High heat of hydrogen adsorption and guest-responsive magnetic modulation in a 3D porous pillared-layer coordination framework†‡
Arpan
Hazra
,
Prakash
Kanoo
and
Tapas Kumar
Maji
*
Molecular Materials Laboratory, Chemistry and Physics of Materials Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore 560 064, India. E-mail: tmaji@jncasr.ac.in; Fax: +91 80 2208 2766; Tel: +91 80 2208 2826
First published on 19th November 2010
Abstract
A bimetallic pillared-layer coordination framework {[Mn3(bipy)3(H2O)4][Cr(CN)6]2·2(bipy)·4(H2O)}n has been constructed using a cyanometallate anion ([Cr(CN)6]3−) and an organic linker (4,4′-bipyridyl) that provides high heat of hydrogen adsorption (∼11.5 kJ mol−1) and shows guest dependent magnetic modulation.
Metal–organic frameworks (MOFs) or porous coordination polymers (PCPs) are promising materials for applications in gas storage, separation, sensing, catalysis, magnetism, drug delivery, etc.1 Multifunctional materials, i.e. materials which combine a set of well defined properties (e.g. porosity and magnetism, porosity and optical) for specific applications, are gaining importance recently.1a,e,2 Such a synergism, where two different functionalities are combined, would open up the possibility and prospect of finding novel physical phenomena for designing smart materials. Magnetism has generic dependence on distance while the porosity is enhanced with long linkers and hence combination of these two is not always easy or straightforward. A rational concept and design approach would be to choose a polycyanometallate anion, [(MA(CN)n]m− (n = 2–8), as a hub which can link to another metal MB to create a magnetic pathway MB–NC–MA–CN–MB.3 Once the magnetic platform has been constructed, it can be further linked by a long organic pillar (e.g.4,4′-bipyridyl) to invoke porosity in the material. The presence of porosity provides the opportunity to study gas storage property, selectivity as well as guest-dependent magnetism4 and magnetic sensing which are of particular interest for applications such as magnetic devices and sensors (Scheme 1).2b,5 Several fascinating properties like guest-dependent spin crossover,4d,6 magnetic ordering sensitive to guest removal/exchange,7 and different magnetic behaviour corresponding to reversible solvent-induced structural changes8 have been reported in porous magnets. On the other hand, the porous property of MOFs is gaining increased attention with time and the framework materials have emerged as an excellent storage alternative to high pressure and liquefied hydrogen tanks.1a,9 In this respect, recent reports indicate that the presence of unsaturated metal site (UMS) is crucial for strong interaction of H2 molecule and also largely enhances the value of enthalpy of adsorption.10 This report tries to combine various aspects: (i) use of cyanometallate anion [Cr(CN)6]3− as magnetic hub; (ii) use of long linker 4,4′-bipyridyl (bipy) to invoke porosity; (iii) generation of UMSs to enhance enthalpy of hydrogen adsorption, together in a single framework in cyanometallate systems for the first time, to the best of our knowledge (Scheme 1). Our continuous efforts towards bimetallic systems11 have resulted in a new MnIICrIII framework {[Mn3(bipy)3(H2O)4][Cr(CN)6]2·2(bipy)·4(H2O)}n (1) (bipy = 4,4′-bipyridyl) that exhibits guest-dependent magnetic modulation and selective sorption of CO2/H2 over N2. It is noteworthy that the presence of UMSs in the framework leads to a very high value of enthalpy of adsorption for H2 and is close to the highest reported value of 13.5 kJ mol−1.10b
 | ||
| Scheme 1 Bimodal functionality in a framework material. | ||
Light yellow block type single crystals of 1 were grown by slow diffusion of K3[Cr(CN)6] and bipy with MnCl2·4H2O in an EtOH/H2O medium (see ESI).‡ The IR spectrum of 1 shows a band at 2125 cm−1 which corresponds to ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) stretch of terminal cyanide groups. The high frequency bands at 2155 and 2170 cm−1 correspond to the bridging cyanide groups (Fig. S1, ESI‡). X-ray single crystal structure determination reveals that 1 has a neutral 3D coordination framework of MnII built by [Cr(CN)6]3− and bipy with the formulation {[Mn3(bipy)3(H2O)4][Cr(CN)6]2·2(bipy)·4(H2O)}n (Fig. S2, ESI‡). The framework of 1 is isomorphous to our previously reported MnIIFeIII system11 where the 2D layers formed by [Cr(CN)6]3− and MnII are pillared by the bipy linkers to generate a 3D pillared-layer framework with two kinds of channel along the c-axis (Fig. 1, S3 and S4, ESI‡). The channels are occupied by water (both coordinated and guest) and bipy molecules. Upon removal of the coordinated water and guest (water and bipy) molecules framework provides 37.7% void space to the total crystal volume with coordinatively unsaturated MnII centers on the pore surface. Despite of rigid connectivity in three directions, 1 has also significant inter- (H-bonding: Cr1–CN⋯O1w⋯CN–Cr1 and Mn2–O2⋯bipy⋯O2–Mn2) and intralayer (H-bonding: Mn1–O1⋯O2–Mn2 and Mn1–O1⋯CN–Cr1) supramolecular interactions (Fig. S5 and Table S4, ESI‡) that would have pronounced influence on its properties.
