On/off luminescence vapochromic selective sensing of benzene and its methylated derivatives by a trinuclear silver(I) pyrazolate sensor†
Manal A.
Rawashdeh-Omary
*a,
Maher D.
Rashdan
a,
Shylaja
Dharanipathi
a,
Oussama
Elbjeirami‡
a,
Prashanth
Ramesh
b and
H. V. Rasika
Dias
*b
aDepartment of Chemistry and Physics, Texas Woman's University, Denton, Texas, 76204, USA. E-mail: momary@twu.edu
bDepartment of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, Texas, 76019, USA. E-mail: dias@uta.edu
First published on 17th November 2010
Abstract
{[3,5-(CF3)2Pz]Ag}3 (1) films exhibit selective/reversible sensing of small-organic-molecule (SAM) vapors, which readily switch-on bright-green (benzene or toluene) or bright-blue (mesitylene) luminescence that switches-off upon vapor removal. Vapors of electron-deficient SAMs or non-aromatic solvents did not attain luminescence switching and were not adsorbed.
SAM vapors are hazardous and highly regulated, some even carcinogenic (e.g., benzene). In the USA, the exposure daily limit for benzene vapor is as low as 0.1 ppm.1 Monitoring exposure of workers to benzenes is technically challenging. The current accepted practices mostly involve air-sampling pumps packed with adsorbents, which are later extracted for chromatographic analysis of benzene content. Recent sensing devices have been based on diode-laser IR absorption spectroscopy, metal oxide semiconductor films, and cross-reactive array “electronic nose” sensing.2,3 While many good analytical techniques exist for benzene levels, their relative expense and inconvenience make the discovery of materials that sense SAMs in a reversible manner with high selectivity and sensitivity a highly-coveted goal. Whereas significant work on molecular SAM sensors has involved relatively expensive, Pt(II)-3 or Au(I)-4based materials, it is surprising that Ag-based complexes have not been contemplated given the well-known affinity of Ag(I) to SAMs.5 Complex {[3,5-(CF3)2Pz]Ag}3 (1) is strongly π acidic, thus readily forms crystalline solvates with benzene and its methylated derivatives.6 Here we communicate luminescence on/off switching and sorption properties upon exposure of non-luminescent thin films of 1 to vapors of benzene, toluene, and mesitylene. The photophysical data are correlated with the structures of the crystalline solvates (Chart 1) and corresponding thin films to understand the sensing mechanism and spectral assignment.
 | ||
| Chart 1 Molecular structure of 1 and two limiting packing motifs for its binary adducts with benzene (middle) and mesitylene (right). | ||
The synthesis of 15e and its π-acid/π-base binary adducts with the SAMs herein6b,d followed literature procedures. The photoluminescence (PL) of unsolvated crystals of 1 was reported at cryogenic temperatures (non-luminescent at room temperature, RT),6a whereas PL characterization of the SAM adducts of 1 has not been described hitherto. Fig. 1a–c show the PL response of unsolvated thin films cast from solutions of 1 in dichloromethane on quartz plates upon exposure and removal of SAM vapors. Immediately after exposure to benzene or toluene vapor, the non-luminescent dry thin film starts to exhibit bright-green PL whereas mesitylene vapor causes bright-blue PL; the peak maxima are ca. 520, 515, and 410 nm, respectively. As shown in Fig. 1a, the intensity of the green PL continues to increase upon increased exposure time of 1 to benzene vapor while subsequent air-drying of the same film results in gradual intensity decrease. Similar changes are seen by toluene and mesitylene exposure (Fig. 1b and c and ESI†). The greater the exposure of the sensor film to the SAM the longer it takes for the PL signal to completely disappear by air-drying, while vacuum- or heat-drying accelerates the process. The on/off process can be repeated multiple cycles; we repeated the experiment up to 20 times without observing any decomposition or compromise in the switching action if the drying step is done via air drying or vacuum. Repeated heating, however, leads to film degradation. The sensitivity to the presence or absence of trace solvent is remarkable. One can determine during reaction workup of 1 the extent of drying needed to completely remove benzene, which is the solvent used in the synthetic procedure.5e,6a Indeed, the phenomenon was discovered when one group of inorganic lab students at TWU attained the expected non-luminescent dry product while another group attained a green-luminescent product that would not have been completely dry; further drying of that sample attained the non-luminescent product. Luminescent crystals of SAM solvates of 1 can be obtained simply by slow evaporation of a solution made by dissolving the non-luminescent dry product in the SAM.6
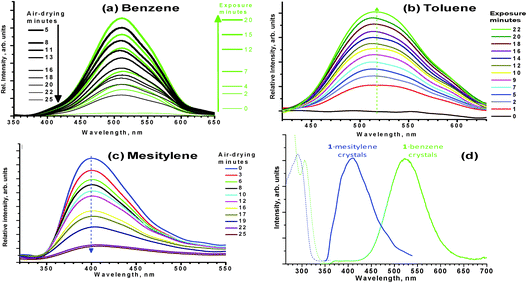 | ||
| Fig. 1 Luminescence vapochromism of a dry film of 1 upon interaction with benzene (a), toluene (b), and mesitylene (c) vapors, and PL spectra at RT (d; dashed/solid = excitation/emission) for crystals of mesitylene and benzene solvates of 1 (Chart 1). More spectral data are in the ESI.† | ||
