Playing the surface game—Diels–Alder reactions on diamond nanoparticles†‡§
Gerald
Jarre
a,
Yuejiang
Liang
a,
Patrick
Betz
a,
Daniel
Lang
a and
Anke
Krueger
*ab
aInstitute for Organic Chemistry, Julius-Maximilian University, Am Hubland, Würzburg, Germany. E-mail: krueger@chemie.uni-wuerzburg.de; Fax: +49 931 318 4606; Tel: +49 931 318 5334
bWilhelm Conrad Röntgen Research Center for Complex Material Systems, Julius-Maximilian University, Am Hubland, Würzburg, Germany
First published on 23rd November 2010
Abstract
Stable covalent C–C bonding of aromatic moieties has been achieved using Diels–Alder reactions on surface-annealed nanodiamond. Subsequent functionalisation leads to tunable surface properties and molecule-like behaviour.
Diamond has long been thought to be an inert and rather unreactive material and its applications were mainly based on its well-known mechanical resistance or its remarkable optical properties. However, in recent years it has been shown, that diamond, both as a CVD-grown film or in the form of nanoparticles has great potential for a variety of applications.1 These include the use in sensing applications such as electrochemistry, fluorescence labeling,2drug delivery,3 magnetic sensing4 and composite materials.5
Most of these applications require a certain termination of the diamond material's surface. In CVD films this is usually achieved by terminating the as-grown film using gas phase reactions directly in the growth chamber.6 But also photochemical reactions7 and subsequent transformations of existing surface groups8 have been used. In the case of nanoscale diamond particles the surface chemistry mostly relies on the existence of surface groups carrying heteroatoms such as oxygen (as in OH and COOH groups),8nitrogen9 or sulfur.10 There are only few examples where a direct C–C coupling of diamond surface atoms with suitable organic reagents has been achieved, although this most stable grafting is highly desirable for the above mentioned applications. Recently, the arylation of diamond nanoparticles using diazonium salts11 as well as subsequent Suzuki coupling have been reported. Furthermore, radical reactions leading to alkylated diamond have been used, too.12
In this report we present a completely new approach to the stable surface grafting of organic moieties onto diamond nanoparticles: the Diels–Alder reaction of o-quinodimethanes onto thermally annealed nanodiamond. So far, this type of reaction has not been reported for diamond. The major application of this reaction is the modification of curved surfaces of fullerenes, where it avoids the otherwise predominant retro-Diels–Alder reaction. The latter is related to the existing curvature of the π-system.13 In preliminary experiments using pretreated nanoscale diamond (see below) and cyclopentadiene or anthracene, respectively, we also observed a rather low coverage with the cyclohexene moieties; especially higher temperatures led to an increased removal due to the retro-Diels–Alder reaction. That is why we decided to turn to the efficient grafting of aromatic rings using o-quinodimethanes (Scheme 1). The latter are generated in situ and lead to the formation of benzene rings, connected to the diamond surface via two methylene bridges. To be able to carry out this reaction, the diamond surface needs at least a partial coverage with π-bonds. As the pristine material (originating from detonation synthesis) in this study1 possesses only small amounts of π-bonds on its surface and carries a variety of oxygenated surface groups (mainly OH, COOH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)1 a thermal annealing step has been carried out. This leads to desorption of the oxygen-containing groups and the formation of π-bonds on the diamond surface.14
O)1 a thermal annealing step has been carried out. This leads to desorption of the oxygen-containing groups and the formation of π-bonds on the diamond surface.14
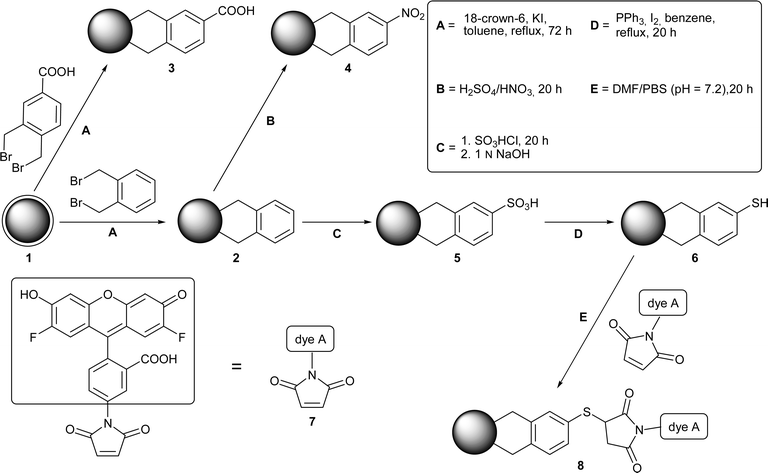 | ||
| Scheme 1 A stable C–C coupling of aromatic moieties with nanodiamond can be achieved by the Diels–Alder reaction of π-bonds on the diamond surface (acting as the dienophile) and an in situ generated o-quinodimethane (acting as the diene). Subsequent reactions on the benzene moieties formed during the grafting enable the flexible functionalisation of the nanodiamond materials. | ||