N) stretch of terminal cyanide groups. The high frequency bands at 2155 and 2170 cm−1 correspond to the bridging cyanide groups (Fig. S1, ESI‡). X-ray single crystal structure determination reveals that 1 has a neutral 3D coordination framework of MnII built by [Cr(CN)6]3− and bipy with the formulation {[Mn3(bipy)3(H2O)4][Cr(CN)6]2·2(bipy)·4(H2O)}n (Fig. S2, ESI‡). The framework of 1 is isomorphous to our previously reported MnIIFeIII system11 where the 2D layers formed by [Cr(CN)6]3− and MnII are pillared by the bipy linkers to generate a 3D pillared-layer framework with two kinds of channel along the c-axis (Fig. 1, S3 and S4, ESI‡). The channels are occupied by water (both coordinated and guest) and bipy molecules. Upon removal of the coordinated water and guest (water and bipy) molecules framework provides 37.7% void space to the total crystal volume with coordinatively unsaturated MnII centers on the pore surface. Despite of rigid connectivity in three directions, 1 has also significant inter- (H-bonding: Cr1–CN⋯O1w⋯CN–Cr1 and Mn2–O2⋯bipy⋯O2–Mn2) and intralayer (H-bonding: Mn1–O1⋯O2–Mn2 and Mn1–O1⋯CN–Cr1) supramolecular interactions (Fig. S5 and Table S4, ESI‡) that would have pronounced influence on its properties.
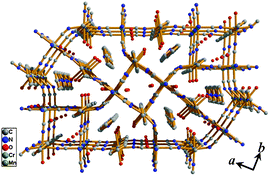 | ||
| Fig. 1 3D view of compound 1 along c direction shows two different type of channels; one square occupied by water molecules and the other distorted triangular occupied by water and guest bipy molecules. | ||
Thermal studies were carried out to analyse the stability and integrity of the framework (Fig. S6, ESI‡). TG analysis under nitrogen flow suggests two-step release of guest and coordinated water molecules (obs. 9.1%; calc. 9.55%) in the temperature range 40–180 °C. However, when 1 is heated at 165 °C under high vacuum (to prepare 1a) we observed the loss of guest bipy together with guest and coordinated water molecules. This was confirmed by IR spectroscopy and elemental analysis (see ESI‡). Powder X-ray diffraction (PXRD) pattern of {[Mn3(bipy)3][Cr(CN)6]2}(1a) recorded after heating the sample at 165 °C under vacuum suggests that the 3D framework structure remains intact even if it sustains loss of water (guest and coordinated) and guest bipy molecules (Fig. S7, ESI‡). However, decrease in intensity of peaks in 1a indicates that crystallinity of the deguest sample decreases.
