Bacteria-based AND logic gate: a decision-making and self-powered biosensor
Zhongjian
Li
a,
Miriam A.
Rosenbaum
a,
Arvind
Venkataraman
a,
Tsz Kin
Tam
b,
Evgeny
Katz
b and
Largus T.
Angenent
*a
aDepartment of Biological and Environmental Engineering, Cornell University, 214 Riley-Robb Hall, Ithaca, NY 14853, USA. E-mail: la249@cornell.edu; Fax: +1 607-255-4449; Tel: +1 607-255-2480
bDepartment of Chemistry and Biomolecular Science, Clarkson University, Potsdam, NY 13699, USA. Fax: +1 315-268-6610; Tel: +1 315-268-4421
First published on 4th January 2011
Abstract
We developed a bacteria-based AND logic gate using a Pseudomonas aeruginosa lasI/rhlI double mutant with two quorum-sensing signaling molecules as the input signals. We showed a distinct electrical output signal, despite the complexity and continuous regulation of metabolic reactions of living cells.
Here, we present bioelectrochemical systems (BESs) based on the electrochemical activity of living bacterial cells to control the electric current generation via built-in biomolecular Boolean logic operations. Biomolecular computing is a next-generation, complex computing approach (i.e., unconventional computing), which is still in its infancy.1 However, enzymatic BESs (e.g., biofuel cells) in which enzymatic reactions were the core parts of various Boolean logic gates (i.e., AND, OR, XOR) to process biochemical input signals by defined chemical reactions have been intensively studied.2–4 In this communication, we report, for the first time, a bioelectrochemical AND logic gate completely based on bacterial signal transduction. Different from enzymatic logic gates, the input signals are processed by living bacteria with all their regulatory and metabolic complexity.
Microbial BESs harbor electrochemically-active bacteria at electrodes to catalyze oxidation and reduction reactions. When a naturally-occurring potential difference between, for example, an anaerobic microbial anode and an aerobic cathode is maintained, electric power can be generated with such BESs (i.e., microbial fuel cells [MFCs]).5 For research purposes, or to foster anaerobic reduction reactions at the cathode, an artificial potential is applied to increase the potential difference between anodes and cathodes.6 By maintaining an appropriate potential at the anode to support a community of microbes that oxidize organic material in wastewater and by artificially elevating the potential at the cathode to reduce CO2, BES has been hailed as a sustainable technology to treat wastewater with low greenhouse gas emissions.7 While three separate MFCs have been electronically connected to provide a Boolean logic output,8 direct bacterial signal transduction in microbial BESs has never been used as a logic system. Boolean AND logic gates based on whole cells of bacteria that were responding to external conditions in an analog mode by, for example, fluorescence signals have been reported.9-11 Here, we report a bacteria-based AND logic gate with a digital response through an electrochemical signal output.
We built a bacteria-based AND logic gate using potentiostatically-controlled and self-powered BESs with a Pseudomonas aeruginosa (PA 14) lasI/rhlI double mutant as the electrochemically-active bacterium. The las and rhl cascade systems are important regulatory systems of cell–cell communication (i.e., quorum sensing [QS]) for P. aeruginosa.
QS is usually defined as a cell-density-dependent regulation system via secreted signaling factors.12 For the P. aeruginosa wildtype strain, the two QS signaling molecules, 3-oxo-dodecanoyl homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), are self-secreted by the LasI and RhlI inducers, respectively. Upon high enough cell density, these secreted compounds are sensed by the LasR and RhlR receptors, respectively, to initiate a concerted cell-response strategy for pathogenesisvia the production of various extracellular virulence factors (e.g., for cystic fibrosis).13 One of the important gene systems under the control of the las/rhl regulatory system is the phzoperon, which codes for the enzymes required for phenazine biosynthesis.14Phenazines are redox-compounds that act as a reversible redox mediator for electric current generation in our engineered BESs.15 The presence of 3-oxo-C12-HSL and C4-HSL initiates the QS cascade, resulting in current generation in BES (Scheme 1).16 The lasI/rhlI double mutant cannot synthesize 3-oxo-C12-HSL and C4-HSL. Therefore, using this double mutant provided us with a bacterial system that is stimulated by biochemical signals.
