The change of corrin-amides to carboxylates leads to altered structures of the B12-responding btuBriboswitch†‡
Sofia
Gallo
,
Stefan
Mundwiler
,
Roger
Alberto
and
Roland K. O.
Sigel
*
Institute of Inorganic Chemistry, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland. E-mail: roland.sigel@aci.uzh.ch; Fax: +41 (0)44 635 6802; Tel: +41 (0)44 635 4652
First published on 10th September 2010
Abstract
By applying four different acid derivatives of vitamin B12, we demonstrate that the H-bonding pattern and the electrostatic environment provided by each side chain of the corrin ring are crucial for the correct structural rearrangement of the btuBriboswitch of E. coli.
Riboswitches are recently described regulatory RNA sequences able to control gene expression by directly binding to cellular metabolites.1–4 Since their discovery, the knowledge on these functional RNA molecules has rapidly increased and has led to detailed insights not only in their distribution and variety5 but also into riboswitch structure as well as into their metabolite-binding modes.6–11 Roughly a dozen riboswitch classes have been described so far, each binding to a specific metabolite and regulating correlated genes.12 The B12-responding riboswitches are larger in size than most other riboswitch classes and encompass aptamer domains of approximately 200 nucleotides.13 This domain, being the B12-binding region of the riboswitch, folds into a highly complex structure13 in order to provide an ideal binding pocket for the largest known metabolite. No three-dimensional structure is available today and secondary structure models of B12-responding riboswitches are currently based on biochemical assays and/or computational assisted modelling.2,13–16 Only little is known on the atomic level of how coenzyme B12 (adenosyl cobalamin, AdoCbl, Fig. 1) binds and switches the B12 riboswitch. Four modes of interaction are feasible of which only a single, several or all might take place: (i) Intermolecular interactions could be mediated by a complex H-bonding network between the RNA and the seven amide-sidechains of the corrin ring, the two riboses and/or the phosphate group of B12. (ii) The upper adenosine moiety could undergo basepairing to a distinct nucleobase of the riboswitch binding pocket. (iii) The two aromatic axial ligands, situated above and below the corrin plane, could bind to the RNAvia intercalation into a helical region or by stacking in general. (iv) B12 binding could be assisted by a “base-off/RNA-on” mode with a RNA-nucleobase coordinated to the cobalt atom similar to the “base-off/His-on” binding mode found in B12-dependent proteins.17 However, this latter “base-off/RNA-on” binding mode can be excluded for the E. coli btuBriboswitch18 but not for other B12 riboswitches, which have not yet been studied in this direction.
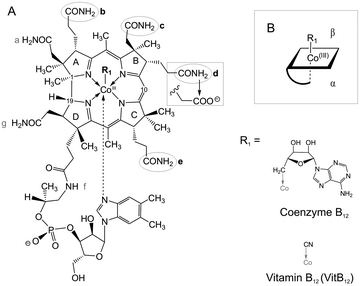 | ||
| Fig. 1 Schematic view of adenosyl cobalamin (AdoCbl) and the VitB12 acid derivatives used in this study. (A) Each acid derivative carries one single modified sidechain at positions b, c, d or e with a carboxylic acid replacing the amide group (sketched at sidechain d). (B) Scheme of AdoCbl: sidechains b, d and e point towards the lower α-side of the corrin, sidechain c is positioned on the upper β-side. | ||
In vivo studies14 and in vitro experiments using in-line probing assays19 suggested the necessity of both apical ligands of B12 for RNA binding.2 However, applying vitamin B12 and adenosyl cobinamide, we recently showed that the removal of either one of the two axial ligands results in a decreased affinity to the btuBRNA by about three log units:18 If added in high enough, i.e. mM, concentrations, both derivatives bind and switch the riboswitch correctly. Consequently, the two large apical ligands provide the affinity to the btuBriboswitch and the corrin ring with its seven amide sidechains is the decisive part to induce its structural rearrangement. Here we address the role of the individual amide groups of four corrin sidechains for switching the btuBriboswitch. In the absence of any three-dimensional structure, the here applied b-, c-, d- and e-acid derivatives of VitB12 provide first insights how the H-bonding properties and the electrostatic environment of the metabolite influence the structural switch of the btuBRNA.