In order to examine the influence of the annealing temperature, we have used detonation diamond annealed at 1000 °C (1a) and 750 °C (1b), respectively. Both materials show an increased amount of carbon in their elemental composition and no significant peaks in the IR spectrum (except for residual C–H). The XRD data and the HRTEM show no extended graphitic structures on the diamond surface as well as the intactness of the diamond core (see ESI§). However, the annealing significantly changes the reactivity of the material towards dienes. In both cases the Diels–Alder reaction using the stem system is successful with the surface loading in compound 2 (0.28 mmol g−1 and 0.15 mmol g−1) depending on the annealing temperature. This proves the increased formation of π-bonds when the temperature during annealing is increased (in the case of 1a a small signal for graphitic structures is visible in the XRD). Particle sizes in the range of 8–40 nm of the arylated nanodiamond show the complete destruction of residual agglomerates in the arylated material 2.
The same is valid when the COOH-functionalized o-quinodimethane is used. However, the surface loadings with benzoic acid is much higher compared to the non-functionalised system. This can be explained by the improved reactivity of the diene component in the Diels–Alder reaction (due to the electron-withdrawing nature of the COOH group) facilitating the attack of the strained π-bonds on the diamond surface. The grafting of highly hydrophilic units on the diamond particles enables the solubilization in polar media such as water and even PBS buffer (a common cell culture medium). Particle size is in the region of 30–50 nm (for COOH terminated DND in DMSO), close to the size of primary diamond particles with a solvate shell.
Both types of arylated nanodiamond can be further reacted. The benzene-carrying material has been subjected to electrophilic aromatic substitution reactions leading to the nitro compound 4 and the sulfonic acid 5. Both have been identified by several spectroscopic and thermal methods (Table 1, see also ESI§). The respective substitution pattern has been elucidated from the fingerprint region of the infrared spectra (see Fig. S2 and S3, ESI§). In order to distinguish the sulfonation of the initial diamond surface from the aromatic substitution, we have carried out the reaction of annealed diamond. From the analytical data it is obvious that the sulfonation takes place on the aromatic rings and not on the diamond directly (see ESI§).
| Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 2a | 2b | 3a | 3b | 4a | 4b | 5a | 5b | 6a | 6b | |
a 50% value by weight.
b Supernatant in water after centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min.
c Of the relevant mass loss step in TGA.
d Supernatant in DMSO after centrifugation at 15 000 rpm for 15 min.
c Of the relevant mass loss step in TGA.
d Supernatant in DMSO after centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min.
e Measured in water (pH 5.8). 000 rpm for 15 min.
e Measured in water (pH 5.8).
|
||||||||||||
| Particle sizea/nm | — | — | 41d | 8d | 47e | 52e | 53e | 86e | 61d | 41d | 76e | 14e |
| ζ-Potb/mV | — | — | 20.4 | 39.8 | −39.7 | −26.0 | −3.4 | 8.2 | −21.3 | −12.7 | 9.0 | 15.2 |
| Δmc (%) | — | — | 2.9 | 1.7 | 26.9 | 10.6 | 4.4 | 2.1 | 6.0 | 2.2 | 4.0 | 1.9 |
| Surface loading/mmol g−1diamond | — | — | 0.28 | 0.15 | 1.82 | 0.72 | 0.29 | 0.14 | 0.30 | 0.13 | 0.29 | 0.23 |
Finally, we have grafted Oregon Green (7), a fluorescent dye, onto the arylated material carrying sulfonic acid groups (5). In a first step sulfonic acid groups have been reduced to thiol functions (6)15 using PPh3 and iodine. According to XPS data, one third is reduced to SH (162.6 eV) whereas two third of the SO3H groups (166.8 eV) remain unchanged. Only the SH-groups have then been reacted with maleiimide-terminated dye 7 (calculated from TGA and elemental analysis, Fig. 1a–d). The conjugate is soluble in organic media and can be purified by column chromatography (Fig. 1e).