Fig. 1d shows PL spectra for crystals whose structures had been verified at the single crystal level viaX-ray diffraction to be [benzene·[1]2·benzene] (i.e., BAAB discrete tetramolecular units; A = π-acid, B = π-base) and {1·mesitylene}∞ (i.e., {BABA}∞ extended chains). At RT, these two crystals exhibit the same bright green and bright blue PL as those seen upon exposing a non-luminescent film of 1 to the corresponding vapor. Interestingly, low-temperature data for [benzene·[1]2·benzene] crystals show both the blue and green bands together; they could be resolved from one another either by excitation wavelength selection or by time resolution (ESI†). The blue emission seen for [benzene·[1]2·benzene] crystals at cryogenic temperatures is remarkably similar in profile, excitation spectra, and lifetime to the blue emissions seen at all temperatures for the {1·mesitylene}∞ crystals. The correlation between photophysical data here and the adduct structures suggests that the blue emissive states correspond to *[BA] localized excitons, as opposed to *{BABA}∞ delocalized states, whereas the green emissive states likely correspond to *[BAAB] excitons. All emissions have long-lived lifetimes in the μs–ms regimes, suggesting phosphorescence and unity intersystem crossing. However, the phosphorescence bands are not assignable to T1 state emission of SAMs, as in systems reported by Haneline and Gabbaï.7 For example, the (0,0) transition of the T1 emission of benzene is in the UV region at 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 cm−1 (ca. 338 nm), far blue-shifted from the visible emissions seen here; substitution leads to only small energy shifts.8 The blue emissions here are associated with ms lifetimes at RT vs. μs for the green emissions. The green emissions have lower-energy excitations, due to dimerization of [BA] units via Ag–Ag interactions in the [BAAB] units.
510 cm−1 (ca. 338 nm), far blue-shifted from the visible emissions seen here; substitution leads to only small energy shifts.8 The blue emissions here are associated with ms lifetimes at RT vs. μs for the green emissions. The green emissions have lower-energy excitations, due to dimerization of [BA] units via Ag–Ag interactions in the [BAAB] units.
The trends of photophysical parameters for solvated single crystals extrapolate to SAM-exposed thin films. Thus, films exposed to benzene vapor attained green PL with (lifetime, λexc) values of (2.9 μs, 310 nm) vs. (18 μs, 310 nm) for [benzene·[1]2·benzene] crystals; likewise, films exposed to mesitylene vapor attained blue PL with (0.74 ms, 290 nm) vs. (4.1 ms, 290 nm) for {1·mesitylene}∞ crystals. Toluene vapor-exposed films exhibit (0.95 μs, 310 nm), qualitatively similar to benzene vapor-exposed films. The shorter lifetimes for the green emissions are likely due to increased metal character in the dimeric excited state. Purely *Ag–Ag excimeric excited states are known to have short μs lifetimes9 whereas external or internal Ag heavy-atom effects usually lead to ms lifetimes.10 Excitation bands for blue emissions in crystal solvates are red-shifted by ca. 3900 cm−1 from the excitation of 1 alone (ca. 260 nm),6 suggesting ground-state interaction between A and B molecules. Further red shift by ca. 2200 cm−1 results from dimerization of BA units to BAAB due to argentophilic interactions.9 The photophysical data, overall, indicate a sensing mechanism involving ground-state π-acid/π-base charge transfer interactions between the sensor and SAM prior to exciton formation. Benzene and toluene vapors intercalate into already dimerized 1 leading to green phosphorescence from BAAB units, whereas mesitylene vapor disrupts the argentophilic interaction to form blue-emitting segregated AB units. Being a stronger π-base than benzene or toluene, mesitylene has the ability to engage in π acid–base interactions that are sufficiently strong so as to overcome the argentophilic interactions and form AB pairs. On the other extreme, SAMs with electron-withdrawing substituents (e.g., chlorobenzene and hexafluorobenzene) or non-aromatic solvents (e.g., acetone, methanol, and THF) do not switch on the luminescence, consistent with lack of interaction with π-acidic 1. Further luminescence vapochromic data are in the ESI.†
In order to gain further structural insights for incorporation of benzene molecules into the sensor film, XRD data have been acquired. Fig. 2a shows that the main diffraction peak at ∼23° diminishes upon exposing the dry thin film of 1 to benzene vapor as it becomes luminescent, but then reappears upon heat-drying and loss of luminescence of the same film. The crystallite size of the ordered domain is calculated as 192.9 Å using the Scherrer equation11 from the Bragg diffraction angle and full width at half maximum for the ∼23° peak in the native drop-cast film. After benzene exposure then drying to re-generate the same peak, a nearly identical value (192.7 Å) is obtained. We note that the simulated powder XRD patterns of the published structures of single crystals of dry 1vs. its benzene solvate likewise show the presence vs. absence of the same peak at ∼23°, respectively (see ESI†). Therefore, diminishment of this peak upon benzene vapor exposure is consistent with the intercalation of guest molecules into the extended stacks of trimer molecules of 1, assuming that such stacks are responsible for the ordered domain—a reasonable assumption given the large grain size of almost 200 Å. The lower-angle XRD peaks appear in both solvated and unsolvated solids (including polymorphs of each; see ESI†) so they likely represent individual molecules of 1.
