 Open Access Article
Open Access ArticleIridium-catalysed condensation of alcohols and amines as a method for aminosugar synthesis†
Ian
Cumpstey
*ab,
Santosh
Agrawal
a,
K. Eszter
Borbas
a and
Belén
Martín-Matute
*a
aDepartment of Organic Chemistry, The Arrhenius Laboratory, Stockholm University, 106 91 Stockholm, Sweden. E-mail: belen@organ.su.se; ian.cumpstey@sjc.oxon.org; Fax: +46 (0)8 15 162477; Tel: +46 (0)8 154908
bICSN-CNRS, 91198 Gif-sur-Yvette CEDEX, France
First published on 8th June 2011
Abstract
Primary carbohydrate amines at primary and secondary carbons are alkylated by alcohols in the presence of [Cp*IrCl2]2. When primary carbohydrate alcohols are used as the coupling partners and in the presence of Cs2CO3, amine-linked pseudodisaccharides are obtained. Secondary carbohydrate alcohols are unaffected under these conditions, which allows regioselective reactions.
In this communication, we address the use of carbohydrates as cheap, renewable and densely functionalised potential building blocks1 for transition metal-catalysed organic synthesis. In particular, we focus on C−N bond formation in the synthesis of structurally varied aminosugars, either by alkylation of carbohydrate primary amines, or by amination of carbohydrate alcohols. Classical methods for amine bond formation, such as alkylation with halides or pseudohalides,2oxidation and reductive amination,3 or Mitsunobu4 chemistry with sulfonamides, result in the production of stoichiometric by-products and involve multistep sequences. A catalytic redox-activated condensation reaction between alcohols and amines to give higher order amines and water is an attractive alternative.5,6 This process was described in the early 1980s,5,7 and has recently gained popularity with the development of efficient homogeneous catalysts based on ruthenium, rhodium and iridium.8–14 To date, the substrate scope has been limited to rather simple molecules. The reactivity of carbohydrate alcohols in the catalytic amination reaction does not appear to be known, while the catalytic alkylation of carbohydrate amines with simple alcohols (as solvents) over heterogeneous catalysts has been reported,15 but not studied in detail. Focussing on the [Cp*IrCl2]2 complex‡ as introduced by Fujita et al.,8 we have examined the scope of the reaction with respect to functionalisation of carbohydrate amines with alcohols; and also the functionalisation of carbohydrate alcohols with amines, including regioselectivity aspects.
First, we considered the reactivity of carbohydrate amines towards non-carbohydrate alcohols. A primary C6 amine with the hydroxyl groups protected (α-Man-1) was used as a test system with cyclohexanol 2a (5 equiv.) as the alkylating reagent (Scheme 1). We found that with a catalyst loading of 1 mol% of [Cp*IrCl2]2, at 120 °C in toluene, the secondary amine α-Man-3 was formed in excellent yield (see ESI† for optimisation of the reaction conditions). The addition of NaHCO3 (25 mol%) did not affect the efficacy of this reaction, which contrasts with earlier results on different substrates (anilines rather than carbohydrate amines).8b
 | ||
| Scheme 1 Alkylation of C6 amine α-Man-1 with cyclohexanol (2a). | ||
In a somewhat more challenging system, hexopyranosides with a primary amine at C3 and with a secondary alcohol unprotected (viz β-Glc-4, β-Man-5 and α-Man-6)§ were tested as substrates (Scheme 2). Here again the alkylation reaction with cyclohexanol (2a) proceeded efficiently to give the secondary amine products for all of the different configurations tested. Products due to redox epimerisation16,17 or amination of the unprotected secondary alcohol groups were not detected. Benzyl alcohol (2b) could also be used as an alkylating reagent, affording β-Glc-7b in excellent yield.
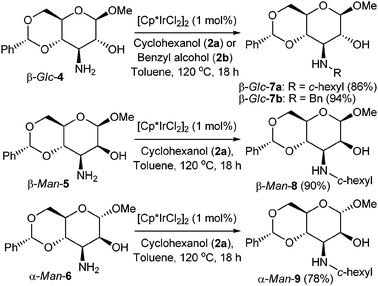 | ||
| Scheme 2 Alkylation of C3 carbohydrate amines with alcohols. | ||
We went on to investigate the alkylation of an essentially unprotected aminosugar α-Man-10. Earlier reactions had been carried out in toluene, but this solvent was unsuitable here as the substrate α-Man-10 was insoluble. Using the alcohol itself as solvent was found to work well. With secondary alcohols 2a and 2c, the products were the secondary amines, while a primary alcohol (2d) gave the tertiary amine as the only product as a result of two consecutive alkylations (Scheme 3).