 | ||
| Scheme 1 Simplified illustration of homoserine lactone-controlled phenazine synthesis by P. aeruginosa. | ||
The bacteria-based AND logic gate was implemented in two different BESs: (1) a potentiostatically-controlled BES (0.3 V vs.Ag/AgCl; VSP, Bio-Logic USA, Knoxville, TN); and (2) a self-powered MFC. For the former BES, we used a temperature-controlled and sealed glass vessel (300 mL, 37 °C), containing a three-electrode setup: a working electrode (carbon fiber cloth with a geometric surface area of 162 cm2, PANEX® 30: PW06, Zoltek, St. Louis), a counter electrode (graphite rod), and a reference electrode (Ag/AgCl sat. KCl). For the latter BES, we used an H-type MFC containing a Nafion 117 membrane (23 cm2), separating the anode and cathode chambers (220 mL). Both the MFC anode and cathode were made out of the carbon fiber cloth that was similar to the working electrode in the potentiostatically-controlled BES. The three-electrode system and the anode chamber of the MFC were operated with AB bacterial minimum medium (pH 6.8, 8 mM (NH4)2SO4, 21 mM Na2HPO4, 11 mM KH2PO4, 26 mM NaCl, 0.39 mM Na2SO4, 0.1 mM CaCl2, 2 μM FeCl3, 30 mM glucose) under oxygen-limited conditions (no active aeration, but headspace was open to atmosphere via a sterile vent filter). The cathode chamber of the MFC was filled with 0.1 M phosphate buffer solution, containing 0.1 M potassium ferricyanide as a terminal electron acceptor. The P. aeruginosa lasI/rhlI mutant was pregrown in Luria-Bertani (LB) medium. 3-oxo-C12-HSL and C4-HSL were applied as input signals. Statistical analysis (a 2-factorial, 2-leveled ANOVA analysis) was performed using Minitab 16 (Minitab Inc., State College, PA).
To build the AND logic gate using the potentiostatically-controlled BES, the absence of 3-oxo-C12-HSL or C4-HSL was considered as logic 0, while their presence at operational concentration (15 μM) was considered as logic 1. The output was defined as 1 (TRUE) when the current produced by the BES was above the threshold value of 0.29 mA and 0 (FALSE) when it was below 0.29 mA (Fig. 1). Application of the input 0,0 did not activate the global gene regulation system due to the absence of both QS signals. Thus, the expression of the phzoperon was not upregulated, resulting in the absence of phenazines, and therefore in a low current of 0.04 ± 0.01 mA. In the presence of both 3-oxo-C12-HSL and C4-HSL (input 1,1), the las and the rhl systems were activated. Consequently, the expression of the phzoperon was fully upregulated by the QS system, which resulted in the highest phenazine production. The resulting current generation was higher than 0.29 mA (0.42 ± 0.06 mA), and therefore the BES generated the output 1. In the presence of only C4-HSL (input 0,1), the rhl system was activated, and the phenazine production was somewhat upregulated, leading to a current generation of 0.22 ± 0.03 mA. With only 3-oxo-C12-HSL (input 1,0), the las system was activated, but the level of upregulation of phenazine production by the las system was lower than by the rhl system (las and rhl work in a cascade mechanism; Scheme 1). For that reason, the current was lower for the input 1,0 compared to 0,1. The current of either of these signal combinations was not high enough to meet the threshold of 0.29 mA, thus, resulting in the output 0 (99.0% confidence). Therefore, the feature of the double mutant-based sensing system corresponds to the equivalent circuit of the AND logic gate (Fig. 1). We confirmed the concerted reaction of the two input signals in the double mutant (leading to AND gate activation) with a 2-factorial, 2-leveled ANOVA analysis (p = 0.001).
 | ||
| Fig. 1 (A) Phenazine-based current production in potentiostatically-controlled BESs for four different input combinations; standard deviation from at least triplicate experiments. The dashed line shows the threshold that separates output 0 and 1. (B) Truth table for the bacteria-based AND logic gate. (C) Equivalent circuit of an AND logic gate. | ||
The same logic operations for the AND logic gate were used in the self-powered MFC for which the power density was logically controlled by phenazine regulation. We used maximum power densities (MPDs), which are obtained from polarization curves, to determine the output. The highest MPDs for all MFC tests were obtained for input 1,1 (1.69 ± 0.41 mW m−2, Fig. 2). The threshold of the Boolean logic AND gate for our MFC system was 1.19 mW m−2 (88.9% confidence). The confidence interval for our self-powered biosensor is lower than for our potentiostatically-controlled BES because the batch-mode MFCs showed experimental limitations (ferricyanide diffusion and accumulation in the anode chamber negatively affected bacterial performance and, therefore, the resulting MPDs). Even under these limitations, however, the ANOVA analysis confirmed the concerted interaction of our input signals (p = 0.015).
