 Open Access Article
Open Access ArticlepH triggered self-assembly of core cross-linked star polymers possessing thermoresponsive cores†
Alexander W.
Jackson
and
David A.
Fulton
*
Chemical Nanoscience Laboratory, School of Chemistry, Bedson Building, Newcastle University, Newcastle upon Tyne, UK. E-mail: d.a.fulton@ncl.ac.uk; Fax: +44 (0)191 222 6929; Tel: +44 (0)191 222 7065
First published on 23rd May 2011
Abstract
Core cross-linked star polymers possessing responsiveness to pH and temperature stimuli have been prepared, and we demonstrate how changes to pH and temperature can be used to trigger the release and uptake of a hydrophobic dye.
Recent years have seen the utilization of dynamic bonds, whether they be non-covalent supramolecular interactions or so-called dynamic covalent bonds, to endow polymeric systems with the abilities to adapt their structures or compositions in response to external stimuli.1 The dynamic covalent bond2 is particularly interesting in this context, as its reversible nature enables polymeric systems incorporating these linkages to modify their architectures by reshuffling, incorporating or releasing their components, in effect providing a mechanism for polymer-based systems to reconfigure their covalent structures and therefore their functional or material properties.3 The strength of the covalent bond also ensures polymeric systems featuring this interaction possess chemical robustness.
We are interested in developing new stimuli-responsive core cross-linked star (CCS) polymers,4 a class of nano-sized polymer assembly whose structural features are defined by a core consisting of cross-linked polymer chains surrounded by a corona of polymeric arms. Much of the interest in CCS polymers is as a consequence of their architectures, possessing cross-linked cores which can be utilized as carriers for small molecules such as drugs or fragrances, and coronal chains which help to solubilize and shield the cargo from its external environment. CCS Polymers also possess solubilities and viscosities similar to linear polymers of relatively low molecular weight, additional properties which make them potentially very useful in a diverse range of fields, most notably drug delivery5 as well as imaging6 and catalysis.7
We describe here dual stimuli-responsive water-soluble CCS polymers which are prepared from cross-linking polymer chains possessing amino and aldehyde functions through the formation of dynamic covalent imine bonds (Fig. 1). These CCS polymers are constructed from linear polymer chains possessing an “inert” block, which prevents macroscopic cross-linking, and a “reactive” block which contains aldehyde or amine functions which facilitate cross-linking of polymer chains through reversible imine bond formation. The reversible nature of this bond allows the position of the imine equilibrium to be modulated by changing the pH, as imine formation is favoured at high pH and disfavoured at low pH.8 Consequently, the assembly and disassembly of aqueous solutions of the CCS polymer can be triggered simply by changing the pH, a process which we demonstrate can lead to the release of hydrophobic dye molecules complexed within the polymer cores. In addition to pH responsiveness, our CCS polymers also possess thermoresponsive cores which endows them with the ability to reversibly switch from hydrophilic to hydrophobic, and we demonstrate here the temperature controlled uptake and release of the same hydrophobic dye.
 | ||
| Fig. 1 Polymers possessing aldehyde and amino groups cross-link at pH 11 to form CCS polymers. Nile Red can be encapsulated within the hydrophobic core of the CCS polymer at 45 °C. The release of Nile Red can be triggered by pH-induced disassembly of the CCS polymer system, or temperature-induced loss of the hydrophobicity of the cross-linked core. Both of these processes are reversible, and the Nile Red can be reencapsulated upon removal of the pH or temperature stimuli. | ||
We prepared (ESI, S3†) our polymer building blocks P1 and P2b using reversible addition–fragmentation chain transfer (RAFT) polymerization.9Polymers P1 and P2b were readily soluble in water, and dynamic light scattering (DLS) studies at 20 °C reveals hydrodynamic radii of 6.6 nm and 6.0 nm respectively, suggesting strongly that these polymers exist as unimolecular species in aqueous solutions and do not form micellar aggregates. This conclusion is supported by 1H NMR spectroscopy (ESI, S8–9†) in D2O of each polymer, which display well-defined signals corresponding to all monomer units, signals which would be expected to diminish in intensity if the polymer chains were packed within the interior of micellar aggregates. As a consequence of their high content of N-isopropylacrylamide monomer units, both polymers display lower critical solution temperatures (LCSTs). The LCST of P1 was measured to be 27 °C, whereas the LCST of the P2b was measured to be 55 °C (ESI, S10†). The differences in measured LCST are simply on account of the relative hydrophobicity of the aromatic aldehyde functions of P1, lowering the LCST, and the hydrophilic amino functions of P2b, which raise the LCST. Above their LCSTs, no evidence was found from DLS for the formation of micellar aggregates as the polymers precipitate from solution.