The desolvated framework 1a was used for gas adsorption studies to establish the permanent porosity of the compound. N2 adsorption at 77 K reveals a type II profile indicating only surface adsorption (Fig. S8, ESI‡). On the contrary, CO2 adsorption measurement at 195 K shows a typical type I profile with a steep uptake at low pressure regions (Fig. 2). The Langmuir surface area calculated from CO2 profile turns out to be 353 m2 g−1. The hysteretic sorption and large isosteric heat of adsorption (qst, ϕ) value of ∼34.3 kJ mole−1 (calculated using Dubinin–Radushkevich equation)12 suggests a strong interaction of CO2 molecules with 1a. This could be correlated with the well established fact that the electric field generated in the framework by UMSs and aromatic π-cloud interacts firmly with the quadrupole moment of CO2 (−1.4 × 10−39 C m2) causing a rapid uptake at low pressure. 1a was also tested for its H2-storage capacity at cryogenic temperature (inset of Fig. 2). High pressure adsorption measurement at 77 K reveals a type I profile with a steep uptake at low pressure and the final uptake volume is ∼81 mL g−1 at ∼45 bar. Interestingly, a careful measurement at 77 K and low pressure (P ≈ 1 atm) (Fig. S8, ESI‡) shows a prominent hysteresis in the adsorption profile suggesting a strong interaction of H2 molecules with the pore surface. The high pressure profiles measured at 77 and 87 K were used for the calculation of enthalpy of adsorption (ΔHads) applying Clausius–Clapeyron equation which provides a value of ∼11.5 kJ mol−1 (Fig. S9–S11, see ESI‡).10a The high value of ΔHads can be linked to the highly polar nature of 1a decorated with cyanide groups (free as well as coordinated) and unsaturated MnII sites (Mn1 and Mn2) which bind H2 molecules strongly in the pore. This is also in line with the hysteresis obtained from the low pressure measurement. It is noteworthy that the value of ΔHads obtained for 1a is one of the highest among the reported values.10 The polar nature of 1a is also reflected in H2O sorption that displays an unusual type I profile (Fig. S12, ESI‡) with a steep uptake at the low pressure region where the framework shows uptake of eight water molecules resulting in {[Mn3(bipy)3][Cr(CN)6]2·8H2O} (1b).
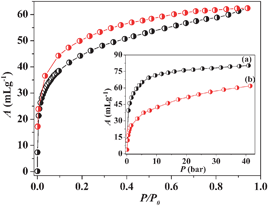 | ||
| Fig. 2 CO2 adsorption isotherm of 1a measured at 195 K. Inset shows the high pressure H2 isotherm (only adsorption) of 1a measured at 77 K (a) and 87 K (b). | ||
We have successively measured magnetic data of as-synthesized 1, 1a and 1b (rehydrated). The dc magnetic susceptibilities are shown in the form of χMvs. T (Fig. S13, ESI‡) and χMT vs. T (1 and 1a) in Fig. 3. The χM value for 1 at 300 K is 0.0531 cm3 mol−1(χMT = 16.17 cm3 mol−1 K) which agrees well with the spin only value (0.0558 cm3 mol−1; 16.74 cm3 mol−1 K) expected for magnetically isolated three MnII (S = 5/2) and two CrIII (S = 3/2) ions. The χMT value gradually decreases with decreasing temperature to reach a minimum value of 14.96 cm3 mol−1 K at 85 K, and then rapidly increases to a maximum value of 2114.96 cm3 mol−1 K at 53 K and then again decreases to 161.65 cm3 mol−1 K at 2.5 K. The 1/χMvs. T plot in the temperature range of 300–150 K obeys the Curie–Weiss law (Fig. S14, ESI‡) with a Weiss constant θ = −39.3 K, which suggests the antiferromagnetic interaction between the adjacent MnII and CrIII ions through cyanide bridges as has already been observed.13 The rapid increase in χMT value indicates a ferrimagnetic ordering, which was determined accurately as 80 K by a dM/dT differential plot (Fig. S15, ESI‡). The decrease in χMT value after 53 K suggests further interaction between the layers through the bipy linker. The magnetic data of 1a also show a typical ferrimagnetic ordering below 50 K and the dM/dT plot suggests that the Tc is about 45.3 K (Fig. S16, ESI‡). The Weiss constant θ was calculated as −59.8 K (300–150 K, Fig. S17, ESI‡) suggesting stronger antiferromagnetic interaction in 1a compared to 1. The change in Weiss constant, Tc and maxima