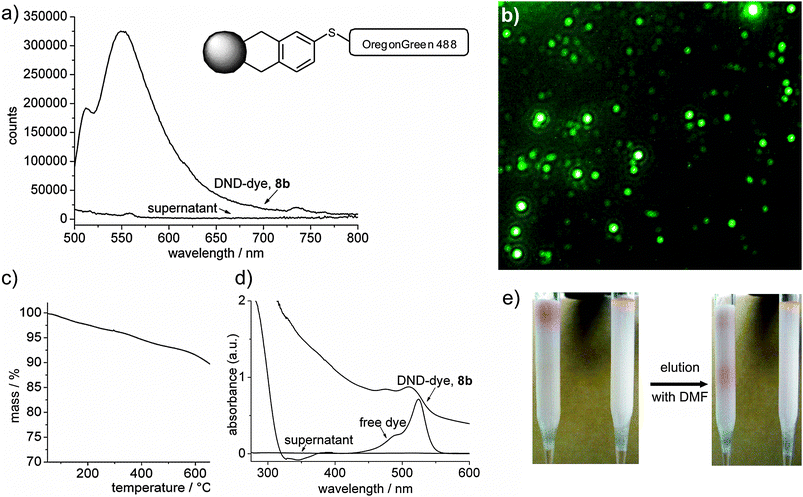 | ||
| Fig. 1 (a) Fluorescence spectrum for a DND–Oregon Green conjugate. No residual luminescence is found in the supernatant solvent after complete precipitation of the conjugate 8; (b) the fluorescence can also be detected in a conventional luminescence microscope for biological studies showing additionally the absence of luminescence from the supernatant medium (overlay with phase contrast image); (c) TGA of DND–Oregon Green (8b); (d) absorption spectra of DND–Oregon Green (8b), free dye and the supernatant solvent; (e) The DND–dye conjugate can be run through a standard column chromatography on alumina: the free dye is not mobile (right, yellow plug on top) whereas the conjugate elutes from the column (left, pinkish front). | ||
In summary, we present here a new and efficient way for the stable grafting of organic moieties onto the surface of diamond nanoparticles using Diels–Alder reactions. We have demonstrated the flexibility of the approach by using different types of o-quinodimethanes and the possibility of subsequent modifications with reactions on the aromatic rings formed during grafting. The material has been modified using a fluorescent dye as a proof of principle. We were also able to purify this diamond–dye conjugate using classical column chromatography, a novelty in the field of nanoscale carbon materials. In the future, we will apply this useful surface modification for the grafting of bioactive moieties to study the application of such diamond conjugates in the field of drug search and delivery.
We acknowledge the financial support of this project by the DFG, the European Commission (contracts EQUIND; Vascubone and DINAMO) and the Fonds der Chemischen Industrie. We thank F. Reinert, A. Schöll and P. Vrdoljak (Phys. Dept. Univ. Würzburg) for XPS measurements, H. Dill (Biochemistry, Univ. Würzburg) for fluorescence microscopy, C. Lambert for access to the fluorescence spectrometer, and N. Stock (Univ. Kiel) for access to a zetapotential analyzer.
Notes and references
- (a) A. Krueger, Chem.–Eur. J., 2008, 14, 1382 CrossRef CAS; (b) A. M. Schrand, S. A. Ciftan Hens and O. A. Shenderova, Crit. Rev. Solid State Mat. Sci., 2009, 34, 18 Search PubMed; (c) V. Y. Dolmatov, Russ. Chem. Rev., 2001, 70, 607 RSC; (d) K. B. Holt, Philos. Trans. R. Soc. London, Ser. A, 2007, 365, 2845 CrossRef CAS; (e) E. Osawa, Diamond Relat. Mater., 2007, 16, 2018 CrossRef CAS.