 | ||
| Scheme 3 Alkylation of amine α-Man-10 with alcohols 2a, 2c–d. | ||
The use of carbohydrate alcohols as latent electrophiles is a challenging goal. The alcohol carbons of carbohydrates are electron-poor, which makes them resistant to oxidation by a transition-metal-catalysed hydrogen-transfer mechanism. To the best of our knowledge, there are no reports on the redox-activated amination reaction of carbohydrate alcohols with either homogeneous or heterogeneous catalysts.‡,§Carbohydrate alcohol α-Glc-13 was treated with carbohydrate amine α-Glc-12 under the reaction conditions used in Scheme 2, but the coupling product was not formed. In this case, the addition of an inorganic base resulted in a significant improvement. With NaHCO3 (25 mol%) at 160 °C, the reaction gave a secondary amine product (Glc,Glc-14), a pseudodisaccharide, in 66% yield. With Cs2CO3, we saw a further improvement, and the secondary amine was formed more quickly at a lower temperature (120 °C) and in higher yield (Scheme 4). This result contrasts again with the observations of Fujita et al. in the coupling of anilines with benzylic alcohols, where better results were obtained with NaHCO3 than with Cs2CO3.8b In a control experiment without added alcohol, the carbohydrate amine α-Glc-12 failed to give a pseudodisaccharide product, indicating that the C2 symmetric diglucose Glc,Glc-14 does arise from a redox condensation involving the carbohydrate alcohol α-Glc-13. A dimannose (Man,Man-16) was similarly formed by reaction of amine α-Man-1 with C6 alcohol α-Man-15, and an unsymmetrical Glc,Man-17 pseudodisaccharide was formed either by condensation of amine α-Man-1 with alcohol α-Glc-13, or by reaction of amine α-Glc-12 with alcohol α-Man-15 (Scheme 4). In these three reactions, the yields were lower, the remainder being unreacted starting materials and unidentified non-polar by-products.
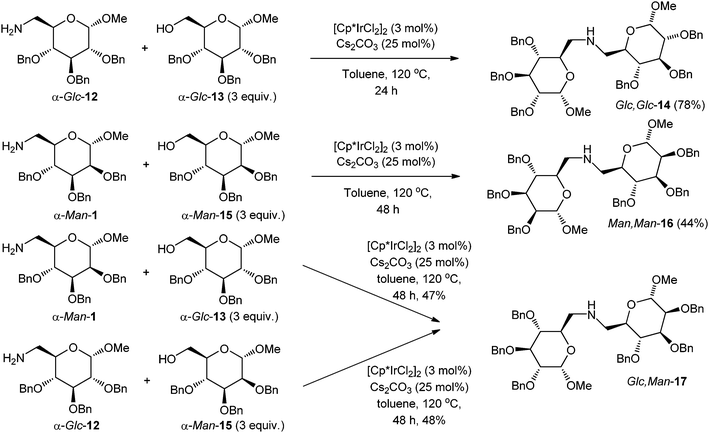 | ||
| Scheme 4 Synthesis of pseudodisaccharides by amination of primary carbohydrate alcohols. | ||
The reactivity of primary carbohydrate alcohols and the unreactive nature of secondary carbohydrate alcohols suggest the possibility of a regioselective amination reaction. Hence the diol β-Glc-18 with free OH4 and OH6 was treated with amine α-Glc-12 under the coupling reaction conditions with Cs2CO3, and a single pseudodisaccharide product (Glc,Glc-19) due to amination of the primary hydroxyl was seen (Scheme 5).
 | ||
| Scheme 5 Regioselective amination of a primary carbohydrate alcohol. | ||
Based on these results, it is possible to draw some provisional conclusions about the behaviour of carbohydrates in the transition-metal-catalysed reaction between alcohols and amines. All products were isolated as single diastereomers. Epimerisation of secondary carbohydrate alcohols was not observed under these conditions, and neither was epimerisation detected at the centres vicinal to the presumed intermediate carbonyl.6 This latter observation contrasts with an earlier result on a protected glycerol.9dCarbohydrate amines can be alkylated in the presence of unprotected carbohydratehydroxyl groups, using non-carbohydrate alcohols as alkylating agents. The primary alcohol of a carbohydrate is more readily functionalised than the secondary alcohols, which allows regioselective functionalisation. The amination of carbohydrate alcohols requires addition of a base, whereas for non-carbohydrate alcohols a base is not required. Cs2CO3 gave good results for the functionalisation of primary carbohydrate alcohols.