CCS Polymers were prepared by mixing equimolar solutions of P1 and P2b in water to afford a solution with a total polymer concentration of 1 wt%. The pH was adjusted to 11.0 with small aliquots (5 μL) of 1M NaOH(aq), and the reaction was left to equilibrate for 16 h at room temperature. No precipitation or macroscopic gelation was observed in the reaction mixture, an observation which suggests macroscopic cross-linking is inhibited by the ‘inert’ blocks within the polymer chains. Gel permeation chromatography (GPC) analysis (Fig. 2a) displayed a significant reduction in the peaks corresponding to the diblock copolymers P1 and P2a10 at retention time ∼22.5 min, and the appearance of a major peak indicating the formation of high molecular weight aggregates at ∼14 min. The minor peaks at ∼20 min and ∼23 min are probably as a consequence of small quantities of CCS polymers possessing very low numbers of arms.11 The progress of this reaction was also followed by DLS (ESI, S11†) at 20 °C, which shows the disappearance of unimolecular polymeric species of Rh = 6–7 nm and the formation of a larger monomodal distribution of aggregates possessing Rh = 18.9 nm. Multi-angle laser light scattering (MALLS) experiments (ESI, S12†) were used to estimate the Mw of the CCS polymer assembly to be 2332 kDa, suggesting that the CCS polymers possess on average about 49 arms,12 and the formation of the CCS polymer architecture was supported by determining the structure sensitive ρ parameter (ρ = Rg/Rh) defined by Burchard and co-workers13 which was found to be ∼1.26, which is consistent14 with monodisperse regular star architectures. The formation of CCS polymers was also supported by 1H NMR spectroscopy. The aldehyde signals of P1, which are evident at pH 5.5, disappear at pH 11.0 suggesting that imine bond formation is occurring. Significant reductions in the intensity of the N-isopropylacrylamide signals relative to N,N′-dimethylacrylamide were observed after the pH increased to 11.0 (ESI, S13†), suggesting the N-isopropylacrylamide units are within the interior of the CCS polymer assembly. All of these observations indicate that the polymer chains cross-link through imine bond formation to form CCS polymers, and are consistent with those reported previously by us for the self-assembly of CCS polymers within an analogous organic-soluble system.15
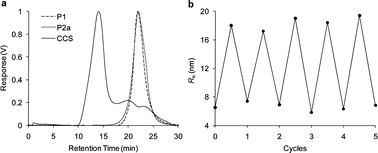 | ||
| Fig. 2 (a) Differential refractive index (dRI) GPC trace (in DMF 0.6 mL min−1) displaying the formation of CCS polymer. (b) pH-Triggered CCS polymer assembly and disassembly, going from pH 5.5 (cycles 0, 1, 2, 3, 4 and 5) to pH 11.0 (cycles 0.5, 1.5, 2.5, 3.5 and 4.5). | ||
The disassembly of the CCS polymers back into their component unimolecular polymer chains was triggered by simply adjusting the pH of the solution to 5.5 with small aliquots (5 μL) of 1 M HCl(aq). After leaving the solution to reequilibriate for 4 h, DLS analysis (ESI, S14†) at 20 °C showed the disappearance of the CCS polymers and the appearance of a monomodal distribution of species possessing hydrodynamic radii of 6–7 nm which corresponds to unimolecular polymer chains. This observation supports the idea that the CCS polymers have disassembled into their component polymer building blocks, a conclusion also supported by GPC analysis (ESI, S15†) which shows the disappearance of the large molecular weight CCS species and the reappearance of the polymer chain building blocks. The disassembly process is entirely reversible, and the ability of this polymeric system to reversibly cycle back and forth between the CCS polymer architecture and single polymer chains was demonstrated (Fig. 2b) by repeatedly switching the pH between 11.0 and 5.5 and monitoring the particle size distribution of the polymer solution by DLS, observations which suggest the system can be switched without any loss in switching efficiency.