in the χMT value suggest that there are changes in the magnetic pathways in 1a. The value of Tc depends on the overall magnetic interaction via covalent (cyanide or as well as bipy bridges) and noncovalent interactions (like H-bond, π–π interactions). In 1a upon removal of the guest water, bipy and coordinated water molecules all the H-bonding interactions (Fig. S5, ESI‡) (vide supra) between the magnetic centres are destroyed. These noncovalent interactions provide ferromagnetic interactions in 1 and their absence in 1a might be responsible for lowering the Curie temperature. Moreover, the structural strain in the deguest phase (like the bent Cr–CN–Mn pathway) also provides the negative contribution of Tc.3c The increase in Tc (59 K) in 1b (Fig. S18–S20, ESI‡) also unequivocally suggests the role of water molecules in ferromagnetic interactions as they are involved in several H-bonding interactions. The field dependence magnetization curve of 1 shows a rapid increase to saturate at 8 kOe which is also the signature of magnetic ordering within the compound (Fig. 4). The saturation magnetization value in 1 is 10 Nβ which is in good agreement with the theoretical value (9 Nβ) expected for a ferrimagnet where antiferromagnetic coupling between CrIII and MnII exists. The decrease in magnetization value (6 Nβ) in 1a at high field suggests strong antiferromagnetic interaction operating between CrIII and MnII which is also in line with observed high Weiss constant and low Tc.
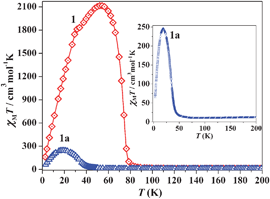 | ||
| Fig. 3 Temperature dependence of the magnetic susceptibility of 1 and 1a in an applied field of 500 Oe under zero field cooled condition. Inset shows the same for 1a to give a clear understanding of ordering at low T. | ||
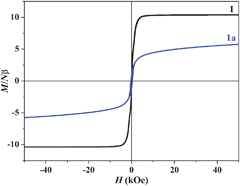 | ||
| Fig. 4 Magnetization plot for 1 and 1a measured at 2.5 K. | ||
In conclusion, we have conceived a rational strategy to assemble 2D magnetic layers into a 3D rigid porous framework that exhibits permanent porosity and ferrimagnetic ordering at low temperature. The highly polar nature of 1a was found to be exceedingly promising for high heat of H2 adsorption and establishes itself to be the first member of cyanometallate systems having heat of H2 adsorption value of ∼11.5 kJ mol−1. The framework demonstrates an interesting guest responsive magnetic modulation with a change in Tc as observed from dc susceptibility measurements. Current efforts are underway to synthesize frameworks with different pillar modules and study their porosity and magnetic properties.
TKM gratefully acknowledges the financial support from DST, Govt. of India (fast track proposal). PK acknowledges CSIR, India for SRF. The authors are thankful to Mr N. Kumar and Prof. A. Sundaresan for helping in magnetic measurements at JNCASR.
Notes and references
- (a) L. J. Murray, M. Dincă and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (b) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (c) J. R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (d) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (e) D. Maspoch, D. Ruiz-Molina and J. Veciana, Chem. Soc. Rev., 2007, 36, 770 RSC; (f) S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Sensors, 2009, 9, 1574 CrossRef CAS; (g) P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774 CrossRef CAS; (h) P. Kanoo, R. Matsuda, M. Higuchi, S. Ktagawa and T. K. Maji, Chem. Mater., 2009, 21, 5860 CrossRef CAS.
- (a) N. R. Champness, Dalton Trans., 2006, 877 RSC; (b) C. J. Kepert, Chem. Commun., 2006, 695 RSC; (c) Z. M. Wang, B. Zhang, Y. J. Zhang, M. Kurmoo, T. Liu, S. Gao and H. Kobayashi, Polyhedron, 2007, 26, 2207 CrossRef CAS; (d) P. Kanoo, K. L. Gurunatha and T. K. Maji, J. Mater. Chem., 2010, 20, 1322 RSC.
- (a) M. Ohba, K. Yoneda and S. Kitagawa, CrystEngComm, 2010, 12, 159 RSC; (b) T. Mallah, S. Thiebaut, M. Verdaguer and P. Veillet, Science, 1993, 262, 1554 CrossRef CAS; (c) W. Kaneko, M. Ohba and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 13706 CrossRef CAS.