We envisage that the methods for the synthesis of structural variants of N-substituted aminosugars described in this communication could find numerous applications in the optimisation of structures for a given purpose, e.g. as ligands for biomolecules. Synthetic N-substituted carbohydrate amines have been shown to inhibit glycosidases;18 and it has been suggested that they could act as ligands for RNA.19 Modification of the structure of natural aminoglycosides could lead to new antibiotics.20
IC and BMM thank Vetenskapsrådet, and BMM thanks the Exselent centre for support. KEB was supported by a Marie Curie fellowship. SA is supported by the Wenner-Gren foundation.
Notes and references
- F. W. Lichtenthaler and S. Mondel, Pure Appl. Chem., 1997, 69, 1853 CrossRef CAS.
- For example, see: (a) T. M. Tagamose and M. Bols, Chem.–Eur. J., 1997, 3, 453; (b) M. J. McDonough, R. V. Stick, D. M. G. Tilbrook and A. G. Watts, Aust. J. Chem., 2004, 57, 233 Search PubMed.
- (a) J. Neumann, S. Weingarten and J. Thiem, Eur. J. Org. Chem., 2007, 1130 Search PubMed; (b) D. J. Walton and J. D. McPherson, Carbohydr. Res., 1987, 167, 123 CrossRef CAS; (c) S. Horii, H. Fukase, T. Matsuo, Y. Kameda, N. Asano and K. Matsui, J. Med. Chem., 1986, 29, 1038 CrossRef CAS.
- (a) A. Zamojski, W. A. Szarek and J. K. N. Jones, Carbohydr. Res., 1972, 23, 460 Search PubMed; (b) J. J. Turner, D. V. Filippov, M. Overhand, G. A. van der Marel and J. H. van Boom, Tetrahedron Lett., 2001, 42, 5763 Search PubMed; (c) T. Akhtar and I. Cumpstey, Tetrahedron Lett., 2007, 48, 8673 Search PubMed.
- For reviews, see: (a) G. Guillena, D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2007, 46, 2358 CrossRef CAS; (b) T. D. Nixon, M. K. Whittlesey and J. M. J. Williams, Dalton Trans., 2009, 753 RSC; (c) R. Yamaguchi, K.-i. Fujita and M. Zhu, Heterocycles, 2010, 81, 1093 CrossRef CAS; (d) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681 CrossRef CAS; (e) G. Gillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611 CrossRef CAS.
- D. Balcells, A. Nova, E. Clot, D. Gnanamgari, R. H. Crabtree and O. Eisenstein, Organometallics, 2008, 27, 2529 CrossRef CAS.
- (a) Y. Watanabe, Y. Tsuji and Y. Ohsugi, Tetrahedron Lett., 1981, 22, 2667 CrossRef CAS; (b) R. Grigg, T. R. B. Mitchell, S. Sutthivaiyakit and N. Tongpenyai, J. Chem. Soc., Chem. Commun., 1981, 611 RSC; (c) S. I. Murahashi, K. Kondo and T. Hakata, Tetrahedron Lett., 1982, 23, 229 CrossRef CAS; (d) A. Arcelli, B.-T. Khai and G. Porzi, J. Organomet. Chem., 1982, 235, 93 CrossRef CAS.
- (a) K.-i. Fujita, Z. Li, N. Ozeki and R. Yamaguchi, Tetrahedron Lett., 2003, 44, 2687 CrossRef CAS; (b) K.-i. Fujita, Y. Enoki and R. Yamaguchi, Tetrahedron, 2008, 64, 1943 CrossRef CAS; (c) R. Kawahara, K.-i. Fujita and R. Yamaguchi, J. Am. Chem. Soc., 2010, 132, 15108 CrossRef CAS.
- (a) G. Cami-Kobeci, P. A. Slatford, M. K. Whittlesey and J. M. J. Williams, Bioorg. Med. Chem. Lett., 2005, 15, 535 CrossRef CAS; (b) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555 CrossRef CAS; (c) M. H. S. A. Hamid and J. M. J. Williams, Chem. Commun., 2007, 725 RSC; (d) M. H. S. A. Hamid, C. L. Allen, G. W. Lamb, A. C. Maxwell, H. C. Maytum, A. J. A. Watson and J. M. J. Williams, J. Am. Chem. Soc., 2009, 131, 1766 CrossRef CAS; (e) O. Saidi, A. J. Blacker, M. M. Farah, S. P. Marsden and J. M. J. Williams, Chem. Commun., 2010, 46, 1541 RSC.
- (a) D. Hollmann, A. Tillack, D. Michalik, R. Jackstell and M. Beller, Chem.–Asian J., 2007, 2, 403 CrossRef CAS; (b) S. Imm, S. Bähn, L. Neubert, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 8126 CrossRef CAS.