To demonstrate that the CCS polymers could be kinetically fixed, the imine bonds were reduced. Excess NaCNBH3 (approx. 100 eq NaCNBH3 per imine bond) was added to a 1% wt solution of CCS polymers at pH 11.0 and the solution was left to stir at room temperature for 2 h. The pH was then adjusted to 5.5 with small aliquots (5 μL) of 1M HCl(aq) and the sample was left overnight. DLS analysis (ESI, S16†) revealed a monomodal particle size distribution whose Rh = 19.4 nm, suggesting that the imine bonds have been successfully reduced to amines, thus preventing the disassembly of the CCS polymer back into its constituent polymer building blocks at low pH.
The position of the imine equilibrium is known to be sensitive to temperature, a factor which might affect the stability of these CCS polymers. To investigate this possibility, a solution of CSS polymers at pH 11.0 were investigated at a range of temperatures (5–50 °C) by DLS analysis (ESI, S17†), displaying near identical hydrodynamic radii across this temperature range.16 This observation confirms that any temperature-induced shift in the position of the imine equilibrium does not lead to the disassembly of CCS polymers. We speculate that because each polymer chain is incorporated into the assembly through multiple imine linkages, then even as the equilibrium shifts away from imine formation, there are still enough imine links present to ensure the integrity of the CCS polymer assembly.
The pH-triggered disassembly was examined further using fluorescence emission spectroscopy and the dye Nile Red as a hydrophobic probe. The CCS polymers possess cores which are composed of copolymers based upon poly-N-isopropylacrylamide, a thermoresponsive polymer which can undergo a reversible phase transition from hydrophilic to hydrophobic at its LCST.17 The possibility therefore exists that above their LCSTs the CCS polymers can reversibly uptake hydrophobic molecules into their cores, and then release these hydrophobes upon the pH-triggered disassembly of the CCS polymers. Nile Red (1 mg per 10 mg of CCS polymer) was added to a 1 wt% solution of CCS polymer at pH 11.0 and the resulting suspension stirred overnight at 45 °C, a temperature above the estimated LCST of the CCS polymer core, followed by filtration to remove excess Nile Red. The obtained solution displayed a bright red/ purple colour (ESI, S18b†), which was analyzed by fluorescence emission spectroscopy to confirm the uptake of Nile Red (Fig. 3a). The pH of the solution was then adjusted to 5.5 to trigger disassembly and the reaction was left overnight at room temperature to re-equilibrate. The solution became noticeably less intense in color (ESI, S18c†) and a purple precipitate was observed. Fluorescence spectroscopy revealed a decrease in the emission intensity (Fig. 3a), observations which suggest the release of Nile Red as the CCS polymer disassembles into its unimolecular polymer chains. The reversibility of this encapsulation process was investigated by fluorescence emission spectroscopy whilst cycling the pH (ESI, S19†), suggesting the complexation and release of hydrophobic Nile Red dye within the core can be triggered over several cycles.
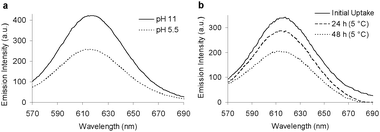 | ||
| Fig. 3 (a) Fluorescence spectra displaying the pH-triggered release of Nile Red from a solution of CCS polymers. (b) Fluorescence spectra as a function of time following the temperature-triggered release of Nile Red from a solution of CCS polymers. | ||
The ability to trigger in response to temperature the uptake and release of guest molecules from the thermoresponsive cores of the CCS polymers was investigated. A sample of CCS polymer containing complexed Nile Red (1 wt%, pH 11.0) was stored at 5 °C for 48 h. After this time a purple precipitate was observed and fluorescence spectroscopy (Fig. 3b) displayed a relative decrease in the emission intensity, suggesting that at low temperatures the core switches from hydrophobic to hydrophilic, releasing Nile Red from the core. The solution was then heated to 45 °C for 24 h and fluorescence spectroscopy (ESI, S20†) confirmed the re-encapsulation of Nile Red. These observations suggest the cross-linked cores can reversibly switch from hydrophilic to hydrophobic, and possess the ability to complex then release hydrophobic guest molecules in response to changes in temperature.