 | ||
| Fig. 2 (a; left) XRD pattern of a dry film of 1vs. interaction with benzene vapor. (b; right) Top: TGA for toluene-exposed (green) vs. dry (black) solids of 1. Bottom: adsorption isotherms for a powder sample of 1 upon exposure to toluene or chloroform. Further related data are in the ESI.† | ||
We also studied the solvent vapor sorption behavior of 1. Thus, Fig. 2b shows thermogravimetric analysis (TGA) data for a phosphorescent solid that had been exposed to toluene (top trace) as well as the adsorption isotherms for a solid powder of 1 upon exposure to toluene and chloroform vapors (bottom trace). The green-phosphorescent film gradually loses its toluene content upon heating and a total loss is attained at ca. 110 °C, corresponding to the boiling point of toluene. Further heating to temperatures > 200 °C leads to melting and decomposition of the phosphorescent solid in a manner similar to that of the non-luminescent dry form of 1; see ESI.† On the other hand, the dry powder of 1 gradually uptakes toluene vapor in more than one adsorption step, reaching an initial plateau at ∼42–45 mg g−1, then a second plateau at ∼66–68 mg g−1, the latter corresponding to ∼0.7 toluene molecules per formula unit of 1. We note that the X-ray crystal structure of the binary adduct of 1 with toluene is known and it features {[1]2·toluene}∞ (i.e., {AAB}∞ extended chains).6d Further increase in relative pressure of toluene vapor leads to much larger weight gains (see ESI†) attributed to condensation on the surface of the crystalline powder of 1, similar to observations by the Yaghi, Qiu, and Gabbaï groups.12 The selectivity of vapor uptake is clearly manifest by lack of chloroform uptake by 1 (Fig. 2b), further reinforcing the luminescence data above.
Finally, a comparison is warranted with some precedent SAM sensors. Mann and co-workers reported Pt(II)- and Ru(II)-based sensors that exhibit remarkable vapoluminescent changed upon exposure to benzene vapor; the authors, however, described the changes as slow, with low sensitivity and/or reversibility.3 The Wang13 and Das14groups reported Zn(II)-based sensors whose exposure to benzene vapor led to luminescence quenching and absorption color change, respectively. Ford and co-workers reported luminescence color change for a tetranuclear CuI cluster upon toluene exposure, which was reversed by exposure to n-pentane.15 In all these examples, the SAM lies in crystal voids as opposed to a bona fide interaction with the chromophore and none entails switching on of PL signal like in 1 here. The Fackler group, however, reported opposite observations to those here (i.e., switched off the PL of a π-basicAu trimer upon interaction with π-acidic C6F6).4d
Reversible, selective, fast, and sensitive sensing of benzene and its methylated derivatives is attained by a silver(I)-based sensor. Luminescence is readily switched on upon vapor exposure of the non-luminescent sensor and off upon vapor removal, which is due to π-acid/π-base interactions. This work, therefore, may pave the way for developing a practical sensing device with unrivaled features for monitoring SAMs.
M.A.R.-O. acknowledges funding by DARPA Young Investigator Award W71B7J-7078-B368, departmental Welch Foundation grant, and ACS-PRF award 49511-UNI3, and greatly appreciates Dr Qian Mather (TA Instruments) and Prof. Bruce Gnade and Mr Michael Perez (Univ. Texas- Dallas) for enabling the adsorption isotherm and XRD experiments. H.V.R.D. acknowledges funding his effort by the Welch Foundation (Y-1289) and NSF (CHE-0845321).
Notes and references
- Centers for Disease Control Web site (accessed March 29, 2010): http://www.cdc.gov/niosh/ershdb/EmergencyResponseCard_29750032.html.
- N. S. Lewis, Acc. Chem. Res., 2009, 37, 663 and references therein.