- (a) B. Blank, M. Madalska and R. Kempe, Adv. Synth. Catal., 2008, 350, 749 CrossRef CAS; (b) B. Blank, S. Michlik and R. Kempe, Adv. Synth. Catal., 2009, 351, 2903 CrossRef CAS; (c) B. Blank and R. Kempe, J. Am. Chem. Soc., 2010, 132, 924 CrossRef CAS; (d) S. Michlik and R. Kempe, Chem.–Eur. J., 2010, 16, 13193 Search PubMed.
- S. Whitney, R. Grigg, A. Derrick and A. Keep, Org. Lett., 2007, 9, 3299 CrossRef CAS.
- (a) R. Martínez, D. J. Ramón and M. Yus, Org. Biomol. Chem., 2009, 7, 2176 RSC; (b) A. Martínez-Asencio, D. J. Ramón and M. Yus, Tetrahedron Lett., 2010, 51, 325 Search PubMed.
- (a) L. U. Nordstrøm and R. Madsen, Chem. Commun., 2007, 5034 RSC; (b) R. N. Monrad and R. Madsen, Org. Biomol. Chem., 2011, 9, 610 RSC and references therein.
- N. Choubdar, R. G. Bhat, K. A. Stubbs, S. Yuzwa and B. M. Pinto, Carbohydr. Res., 2008, 343, 1766 CrossRef CAS.
- (a) J. S. M. Samec, J.-E. Bäckvall, P. G. Andersson and P. Brandt, Chem. Soc. Rev., 2006, 35, 237 RSC; (b) G. Csjernyik, K. Bogár and J.-E. Bäckvall, Tetrahedron Lett., 2004, 45, 6799 CrossRef CAS.
- C. Ramstadius, A. M. Träff, P. Krumlinde, J.-E. Bäckvall and I. Cumpstey, Eur. J. Org. Chem DOI:10.1002/ejoc.201100504.
- V. L. Maxwell, E. L. Evinson, D. P. G. Emmerson and P. R. Jenkins, Org. Biomol. Chem., 2006, 4, 2724 RSC.
- M. Madalinski, M. Stoll, U. Dietrich and H. Kunz, Synthesis, 2008, 1106 Search PubMed.
- (a) T. Hermann, Cell. Mol. Life Sci., 2007, 64, 1841 CrossRef CAS; (b) M. Hainrichson, I. Nudelman and T. Baasov, Org. Biomol. Chem., 2008, 6, 227 RSC.
- (a) M. Bierenstiel and M. Schlaf, Eur. J. Org. Chem., 2004, 1474 Search PubMed; (b) M. Saburi, Y. Ishii, N. Kaji, T. Aoi, I. Sasaki, S. Yoshikawa and Y. Uchida, Chem. Lett., 1989, 563 Search PubMed; (c) G. Descotes, J.-P. Praly and D. Sinou, J. Mol. Catal., 1979, 6, 421 Search PubMed; (d) G. Descotes, D. Sinou and J.-P. Praly, Carbohydr. Res., 1980, 78, 25 Search PubMed.
- (a) I. Isaac, G. Aizel, I. Stasik, A. Wadouachi and D. Beaupère, Synlett, 1998, 475 CrossRef CAS; (b) I. Isaac, I. Stasik, D. Beaupère and R. Uzan, Tetrahedron Lett., 1995, 36, 383 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Optimisation of reaction conditions, experimental details and spectra of new compounds. See DOI: 10.1039/c1cc12800k |
| ‡ We found that other organometallic complexes that have been reported for the amine–alcohol redox condensation failed to give good results with carbohydrate alcohols (amongst others, Ru(p-cymene)dppf, K2CO39d and Ru3(CO)12, P(o-tol)310b), giving either no reaction at all, or traces (< 5%) of the aminated product. For the reaction between carbohydrate amines and non-carbohydrate alcohols, some reactivity was seen using Ru(p-cymene)dppf, K2CO3, toluene, 4 Å sieves, 120 °C, 36 h: amine α-Glc-12 and n-butanol gave the corresponding tertiary amine (65%), but this system was not investigated in detail. |
| § Oxidation of the anomeric hydroxyl of hemiacetals or of primary alcohols by hydrogen-transfer to form lactones has been demonstrated using ruthenium21 and rhodium22catalysts with partially protected or unprotected carbohydrates or alditols as substrates. Reaction of secondary hydroxyl groups is most unusual.17,21d |
| This journal is © The Royal Society of Chemistry 2011 |
