 Open Access Article
Open Access ArticleTemplating a polymer-scaffolded dynamic combinatorial library†
Clare S.
Mahon
,
Alexander W.
Jackson
,
Benjamin S.
Murray
and
David A.
Fulton
*
Chemical Nanoscience Laboratory, School of Chemistry, Newcastle University, Newcastle upon Tyne, NE1 7RU, United Kingdom. E-mail: d.a.fulton@ncl.ac.uk; Fax: +44 (0) 191 222 6929; Tel: +44 (0) 191 222 7065
First published on 24th May 2011
Abstract
A water soluble polymer-scaffolded dynamic combinatorial library whose members can interconvert through acylhydrazone exchange was prepared and shown to re-equilibrate in the presence of macromolecular templates.
Dynamic combinatorial chemistry1 (DCC) has emerged in recent years as a powerful tool for the discovery of molecular receptors. DCC uses reversible covalent reactions to link together building blocks, forming libraries of compounds whose product distributions are under thermodynamic control. The reversible nature of the linkages enables the library members to reconfigure their structures by exchanging and reshuffling their building blocks. Equilibrium perturbations, such as the addition of a template, can result in the structural adaptation of the library, amplifying the concentration of those library members which are stabilized by the template. The library can be kinetically ‘fixed’—often by simply quenching the catalyst involved in catalysing the dynamic exchange—allowing library members to be isolated and characterized. The appeal of the dynamic combinatorial approach lies in its inherent ability to combine library synthesis and affinity screening into a single step, making it a potentially rapid, simple and cost-effective method for the discovery of receptors. DCC has demonstrated great promise for the discovery of small molecule receptors,2–6 but its potential for the discovery of macromolecular counterparts has yet to be investigated.
Several years ago, one of us described7 a new class of DCL—the so-called “polymer-scaffolded dynamic combinatorial library” (PS-DCL)—which was designed to apply concepts from the field of DCC towards the discovery of macromolecular receptors. PS-DCLs are based upon a synthetic polymer scaffold where functionalized residues are grafted onto the scaffold through dynamic covalent linkages. The reversibility of these linkages allows residues to exchange with one another and reshuffle their positions upon the polymer scaffold, presenting a mechanism for the members of the library to adapt their primary structures. We hypothesize that a polymer-scaffolded DCL should be able to respond to the addition of a template in the same manner as that demonstrated by other DCLs,5,6 inducing the system to re-equilibrate and amplifying the concentration of library members which best bind the template. This wholly thermodynamically controlled templation process provides a mechanism to correct and refine binding sites, a virtue absent in molecularly imprinted polymers8,9 (MIPs) where the molecular imprinting process is kinetically controlled and there is no scope for error-correction during the templating procedure. This deficiency leads to significant heterogeneity and binding pockets which are not optimised, with detrimental effects on molecular recognition.10–12 The PS-DCL concept can be contrasted to the conventional combinatorial approach utilised by Schrader and co-workers13 for the discovery of polymers for use in protein recognition. In this work, a small set of functionalized monomers was used to prepare libraries of copolymers which were screened successfully to find selective binders for arginine-rich proteins in aqueous solution, a process requiring extensive synthesis and screening. As with MIPs, the recognition units are incorporated irreversibly into the polymer scaffold and there is no available mechanism for the optimisation of binding sites. Recently Ghadiri and co-workers14 have arguably demonstrated the validity of aspects of the PS-DCL concept within their impressive work on sequence-adaptive oligonucleotides. We report here the generation of an aqueous PS-DCL based on acylhydrazone exchange upon a simple synthetic polyacrylamide scaffold, and demonstrate that this library can re-equilibrate in the presence of macromolecular templates, work which we believe represents important first steps towards the realisation of the PS-DCL concept as a general method for the discovery of truly macromolecular receptors.