The monocarboxylic b-, d- and e-acid derivatives of VitB12 (Fig. 1) were synthesized as a mixture by mild hydrolysis of VitB12 and separated with an aminopropyl column followed by HPLC as described.20,21 The isolated acids were desalted by phenol extraction and dissolved in 50 mM Tris–HCl (pH 8.3, 25 °C). Analysis of the 13C-signals (D2O, 293 K) between 25 and 40 ppm allowed the unambiguous assignment of each of the three acid derivatives. c-acid VitB12 (Fig. 1) was synthesized separately via the 7,8-lactone intermediate according to Brown et al.22
In this study, we performed so-called in-line probing experiments19 with the 202 nt long aptamer sequence of the btuBriboswitch in the presence of each of the above listed four acid derivatives. This method visualizes the structural rearrangement of the btuBriboswitch upon binding to B12 by appearance or disappearance of nine distinct cleavage-bands distributed over the whole 202 nt long btuBaptamer sequence (Fig. 2).2,18
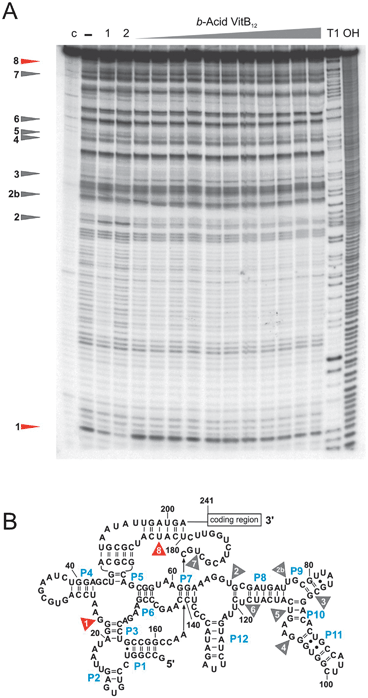 | ||
| Fig. 2 In-line probing experiments with the 202 nt long btuBriboswitch. (A) The structural changes of the RNA induced upon addition of b-acid VitB12 are limited to site 1 and 8 (marked in red), while the other seven sites (marked in grey) are not affected. (B) Cleavage sites mapped onto the proposed secondary structure showing that the full right-hand side of the riboswitch (P8 to P11) remains unaffected by binding of b-acid VitB12. 7 nM of btuBRNA was incubated for 40 hours in 50 mM Tris–HCl, pH 8.3, 20 mM MgCl2 and 100 mM KCl in the absence of B12 (−) or in the presence of 10 μM, 50 μM, 100 μM, 0.5 mM, 0.75 mM, 1 mM, 2 mM, 4 mM, 5 mM, 7.5 mM and 9 mM of b-acid vitamin B12. Lane (c) contains the unreacted RNA, T1 shows the RNAse T1 digestion and OH the alkaline digestion ladder. Lane (1) indicates the btuBRNA incubated in the presence of 5 mM VitB12, lane (2) the RNA incubated with 10 μM AdoCbl for comparison. | ||
Incubation with the b-, d- and e-acid derivatives of VitB12 leads to a reduced cleavage pattern of the RNA sequence compared to the one induced by AdoCbl and derivatives thereof having an intact corrin ring (Fig. 2 and ESI‡, Fig. S1 and S2). These three acid derivatives induce the expected intensity change only at sites 1 and 8, while the other seven sites remain mostly unaffected. b-Acid VitB12 additionally slightly influences site 5 and e-acid VitB12 sites 4, 6 and 7 (ESI‡, Fig. S2 and Table S1). These changes at sites 5, and 4, 6, and 7 are however within the error limits of this method. None of these three acids induces any further intensity changes within the rest of the cleavage pattern. The KD-values calculated from the intensity changes at sites 1 and 8 are 3.16 ± 0.80 mM (b-acid VitB12), 524 ± 48 μM (d-acid VitB12), and 263 ± 12 μM (e-acid VitB12). Hence, the binding strength is mostly in the same order of magnitude as found for VitB12 (KD = 316 ± 58 μM; ESI‡, Table S1) illustrating that indeed the axial ligands are largely responsible for the affinity.
For all three acid derivatives the maximal cleavage intensity changes at sites 1 and 8 are generally lower than observed for AdoCbl or VitB12 but still distinct (ESI‡, Fig. S3). Hence, each modification at the three sidechains b, d and e prevents the riboswitch from complete and concerted structural rearrangement. The reason for the inability of these three acid derivatives to fully restructure the riboswitch might be twofold: first, the change from a hydrogen bond donor (amide) to a H-bond acceptor (acid) completely changes the hydrogen bonding network. Second, the additional negative charge of the carboxylate on the corrin could well lead to an electrostatic repulsion between the B12 molecule and the phosphodiester backbone of the RNA. Although in-line probing provides no direct information on the localization of B12 within the RNA, our results indicate a direct interaction between the lower α-side of the corrin and the region between P6 and P12 of the riboswitch. These 100 nucleotides remain mostly unaffected upon incubation with b-, d- and e-acid VitB12. In order to test if high concentrations of B12 do not generally affect RNA structure, we have also incubated an unrelated RNA sequence with our acid-VitB12 derivatives (ESI‡, Fig. S4). No change in cleavage pattern was observed illustrating the specificity of the B12–btuB interaction.
