Metal binding to a zinc-finger peptide: a comparison between solution and the gas phase†‡
Yana
Berezovskaya
a,
Craig T.
Armstrong
b,
Aimee L.
Boyle
b,
Massimiliano
Porrini
a,
Derek N.
Woolfson
*bc and
Perdita E.
Barran
*a
aSchool of Chemistry, University of Edinburgh, Edinburgh, UK. E-mail: Perdita.Barran@ed.ac.uk; Fax: 13 1650 7533; Tel: 13 1650 7533
bSchool of Chemistry, University of Bristol, Bristol, UK
cDepartment of Biochemistry, University of Bristol, Bristol, UK. E-mail: D.N.Woolfson@bristol.ac.uk; Fax: 11 7929 8611; Tel: 11 7954 6347
First published on 20th September 2010
Abstract
Solution-phase spectroscopy and mass spectrometry are used to probe interactions between divalent metal ions and a synthetic Cys2His2 zinc-finger peptide (vCP1). Both methods provide the same order of binding affinity, zinc ≥ cobalt ≫ copper ≫ calcium. Collision-cross-section measurements show that both apo and holo forms are compact. This is corroborated by molecular-dynamics simulations.
Advances in mass spectrometry (MS) have placed it at the forefront of techniques for characterising proteins. For the past three decades, mass spectrometry has been increasingly used to provide masses of single proteins, and of protein fragments as part of proteomics and protein-sequencing studies. More recently, developments in soft ionization methods have allowed researchers to observe protein complexes in the gas phase, signifying a move from studying single proteins to protein systems. Moreover, it has been possible to record the time taken for proteins to move through inert gases in a drift cell using ion mobility mass spectrometry (IM-MS), allowing users to calculate collision cross sections and hence obtain valuable information on the conformations adopted in the gas phase.
We employ mass spectrometry in combination with the related technique of ion mobility mass spectrometry to elucidate conformations of charged biomolecules in the gas phase.2,3 IM-MS has already proven useful in fundamental studies of sequence-to-structure relationships in polypeptides, since structures observed in vacuo are defined intrinsically by the sequence.4,5 Ion-mobility instrumentation determines the time it takes ions to pass through a drift cell containing an inert gas under the influence of a weak electric field.6 The mobility of an ion is the constant of proportionality between the velocity at which it moves and the applied electric field, and is inversely proportional to the collision cross section (Ω) of the gas-phase ion:
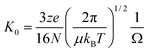 | (1) |
Here we use MS and IM-MS to examine a synthetic peptide for a consensus zinc finger sequence, vCP1, derived from the work of Berg et al.7 Zinc fingers are found widely in Nature where they recognise DNA and perform other functions. The key zinc-binding residues are conserved cysteines (Cys, thiol containing) and histidines (His, imidazole containing), which coordinate metal and drive the folding of the polypeptide chain,8Fig. 1. vCP1 comprises just 26 amino acids making it a good model for studying peptide–metal interactions in solution and the gas phase.
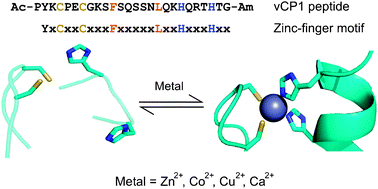 | ||
| Fig. 1 The vCP1 peptide. Sequence, design criteria and proposed equilibrium in the presence of divalent metal ions. The sequences show key cysteine and histidine residues in yellow and blue, respectively. The residues shown in orange form a small hydrophobic core. The schematic shows the proposed rearrangement of a zinc finger when the two cysteine (sulfhydryl groups in yellow) and two histidine residues (imidazole nitrogen atoms in blue) coordinate a divalent metal ion (grey).1 Adapted from PDB entries 1Z60 and 2DRP. | ||
To confirm the secondary structure and metal binding of vCP1 in solution, we used circular dichroism (CD) spectroscopy and ultraviolet (UV) spectroscopy, respectively. The CD spectra in the absence of metal ions confirm that the peptide was largely unstructured, whereas upon the addition of zinc the peptide folded to give a spectrum consistent with that reported for naturally occurring zinc fingers, Fig. 2a.
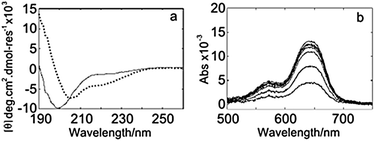 | ||
| Fig. 2 Solution-phase spectra for vCP1. (a) CD spectra of 25 μM peptide in the absence (solid line) and presence (dotted line) of 50 μM zinc. (b) UV spectra for a titration of 4.4–47.4 μM Co2+ into 11.9 μM vCP1. Buffer: 5% isopropanol; pH 7.2; 20 mM ammonium acetate; 500 μM TCEP. | ||
Addition of cobalt effected a similar change to that seen with zinc; in contrast, addition of copper and calcium changed the conformation of the peptide in a less pronounced manner (Supporting Information). The addition of cobalt to vCP1 was also followed by UV spectroscopy, Fig. 2b. The spectra are consistent with Cys2His2 tetrahedral coordination of the metal,9 and a binding constant of 0.8 μM (see Supporting Information). A competitive-binding assay for zinc against the cobalt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vCP1 complex revealed sub-nM binding of zinc. These data confirm that vCP1 is a good model for metal binding studies under the solution conditions needed to best preserve intact complexes into the solvent-free environment of a mass spectrometer.
vCP1 complex revealed sub-nM binding of zinc. These data confirm that vCP1 is a good model for metal binding studies under the solution conditions needed to best preserve intact complexes into the solvent-free environment of a mass spectrometer.