Acylhydrazone exchange was chosen as the dynamic reaction to append organic residues to suitably functionalized polymer scaffolds as it is a well-studied and successful reaction in dynamic covalent chemistry.1 Acylhydrazone linkages are formed from the acid-catalysed condensation of acylhydrazides with aldehydes, and the resulting acylhydrazone bonds can readily undergo component exchange, with optimum rates of formation and exchange observed at pH 4.5.15 Many DCLs have been reported based around acylhydrazone exchange in organic media,2–6 however, there have been relatively few reports of dynamic systems based upon acylhydrazone exchange in aqueous solution.16,17
Our water-soluble PS-DCL was constructed upon the polymer scaffold P1, which is a polyacrylamide derivative featuring reactive aromatic aldehyde functions. Polymer P1 was prepared (see ESI†) by the RAFT copolymerization of N,N′-dimethylacrylamide and the monomer N-ethylacrylamide-2-(4-formylbenzamide), affording a polymer possessing a degree of polymerization of approximately 85 and displaying approximately 14 aldehyde functions (by 1H NMR spectroscopy).
Because of the limited water-solubility of this polymer, our PS-DCL was prepared (Scheme 1) by a two stage process. Conjugation of excess Girard's reagent T (1) onto P1 through hydrazone bonds drives the acylhydrazone formation equilibrium to completion and produces a water-soluble polymer (P2). Upon addition of a second acylhydrazide derivative (2), the polymer undergoes component exchange to produce the PS-DCL which is composed of an inter-converting mixture of polymers adorned with varying amounts of the residues 1 and 2. All experiments were performed using 50 mM concentrations of 1 and 2 in buffered D2O (NH4OAc/AcOH pH 4.5). The composition of the residues conjugated to the polymer scaffolds cannot be monitored directly by 1H NMR spectroscopy because the diagnostic signals corresponding to conjugated residues overlap. Instead, we determined the residual composition indirectly by using 1H NMR spectroscopy to measure the relative concentrations of unconjugated residues 1 and 2 in solution, thus allowing the residual composition upon the polymer scaffolds to be ascertained. Equilibrium was reached after 16 h, with 1H NMR spectroscopy revealing both unconjugated acylhydrazides were present in solution in a 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 ratio, implying the residual composition upon the polymer scaffolds is also 1.0
1.0 ratio, implying the residual composition upon the polymer scaffolds is also 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. No aldehyde signal was observed, indicating that the polymer is fully functionalized with acylhydrazone residues. The PS-DCL composition was monitored over a period of 48 h, with no further deviation from this composition observed. This observation suggests that in the absence of any template, the polymer scaffold displays no particular preference for the incorporation of either residue 1 or 2.
1.0. No aldehyde signal was observed, indicating that the polymer is fully functionalized with acylhydrazone residues. The PS-DCL composition was monitored over a period of 48 h, with no further deviation from this composition observed. This observation suggests that in the absence of any template, the polymer scaffold displays no particular preference for the incorporation of either residue 1 or 2.
 | ||
| Scheme 1 Preparation of a PS-DCL. | ||
We hypothesized that a macromolecular template with the capacity to participate in multivalent interactions with a polymeric receptor would be most suited to our system and so a 70 kDa poly(sodium-4-styrene sulphonate) and the protein Bovine Serum Albumin (BSA) were chosen as macromolecular templates. Upon addition of poly(sodium-4-styrene sulphonate),‡ changes in the residual composition of the PS-DCL as a function of time were monitored by 1H NMR spectroscopy, which revealed (Fig. 1) an increase in the ratio of free acylhydrazide 2 relative to 1 of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 from an initial ratio of 1.0
1.0 from an initial ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. This observation suggests that the PS-DCL has responded to the addition of the template, re-equilibrating to incorporate a greater proportion of residue 1 onto the polymer scaffold and rejecting residue 2 (Fig. 2). We postulate that the observed templating effect is a consequence of favourable multivalent ion-ion interactions between residues of 1 conjugated to the polymer scaffold and the poly(sodium-4-styrene sulphonate) template.