Sidechain c points towards the upper β-side of the corrin ring (Fig. 1). Interestingly, c-acid VitB12 induces a completely different cleavage pattern of the btuBriboswitch (Fig. 3) than any other B12 derivative tested so far. In addition, strong, site-specific degradation of the RNA is observed at higher concentrations of c-acid VitB12 as is obvious from the strong decrease in intensity of the full-length band. This strong degradation of the full-length band prevents the determination of affinity constants. New cleavage-sites are situated at U16, U19, C52, U58, U82, and within P10 (named A, B, C, D, E, and F; Fig. 3). Two further cleavage sites correlate with sites 5 and 6, where the cleavage is increased in accordance to the addition of AdoCbl. The modification of sidechain c does therefore not prevent binding, but leads to a completely different structure of the btuBriboswitch. It follows that the amide group at sidechain c is crucial for a (at least partially) correct rearrangement of the btuBRNA.
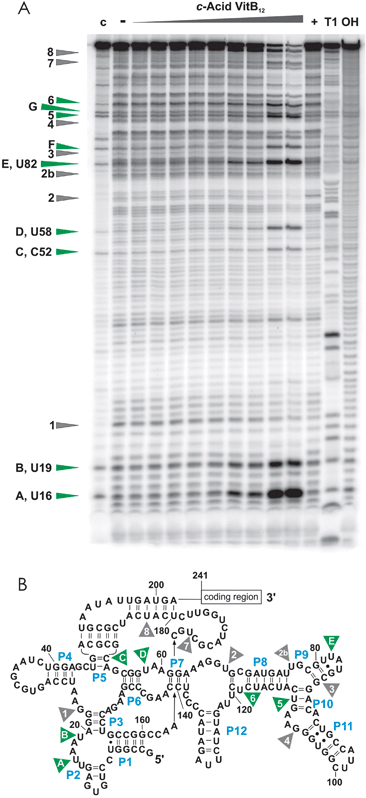 | ||
| Fig. 3 In-line probing experiments with c-acid VitB12. (A) In the presence of high concentrations of c-acid VitB12, the btuBRNA is strongly cleaved at specific sites marked in green. These sites are situated all over the RNA sequence (B) but do, besides sites 5 and 6, not correspond to the cleavage sites induced by AdoCbl (shown in grey). 30 nM of the btuBRNA was incubated for 41 hours in 100 mM Tris–HCl, pH 8.3, 20 mM MgCl2 and 100 mM KCl in the absence of B12 (−) or with 1 μM, 10 μM, 50 μM, 100 μM, 250 μM, 0.5 mM, 0.75 mM, 1 mM, and 5 mM of c-acid VitB12. Lane (+) is the positive control by incubation with 10 μM AdoCbl, lane (c) is the unreacted RNA, lane (T1) is the RNAse T1 digestion and lane (OH) is the alkaline hydrolysis ladder. | ||
Although highly fascinating, one can at present only speculate why c-acid VitB12 has such a different sculpturing effect on the btuBriboswitch. Several explanations are feasible: (i) The carboxylate group of c-acid VitB12 is the only one out of the here examined derivatives, that is positioned at the β-side. If localised in very close neighbourhood to the RNA, any change in H-bonding properties and/or electric charge at this site would lead to a local destabilization of the RNA structure and subsequently to an overall different structure. (ii) Carboxylates are well known ligands for Mg2+. A new Mg2+ binding site might thus be created, consequently not only changing the local structure, but also cleaving the RNA efficiently. Taken together, the β-side of the corrin ring seems to be more important for correct btuBRNA switching than the α-side.
In the absence of a crystal structure, our results provide first insights into the importance of single atoms of the B12 metabolite to switch the btuBRNA. In general, our experiments demonstrate an unprecedented behaviour of metabolite binding to any riboswitch. Alterations on the metabolite usually lead to a strongly decreased or completely abolished binding to the riboswitch.2 To the best of our knowledge, this study demonstrates for the first time in general, that the change of a single functional group, i.e. –NH2 to –O−, does not hamper binding, but instead induces a different structural change on the btuBriboswitch of E. coli. These findings are also important in the light of future new classes of antibiotics targeting riboswitches,23,24 which, e.g., should bind with high affinity but induce a wrong structure.