- (a) X. N. Cheng, W. X. Zhang, Y. Y. Lin, Y. Z. Zheng and X. M. Chen, Adv. Mater., 2007, 19, 1494 CrossRef CAS; (b) M. Kurmoo, H. Kumagai, K. W. Chapman and C. J. Kepert, Chem. Commun., 2005, 3012 RSC; (c) D. Maspoch, D. Ruiz-Molina, K. Wurst, N. Domingo, M. Cavallini, F. Biscarini, J. Tejada, C. Rovira and J. Veciana, Nat. Mater., 2003, 2, 190 CrossRef CAS; (d) G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS.
- (a) D. Maspoch, D. Ruiz-Molina and J. Veciana, J. Mater. Chem., 2004, 14, 2713 RSC; (b) E. Coronado, F. Palacio and J. Veciana, Angew. Chem., Int. Ed., 2003, 42, 2570 CrossRef CAS.
- (a) V. Niel, A. L. Thompson, M. C. Munoz, A. Galet, A. S. E. Goeta and J. A. Real, Angew. Chem., Int. Ed., 2003, 42, 3760 CrossRef CAS; (b) Z. M. Wang, B. Zhang, H. Fujiwara, H. Kobayashi and M. Kurmoo, Chem. Commun., 2004, 416 RSC; (c) M. Kurmoo, H. Kumagai, M. Akita-Tanaka, K. Inoue and S. Takagi, Inorg. Chem., 2006, 45, 1627 CrossRef CAS.
- (a) M. H. Zeng, X. L. Feng, W. X. Zhang and X. M. Chen, Dalton Trans., 2006, 5294 RSC; (b) L. G. Beauvais and J. R. Long, J. Am. Chem. Soc., 2002, 124, 12096 CrossRef CAS.
- (a) K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281 CrossRef CAS; (b) C. Livage, N. Guillou, J. Chaigneau, P. Rabu, M. Drillon and G. Férey, Angew. Chem., Int. Ed., 2005, 44, 6488 CrossRef CAS; (c) C. Serre, F. Millange, C. Thouvenot, M. Nogues, G. Marsolier, D. Louer and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519 CrossRef CAS.
- K. M. Thomas, Dalton Trans., 2009, 1487 RSC.
- (a) S. S. Kaye and J. R. Long, J. Am. Chem. Soc., 2005, 127, 6506 CrossRef; (b) J. G. Vitillo, L. Regli, S. Chavan, G. Ricchiardi, G. Spoto, P. D. C. Dietzel, S. Bordiga and A. Zecchina, J. Am. Chem. Soc., 2008, 130, 8386 CrossRef CAS; (c) B. Chen, X. Zhao, A. Putkham, K. Hong, E. B. Lobkovsky, E. J. Hurtado, A. J. Fletcher and K. M. Thomas, J. Am. Chem. Soc., 2008, 130, 6411 CrossRef CAS; (d) S. Mohapatra, K. Hembram, U. Waghmare and T. K. Maji, Chem. Mater., 2009, 21, 5406 CrossRef CAS.
- T. K. Maji, S. Pal, K. L. Gurunatha, A. Govindaraj and C. N. R. Rao, Dalton Trans., 2009, 4426 RSC.
- M. M. Dubinin, Chem. Rev., 1960, 60, 235 CrossRef CAS.
- (a) Y. Yoshida, K. Inoue and M. Kurmoo, Chem. Lett., 2008, 586 CrossRef CAS; (b) T. Glaser, M. Heidemeier, T. Weyhermüller, R. D. Hoffmann, H. Rupp and P. Müller, Angew. Chem., Int. Ed., 2006, 45, 6033 CrossRef CAS; (c) A. Marvilliers, S. Parsons, E. Rivière, J. P. Audière and T. Mallah, Chem. Commun., 1999, 2217 RSC.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Extensive figures and full experimental details of crystallography, physical measurements, magnetic measurements. CCDC 784351. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc02490b |
| This journal is © The Royal Society of Chemistry 2011 |