In conclusion, we have prepared interesting water-soluble CCS polymer systems possessing pH- and thermoresponsiveness, and demonstrated how both of these stimuli can be used to trigger the release and uptake of a hydrophobic dye molecule. We are currently investigating the kinetics of guest uptake and release by the assembly-disassembly and hydrophilic-hydrophobic mechanisms, and investigating the capacities of the CCS polymers to encapsulate different guests.
Research Councils UK (fellowship to DAF), Newcastle University (Studentship to AWJ) and One North East are gratefully thanked for support.
Notes and references
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2011, 10, 14–27 CrossRef CAS.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef.
- (a) J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814–831 CrossRef CAS; (b) J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151–160 RSC.
- (a) H. Gao and K. Matyjaszewski, Prog. Polym. Sci., 2009, 34, 317–350 CrossRef CAS; (b) A. Blencowe, J. F. Tan, T. K. Goh and G. G. Qiao, Polymer, 2009, 50, 5–32 CrossRef CAS.
- J. T. Wiltshire and G. G. Qiao, Aust. J. Chem., 2007, 60, 699–705 CrossRef CAS.
- K. Fukukawa, R. Rossin, A. Hagooly, E. Pressly, J. Hunt, B. Messmore, K. Wooley, M. Welch and C. J. Hawker, Biomacromolecules, 2008, 9, 1329–1339 CrossRef CAS.
- (a) T. Terashima, M. Kamigaito, K.-Y. Baek, T. Ando and M. Sawamoto, J. Am. Chem. Soc., 2003, 125, 5288–5289 CrossRef CAS; (b) B. Helms, S. J. Guillaudeu, Y. Xie, M. McMurdo, C. J. Hawker and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2005, 44, 6384–6387 CrossRef CAS; (c) T. Terashima, M. M. Ouchi, T. Ando, M. Kamigaito and M. Sawamoto, Macromolecules, 2007, 40, 3581–3588 CrossRef CAS.
- C. Godoy-Alcántar, A. K. Yatsimirsky and J.-M. Lehn, J. Phys. Org. Chem., 2005, 18, 979–985 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 59, 669–692.
- GPC Analysis was performed on polymer 2a (the N-Boc derivative of 2b) as polymer 2b, like many polyacrylamides does not chromatograph well.
- Previous work by us has identified two-armed core cross-linked species as a minor by-product of CCS polymer formation by the approach used here: see ref. 15.
- Calculated by dividing Mw for CCS polymers by the average Mw of P1 and P2b.
- P. Lang, W. Burchard, M. S. Wolfe, H. J. Spinelli and L. Page, Macromolecules, 1991, 24, 1306–1314 CrossRef.
- W. Burchard, M. Schmidt and W. H. Stockmayer, Macromolecules, 1980, 13, 1265–1272 CrossRef CAS.
- A. W. Jackson and D. A. Fulton, Chem. Commun., 2010, 46, 6051–6053 RSC.
- The hydrodynamic radius of the CCS polymer would be expected to ‘shrink’ as the core passes through its LCST. We speculate that no ‘shrinking’ of the CCS polymer assembly is observed on account of the relatively high cross-linking density of the core, making the core rigid, and the relatively small size of the core, meaning any ‘shrinkage’ may not be within the limits of detection of dynamic light scattering.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, reaction schemes, spectroscopic data, GPC traces, temperature-turbidity curves, MALLS data and DLS analysis. See DOI: 10.1039/c1cc11785h |
| This journal is © The Royal Society of Chemistry 2011 |