Financial support by the Swiss National Science Foundation (R.K.O.S.), the State Secretariat for Education and Research (COST-D39) and the University of Zürich is gratefully acknowledged. We thank Dr. Pilar Ruiz Sànchez for very fruitful discussions and her help in the separation of the VitB12 acids.
Notes and references
- A. S. Mironov, I. Gusarov, R. Rafikov, L. E. Lopez, K. Shatalin, R. A. Kreneva, D. A. Perumov and E. Nudler, Cell (Cambridge, Mass.), 2002, 111, 747–756 CrossRef CAS.
- A. Nahvi, N. Sudarsan, M. S. Ebert, X. Zou, K. L. Brown and R. R. Breaker, Chem. Biol., 2002, 9, 1043–1049 CrossRef CAS.
- W. C. Winkler, S. Cohen-Chalamish and R. R. Breaker, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15908–15913 CrossRef CAS.
- W. Winkler, A. Nahvi and R. R. Breaker, Nature, 2002, 419, 952–956 CrossRef CAS.
- W. C. Winkler and R. R. Breaker, Annu. Rev. Microbiol., 2005, 59, 487–517 CrossRef CAS.
- A. Serganov, A. Polonskaia, A. T. Phan, R. R. Breaker and D. J. Patel, Nature, 2006, 441, 1167–1171 CrossRef CAS.
- S. Thore, M. Leibundgut and N. Ban, Science, 2006, 312, 1208–1211 CrossRef CAS.
- R. K. Montange and R. T. Batey, Nature, 2006, 441, 1172–1175 CrossRef CAS.
- D. J. Klein and A. R. Ferré-D'Amaré, Science, 2006, 313, 1752–1756 CrossRef CAS.
- A. Serganov, Y. R. Yuan, O. Pikovskaya, A. Polonskaia, L. Malinina, A. T. Phan, C. Höbartner, R. Micura, R. R. Breaker and D. J. Patel, Chem. Biol., 2004, 11, 1729–1741 CrossRef CAS.
- R. T. Batey, S. D. Gilbert and R. K. Montange, Nature, 2004, 432, 411–415 CrossRef CAS.
- A. Serganov and D. J. Patel, Nat. Rev. Genet., 2007, 8, 776–790 CrossRef CAS.
- A. Nahvi, J. E. Barrick and R. R. Breaker, Nucleic Acids Res., 2004, 32, 143–150 CrossRef CAS.
- X. W. Nou and R. J. Kadner, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7190–7195 CrossRef CAS.
- S. Ravnum and D. I. Andersson, Mol. Microbiol., 2001, 39, 1585–1594 CrossRef CAS.
- A. G. Vitreschak, D. A. Rodionov, A. A. Mironov and M. S. Gelfand, RNA, 2003, 9, 1084–1097 CrossRef CAS.
- C. L. Drennan, S. Huang, J. T. Drummond, R. G. Matthews and M. L. Lidwig, Science, 1994, 266, 1669–1674 CAS.
- S. Gallo, M. Oberhuber, R. K. O. Sigel and B. Kräutler, ChemBioChem, 2008, 9, 1408–1414 CrossRef CAS.
- G. A. Soukup and R. R. Breaker, RNA, 1999, 5, 1308–1325 CrossRef CAS.
- B. Spingler, S. Mundwiler, P. Ruiz-Sánchez, D. R. van Staveren and R. Alberto, Eur. J. Inorg. Chem., 2007, 2641–2647 CrossRef CAS.
- P. M. Pathare, D. S. Wilbur, S. Heusser, E. V. Quadros, P. McLoughlin and A. C. Morgan, Bioconjugate Chem., 1996, 7, 217–232 CrossRef CAS.
- K. L. Brown, S. Cheng and H. M. Marques, Inorg. Chem., 1995, 34, 3038–3049 CrossRef CAS.
- K. F. Blount and R. R. Breaker, Nat. Biotechnol., 2006, 24, 1558–1564 CrossRef CAS.
- E. Ott, J. Stolz, M. Lehmann and M. Mack, RNA Biol., 2009, 6, 276–280 Search PubMed.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Additional figures. See DOI: 10.1039/c0cc02447c |
| This journal is © The Royal Society of Chemistry 2011 |