- C.-C. Fu, H.-Y. Lee, K. Chen, T.-S. Lim, H.-Y. Wu, P.-K. Lin, P.-K. Wei, P.-H. Tsao, H.-C. Chang and W. Fann, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 727 CrossRef CAS.
- (a) R. Lam, M. Chen, E. Pierstorff, H. Huang, E. Osawa and D. Ho, ACS Nano, 2008, 2, 2095 CrossRef CAS; (b) N. Kossowsky, A. Gelman, H. J. Hnatyszyn, S. Raiguru, R. L. Garrell, S. Torbati, S. S. Freitas and G. M. Chow, Bioconjugate Chem., 1995, 6, 507 CrossRef CAS.
- G. Balasubramanian, I. Y. Chan, R. Kolesov, M. Al-Hmoud, J. Tisler, C. Shin, C. Kim, A. Wojcik, P. R. Hemmer, A. Krueger, T. Hanke, A. Leitenstorfer, R. Bratschitsch, F. Jelezko and J. Wrachtrup, Nature, 2008, 455, 648 CrossRef CAS.
- (a) O. Shenderova, T. Tyler, G. Cunningham, M. Ray, J. Walsh, M. Casulli, S. Hens, G. McGuire, V. Kuznetsov and S. Lipa, Diamond Relat. Mater., 2007, 16, 1213 CrossRef CAS; (b) L. Li, J. L. Davidson and C. M. Lukehart, Carbon, 2006, 44, 2308 CrossRef CAS.
- B. Rezek, D.-C. Shin, T. Nakamura and C. E. Nebel, J. Am. Chem. Soc., 2006, 128, 3884 CrossRef CAS.
- T. L. Clare, B. H. Clare, B. M. Nichols, N. L. Abbott and R. J. Hamers, Langmuir, 2005, 21, 6344 CrossRef CAS.
- K. Ushizawa, Y. Sato, T. Mitsumori, T. Machinami, T. Ueda and T. Ando, Chem. Phys. Lett., 2002, 351, 105 CrossRef CAS.
- S. Ciftan Hens, G. Cunningham, T. Tyler, S. Moseenkov, V. Kuznetsov and O. Shenderova, Diamond Relat. Mater., 2008, 17, 1858 CrossRef CAS.
- T. Nakamura, T. Ohana, Y. Hagiwara and T. Tsubota, Phys. Chem. Chem. Phys., 2009, 11, 730 RSC.
- (a) W. S. Yeap, S. Chen and K. P. Loh, Langmuir, 2009, 25, 185 CrossRef CAS; (b) Y. Liang, M. Ozawa and A. Krueger, ACS Nano, 2009, 3, 2288 CrossRef CAS; (c) Y. Liang, T. Meinhardt, G. Jarre, M. Ozawa, P. Vrdoljak, A. Schöll, F. Reinert and A. Krueger, J. Colloid Interface Sci., 2010 DOI:10.1016/j.jcis.2010.10.044 , in press.
- T. Nakamura, M. Ishihara, T. Ohana and Yoshinori Koga, Chem. Commun., 2003, 900 RSC.
- P. Belik, A. Gügel, A. Kraus, M. Walter and K. Müllen, J. Org. Chem., 1995, 60, 3307 CrossRef CAS.
- (a) J. Chen, S. Z. Deng, J. Chen, Z. X. Yu and N. S. Xu, Appl. Phys. Lett., 1999, 74, 3651 CrossRef CAS; (b) A. V. Okotrub, L. G. Bulusheva, V. L. Kuznetsov, Yu. V. Butenko, A. L. Chuvilin and M. I. Heggie, J. Phys. Chem. A, 2001, 105, 9781 CrossRef CAS.
- S. Oae and H. Togo, Bull. Chem. Soc. Jpn., 1983, 56, 3802 CAS.
Footnotes |
| † Dedicated to Prof. Henning Hopf on the occasion of his 70th birthday. |
| ‡ This article is part of the ‘Emerging Investigators’ themed issue for ChemComm |
| § Electronic supplementary information (ESI) available: Experimental procedures, complete spectroscopic data for all materials, fluorescence images. See DOI: 10.1039/c0cc02931a |
| This journal is © The Royal Society of Chemistry 2011 |