1.0. This observation suggests that the PS-DCL has responded to the addition of the template, re-equilibrating to incorporate a greater proportion of residue 1 onto the polymer scaffold and rejecting residue 2 (Fig. 2). We postulate that the observed templating effect is a consequence of favourable multivalent ion-ion interactions between residues of 1 conjugated to the polymer scaffold and the poly(sodium-4-styrene sulphonate) template.
 | ||
| Fig. 1 1H NMR spectroscopic analysis (500 MHz, D2O, pH 4.5) of PS-DCL before (t = 0 h) and after (t = 17 h) addition of poly(sodium-4-styrene sulphonate) highlighting the changes in intensity of the diagnostic signals within the residues 1 and 2 17 h after the addition of poly(sodium-4-styrene sulphonate) template. | ||
 | ||
| Fig. 2 Effect of addition of poly(sodium-4-styrene sulphonate) to a PS-DCL upon the concentrations of unconjugated 1 and 2 as a function of time (diamonds). There is no observed in change in the ratio of 1 and 2 in the absence of template (squares) or in the absence of polymer (triangles). | ||
The templating effect of BSA upon the PS-DCL was also investigated. BSA has an isoelectronic point (pI) of 5.518 indicating its surface is positively charged under the experimental conditions. Upon addition of BSA, 1H NMR spectroscopy revealed an increase in the concentration of unconjugated acylhydrazide 1 relative to 2 of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 from an initial ratio of 1.0
0.8 from an initial ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (Fig. 3). This observation suggests the PS-DCL has re-equilibrated to incorporate a greater proportion of residue 2 onto the polymer scaffold and rejected residue 1. We postulate that this templating effect may be a result of favourable ion–dipole interactions between BSA and ethylene glycol residues on the polymer scaffold, or in avoidance of unfavourable cation–cation interactions between BSA and residues of 1 present on the polymer scaffold. To verify that the effects observed were indeed as a consequence of the templating effects of poly(sodium-4-styrene sulphonate) or BSA, control experiments were performed. For PS-DCLs to which no template was added, the library maintained a 1.0
1.0 (Fig. 3). This observation suggests the PS-DCL has re-equilibrated to incorporate a greater proportion of residue 2 onto the polymer scaffold and rejected residue 1. We postulate that this templating effect may be a result of favourable ion–dipole interactions between BSA and ethylene glycol residues on the polymer scaffold, or in avoidance of unfavourable cation–cation interactions between BSA and residues of 1 present on the polymer scaffold. To verify that the effects observed were indeed as a consequence of the templating effects of poly(sodium-4-styrene sulphonate) or BSA, control experiments were performed. For PS-DCLs to which no template was added, the library maintained a 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 composition of 1 and 2 in solution over a period of 17 h, as monitored by 1H NMR spectroscopy (Fig. 2 and 3). Templates were also added to 50 mM solutions of 1 and 2 in the absence of polymer scaffold and monitored for 17 h by 1H NMR spectroscopy. We observed no differences in chemical shifts or signal broadening, evidence which suggests there are no significant interactions between either template and the acylhydrazides 1 and 2. These observations indicate that the re-equilibriation processes observed are indeed as a consequence of the PS-DCL interacting with the template.
1.0 composition of 1 and 2 in solution over a period of 17 h, as monitored by 1H NMR spectroscopy (Fig. 2 and 3). Templates were also added to 50 mM solutions of 1 and 2 in the absence of polymer scaffold and monitored for 17 h by 1H NMR spectroscopy. We observed no differences in chemical shifts or signal broadening, evidence which suggests there are no significant interactions between either template and the acylhydrazides 1 and 2. These observations indicate that the re-equilibriation processes observed are indeed as a consequence of the PS-DCL interacting with the template.