- Examples: (a) S. M. Drew, L. I. Smith, K. A. McGee and K. R. Mann, Chem. Mater., 2009, 21, 3117 CrossRef CAS; (b) K. A. McGee, B. J. Marquardt and K. R. Mann, Inorg. Chem., 2008, 47, 9143 CrossRef CAS.
- Examples: (a) A. Luquin, C. Elosúa, E. Vergara, J. Estella, E. Cerrada, C. Bariáin, I. R. Matías, J. Garrido and M. Laguna, Gold Bull., 2007, 40, 225 CAS; (b) E. J. Fernández, J. M. López de Luzuriaga, M. Monge, M. Montiel, M. E. Olmos, J. Pérez, A. Laguna, F. Mendizábal, A. A. Mohamed and J. P. Fackler Jr., J. Am. Chem. Soc., 2003, 125, 2022 CrossRef CAS; (c) M. A. Mansour, W. B. Connick, R. J. Lachicotte, H. J. Gysling and R. Eisenberg, J. Am. Chem. Soc., 1998, 120, 1329 CrossRef CAS; (d) M. A. Rawashdeh-Omary, M. A. Omary, J. P. Fackler, Jr., R. Galassi, B. R. Pietroni and A. Burini, J. Am. Chem. Soc., 2001, 123, 9689 CrossRef CAS.
- (a) A. E. Hill, J. Am. Chem. Soc., 1921, 43, 254 CrossRef CAS; (b) R. S. Mulliken, J. Am. Chem. Soc., 1952, 74, 811 CrossRef CAS; (c) R. W. Turner and E. L. Amma, J. Am. Chem. Soc., 1966, 88, 3243 CrossRef CAS; (d) A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk and M. Schröder, Coord. Chem. Rev., 2001, 222, 155 CrossRef CAS; (e) H. V. R. Dias, S. A. Polach and Z. Wang, J. Fluorine Chem., 2000, 103, 163 CrossRef CAS.
- (a) M. A. Omary, M. A. Rawashdeh-Omary, M. W. A. Gonser, O. Elbjeirami, T. Grimes, T. R. Cundari, H. V. K. Diyabalanage, C. S. P. Gamage and H. V. R. Dias, Inorg. Chem., 2005, 44, 8200 CrossRef CAS; (b) H. V. R. Dias and C. S. P. Gamage, Angew. Chem., Int. Ed., 2007, 46, 2192 CrossRef CAS; (c) D. M. M. Krishantha, C. S. P. Gamage, Z. A. Schelly and H. V. R. Dias, Inorg. Chem., 2008, 47, 7065 CrossRef CAS and references therein (d) H. V. R. Dias, C. S. P. Gamage, J. Keltner, H. V. K. Diyabalanage, I. Omari, Y. Eyobo, N. R. Dias, N. Reohhr, L. Mckinney and T. Poth, Inorg. Chem., 2007, 46, 2979 CrossRef CAS.
- M. R. Haneline and F. P. Gabbaï, Angew. Chem., Int. Ed., 2004, 43, 5471 CrossRef CAS.
- S. P. McGlynn, T. Azumi and M. Kinoshita, Molecular Spectroscopy of the Triplet State, Prentice Hall, Englewood Cliffs, New Jersey, 1969 Search PubMed.
- M. A. Omary and H. H. Patterson, J. Am. Chem. Soc., 1998, 120, 7696 CrossRef CAS.
- M. A. Omary, O. Elbjeirami, C. S. P. Gamage, K. M. Sherman and H. V. R. Dias, Inorg. Chem., 2009, 48, 1784 CrossRef CAS.
- B. D. Cullity and S. R. Stock, Elements of X-Ray Diffraction, Prentice-Hall, 3rd edn, 2001, pp. 167–171 Search PubMed.
- (a) M. Eddaoudi, H. L. Li and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391 CrossRef CAS; (b) Q. Fang, G. Zhu, M. Xue, J. Sun, F. Sun and S. Qiu, Inorg. Chem., 2006, 45, 3582 CrossRef CAS; (c) T. J. Taylor, V. I. Bakhmutov and F. P. Gabbaï, Angew. Chem., Int. Ed., 2006, 45, 7030 CrossRef CAS.
- J. Pang, E. J.-P. Marcotte, C. Seward, R. S. Brown and S. Wang, Angew. Chem., 2001, 113, 4166 CrossRef.
- S. Das and P. K. Bharadwaj, Inorg. Chem., 2006, 45, 5257 CrossRef CAS.
- E. Cariati, X. Bu and P. C. Ford, Chem. Mater., 2000, 12, 3385 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Further experimental details and movie files showing the vapochromic changes. See DOI: 10.1039/c0cc03964k |
| ‡ Current address: King Fahd University of Petroleum & Minerals, Dahran, Saudi Arabia. |
| This journal is © The Royal Society of Chemistry 2011 |
