers, shortening or stiffening of discrete parts of the framework can be achieved. Discussions following the lecture revolved around future challenges and opportunities for the growing field, for example the potential for light switchable or redox active frameworks, and direct control of biological processes using designer DNA nanostructures. That afternoon there was again a series of 5 short appetizer lectures preceding a second poster session. A diverse series of topics were presented ranging from helical supramolecular chirality, polyisocyanopeptide nanoworms to innovative strategies for catalytic site-selective modification of carbohydrates. An energetic poster session followed.
 | ||
| Fig. 5 Sleiman's DNA architechtures tailored with ligands capable of detecting metals in their various oxidation states. | ||
After dinner the audience were treated to a seminar of a rather different nature. Marcel Mayor (University of Basel) introduced by Jan van Esch (Delft University of Technology) presented a thought provoking lecture. We were introduced to a tree connecting the physical sciences and the philosophy of making designer molecules to explore physical questions. Elegant and technically challenging experiments exploiting single molecule rectifiers (Fig. 6) were explained. Perhaps the most exciting experiments described were those where Mayor pondered and demonstrated the wave particle duality of molecules as large as 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 amu. To achieve this, molecules limited in their conformational degrees of freedom were designed and synthesised, vaporised and focused through a series of slits enabling the molecular interferometry to be observed. The question was raised as to at what mass the end of quantum mechanics would be reached, as surely a molecule of 10
123 amu. To achieve this, molecules limited in their conformational degrees of freedom were designed and synthesised, vaporised and focused through a series of slits enabling the molecular interferometry to be observed. The question was raised as to at what mass the end of quantum mechanics would be reached, as surely a molecule of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 amu could now be considered to be an object.
123 amu could now be considered to be an object.
 | ||
| Fig. 6 The Mayor single molecule rectifier. | ||
The final talks of the conference were as exciting as the first, introduced by moderator Kay Severin (EPFL). The penultimate speaker was Wilhelm Huck (Radboud University Nijmegen) who presented the issue of crowding and limited diffusion in the intracellular environment from the perspective of statistical mechanics and its impact on enzyme mechanism or protein complex formation in living systems. He proposed leveraging the tools of microfluidics, in which his lab is a world leader, to enable extreme high-throughput single-molecule or single-cell analysis of stochastic events in complex phenomena such as transcriptional activation. In support of this idea, Wilhelm entertained the audience with his lab’s contributions to droplet microfluidics, and the amazing manipulations of picolitre volumes that may be achieved by careful device design. Bringing the conference to a close with the final lecture on day six was Ivan Huc (University of Bordeaux, CNRS), who echoed earlier talks in his view that both careful design and serendipitous discovery have vital roles to play in exploring new science. Ivan provided a barnstorming overview of his work on artificial helical structures, ranging from designer supramolecules with tunable self-assembly properties to complex multi-helical materials with exciting prospects for self-replication. The development of organic chemistry was a constant presence just below the surface of the talk, and the importance of highly convergent synthetic methodologies to make ever larger molecules was evident in the remarkable results presented.
The president of the organising committee, Jeremy Sanders, brought proceedings to a close by thanking all those responsible for this excellent conference with particular thanks to the secretary of the committee, Jérôme Lacour. He stated that whilst conventional stereochemistry is limited to three dimensions Bürgenstock has many more. Jeremy Sanders finished by passing his role on to next year's president Andreas Pfaltz (University of Basel), with Luisa De Cola (University of Münster) as vice-president. They now take on the exciting challenge of delivering a conference that matches the high standards of this most diverse, engaging and interesting 2011 Bürgenstock conference.
EWT and RJMG thank the conference organisers for Junior Scientist Programme awards.
| This journal is © The Royal Society of Chemistry 2011 |