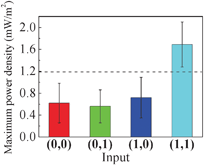 | ||
| Fig. 2 Phenazine-based MPDs in MFCs for four different input combinations; standard deviation from quadruplicate experiments. The dashed line shows the threshold that separates output 0 and 1. | ||
Bacterial metabolic reactions are very complex. They are comprised of constant changes in multiple gene expression and protein levels. Despite this complex metabolic network, we showed that two simple input signals were converted into one clear digital output signal. However, the time scale of this bacteria-based biocomputing system is long (∼115 h). The MFC integration with a logic gate has the potential to fit the requirement of a self-powered and decision-making biosensor. P. aeruginosa is an opportunistic pathogen, which infects humans, other animals, and plants. Based on the detection and biomolecular computing of two chemical input signals, our work demonstrated the feasibility of developing a biosensor for the detection or long-term monitoring of P. aeruginosa infections (e.g., in in vitro human tissue of the respiratory tract) viahomoserine lactone sensing. Other highly promising applications for bacteria-based biocomputing systems may be the monitoring and controlling of fermentation, wastewater treatment, or remediation processes, possibly with other electrochemically-active bacteria.
This work was supported through NSF Career grant # 0939882 to L.T.A. Z.L. gratefully acknowledges the Chinese Scholarship Council for providing a research scholarship for his stay at Cornell University. The authors thank Dr Deborah A. Hogan at Dartmouth Medical School for strain acquisition.
Notes and references
- E. Katz and V. Privman, Chem. Soc. Rev., 2010, 39, 1835–1857 RSC.
- M. Zhou, Y. Du, C. G. Chen, B. L. Li, D. Wen, S. J. Dong and E. K. Wang, J. Am. Chem. Soc., 2010, 132, 2172–2174 CrossRef CAS.
- T. K. Tam, G. Strack, M. Pita and E. Katz, J. Am. Chem. Soc., 2009, 131, 11670–11671 CrossRef CAS.
- L. Amir, T. K. Tam, M. Pita, M. M. Meijler, L. Alfonta and E. Katz, J. Am. Chem. Soc., 2009, 131, 826–832 CrossRef CAS.
- Z. He, S. D. Minteer and L. T. Angenent, Environ. Sci. Technol., 2005, 39, 5262–5267 CrossRef CAS.
- T. H. Pham, P. Aelterman and W. Verstraete, Trends Biotechnol., 2009, 27, 168–178 CrossRef CAS.
- K. Rabaey and R. A. Rozendal, Nat. Rev. Microbiol., 2010, 8, 706–716 Search PubMed.
- J. Greenman, I. Ieropoulos and C. Melhuish, Int. J. Unconv. Comput., 2008, 4, 23–32 Search PubMed.
- K. I. Ramalingam, J. R. Tomshine, J. A. Maynard and Y. N. Kaznessis, Biochem. Eng. J., 2009, 47, 38–47 CrossRef.
- A. Goñi-Moreno, M. Redondo-Nieto, F. Arroyo and J. Castellanos, Nat. Comput, 2000 DOI:10.1007/s11047-010-9184-2.
- A. Tamsir, J. J. Tabor and C. A. Voigt, Nature, 2010 DOI:10.1038/nature09565.
- P. Williams and M. Camara, Curr. Opin. Microbiol., 2009, 12, 182–191 CrossRef CAS.
- C. Winstanley and J. L. Fothergill, FEMS Microbiol. Lett., 2009, 290, 1–9 CrossRef CAS.
- L. E. P. Dietrich, A. Price-Whelan, A. Petersen, M. Whiteley and D. K. Newman, Mol. Microbiol., 2006, 61, 1308–1321 CrossRef CAS.
- A. Price-Whelan, L. E. P. Dietrich and D. K. Newman, Nat. Chem. Biol., 2006, 2, 71–78 CrossRef CAS.
- A. Venkataraman, M. Rosenbaum, J. B. A. Arends, R. Halitschke and L. T. Angenent, Electrochem. Commun., 2010, 12, 459–462 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