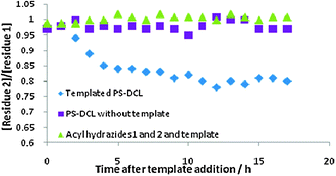 | ||
| Fig. 3 Effect of addition of bovine serum albumin to a PS-DCL upon the concentrations of unconjugated residues 1 and 2 as a function of time (diamonds). There is no observed change in the ratio of 1 and 2 in the absence of template (squares) or in the absence of polymer (triangles). | ||
In conclusion, we have prepared an aqueous PS-DCL in which library members interconvert through acylhydrazone exchange and shown how the exchange of side-chain residues has been observed indirectly using 1H NMR spectroscopy. The PS-DCL has also been shown to respond to the addition of two macromolecular template species in a manner that may be rationalised. It could be argued that the actual effects of templating on the polymer composition are small in comparison to the large amplifications of binders associated with macrocyclic DCLs.1 However, interactions between polymer and macromolecular templates are likely to involve localised regions, analogous to protein-protein binding through ‘hot spots,’ where the substitution of a small number of residues has been observed to drastically reduce binding affinity.19 We are now attempting to establish if the re-equilibration process upon templation leads to an enhancement in binding affinity between the members of the PS-DCL and the templates, and developing more complex PS-DCLs and HPLC methods to study changes in the library during templation. Our strategy could lead to the development of wholly synthetic receptors for the selective recognition of proteins, or to the generation of low-cost antibody mimics.
We wish to thank EPSRC and One North East for considerable support, and acknowledge the assistance of the EPSRC National Mass Spectrometry Service Centre, Swansea. We also thank Dr C. Y. Wills for assistance with 1H NMR experiments.
Notes and references
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS.
- R. L. E. Furlan, Y.-F. Ng, S. Otto and J. K. M. Sanders, J. Am. Chem. Soc., 2001, 123, 8876–8877 CrossRef CAS.
- S. L. Roberts, R. L. E. Furlan, S. Otto and J. K. M. Sanders, Org. Biomol. Chem., 2003, 1, 1625–1633 RSC.
- R. L. E. Furlan, G. R. L. Cousins and J. K. M. Sanders, Chem. Commun., 2000, 1761–1762 RSC.
- G. R. L. Cousins, R. L. E. Furlan, Y. F. Ng, J. E. Redman and J. K. M. Sanders, Angew. Chem., Int. Ed., 2001, 40, 423–428 CrossRef CAS.
- J. M. Klein, V. Saggiomo, L. Reck, M. McPartlin, G. D. Pantos, U. Luning and J. K. M. Sanders, Chem. Commun., 2011, 47, 3371–3373 RSC.
- D. A. Fulton, Org. Lett., 2008, 10, 3291–3294 CrossRef CAS.
- G. Wulff, W. Vesper, R. Grobe-Einsler and A. Sarhan, Makromol. Chem., 1977, 178, 2799–2816 CAS.
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812–1832 CrossRef CAS.
- L. Ye and K. Mosbach, J. Am. Chem. Soc., 2001, 123, 2901–2902 CrossRef CAS.
- C. A. Carlson, J. A. Lloyd, S. L. Dean, N. R. Walker and P. L. Edmiston, Anal. Chem., 2006, 78, 3537–3542 CrossRef CAS.
- S. C. Zimmerman and N. G. Lemcoff, Chem. Commun., 2004, 5–14 RSC.
- S. J. Koch, C. Renner, X. Xie and T. Schrader, Angew. Chem., Int. Ed., 2006, 45, 6352–6355 CrossRef CAS.
- Y. Ura, J. M. Beierle, L. J. Leman, L. E. Orgel and M. R. Ghadiri, Science, 2009, 325, 73–77 CrossRef CAS.
- A. Dirksen, S. Dirksen, T. M. Hackeng and P. E. Dawson, J. Am. Chem. Soc., 2006, 128, 15602–15603 CrossRef CAS.
- B. Levrand, Y. Ruff, J.-M. Lehn and A. Herrmann, Chem. Commun., 2006, 2965–2967 RSC.
- Z. Rodriguez-Docampo and S. Otto, Chem. Commun., 2008, 5301–5303 RSC.
- F. Torrens, G. Castellano, A. Campos and C. Abad, J. Mol. Struct., 2009, 924–926, 274–284 CrossRef CAS.
- T. Clackson and J. A. Wells, Science, 1995, 267, 383–386 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, GPC traces. See DOI: 10.1039/c1cc11998b |
| ‡ Addition of a small aliquot of MeOH-d4 was necessary to prevnt precipitation of the PS-DCL and poly(sodium-4-styrene sulphonate). MeOH-d4 was also added to relevant control reactions. |
| This journal is © The Royal Society of Chemistry 2011 |
