Chiral squaramide-catalyzed highly diastereo- and enantioselective direct Michael addition of nitroalkanes to nitroalkenes†
Abstract
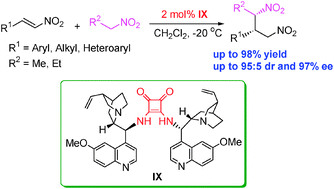
An efficient highly diastereo- and enantioselective direct
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...