Fig. 3 shows typical nESI mass spectra for vCP1 without (Fig. 3a) and with (Fig. 3b–e) metals added; the regions for the +3 charge state(s) of the peptide are shown. The [M + 3H]3+ or equally charged [M + X + H]3+, where X = metal, were the dominant peaks in the spectra, ∼8 times more intense than the +2 and +4 species. Use of TCEP (tris(2-carboxyethyl)phosphine) maintained approximately 99% of the cysteines in the reduced state as evidenced by the isotopic cluster analysis. Theoretical fitting for the elemental compositions of the fully reduced peptide is superimposed on the experimental isotopic distribution, which allowed us to conclude that under these conditions the metals are coordinated by thiolates (S−). Impurity peaks annotated on Fig. 3a are the common adducts of oxygen (*), sodium (**) and calcium (***). The ratio between the intensity of the 12C peak for both apo and holo species was determined for each spectrum, which gave the order of metal affinity for apo-vCP1, Zn2+ > Co2+ ≫ Cu2+ ≫ Ca2+, consistent with the solution-phase data.
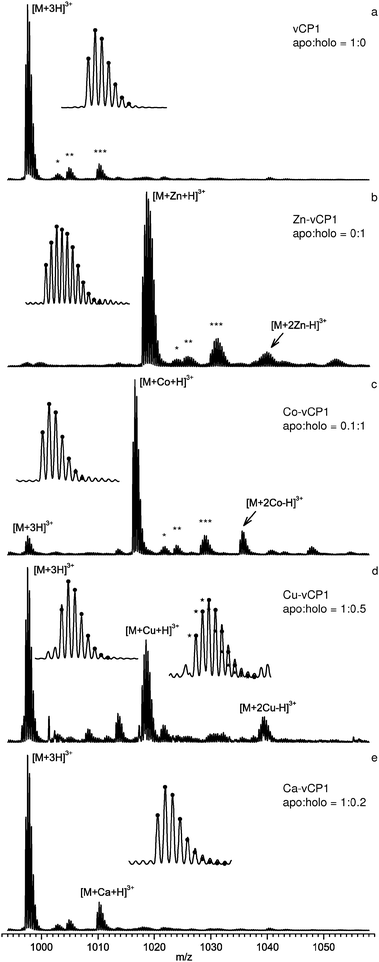 | ||
| Fig. 3 nESI mass spectra of the +3 charge state of vCP1. For (a) the apo state, and with: (b) Zn2+, (c) Co2+, (d) Cu2+ and (e) Ca2+. Ratios of apo to holo form were calculated from the intensities of the mono-isotopic peaks. Insets show the resolved isotopic clusters for the free and bound states along with theoretical fitting (●). Isotopic cluster analysis indicated that 99% of the peptide is reduced, except in the case of Cu2+. Conditions: 20 μM peptide; 10 mM ammonium acetate; 5% isopropanol; pH 6.8; 200 μM TCEP; 100 μM metal acetate salts. | ||
Preferences for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vCP1
1 vCP1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal binding were observed for Zn2+ (Fig. 3b), and to a slightly lesser extent for Co2+ (Fig. 3c) compared to the other metal ions. For both of these metals the same set of adducts, and at similar relative intensities as observed for apo-vCP1, were present with the holo-forms. A small fraction of vCP1 bound metal is seen in a 1
metal binding were observed for Zn2+ (Fig. 3b), and to a slightly lesser extent for Co2+ (Fig. 3c) compared to the other metal ions. For both of these metals the same set of adducts, and at similar relative intensities as observed for apo-vCP1, were present with the holo-forms. A small fraction of vCP1 bound metal is seen in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. This was most pronounced for Cu2+. Perhaps not surprisingly, in the presence of Cu2+ there was a higher fraction of the oxidised form of apo-vCP1, as revealed by the isotopic cluster analysis (Fig. 3d, inset), despite the 50-fold excess of TCEP.
2 ratio. This was most pronounced for Cu2+. Perhaps not surprisingly, in the presence of Cu2+ there was a higher fraction of the oxidised form of apo-vCP1, as revealed by the isotopic cluster analysis (Fig. 3d, inset), despite the 50-fold excess of TCEP.
We found Ca2+ to bind vCP1 with lowest affinity in both the gas and solution phase. A small amount of calcium (a contaminant of lab-ware, reagents, and even deionised water) was found to bind the peptide even in the presence of Zn2+, leading us to conclude that Ca2+ binds vCP1 non-specifically, and probably away from the Cys2His2 binding site. We observed a low μM affinity for Zn2+ from MS analysis (see Supporting Information), considerably lower than found in solution. This difference may be attributable to desolvation effects, although the relative affinities for different metals are expected to be similar; this effect will be explored elsewhere.
By performing IM-MS experiments for all of the apo and the holo forms of vCP1 shown in Fig. 3, we obtain arrival time distributions (ATDs) at a range of drift voltages which are converted to collision cross sections following eqn (1) (Table 1). The packing found for the apo-vCP1 and Zn-vCP1 gave the lowest, and surprisingly similar, values. However all the values for the apo- and metal-bound forms were similar, and within experimental error. We expect to see more difference in collision cross section at elevated temperatures in the drift cell and thus obtain more information about the binding energies of this system in our future work.
| Apo-vCP1 (Å2) | Holo-vCP1 (Å2) | |
|---|---|---|
| vCP1 | 488 ± 22 | — |
| Zn-vCP1 | 487 ± 24 | 488 ± 21 |
| Co-vCP1 | 518 ± 2 | 492 ± 17 |
| Cu-vCP1 | 499 ± 12 | 493 ± 13 |
| Ca-vCP1 | 528 ± 8 | 517 ± 2 |
To investigate these phenomena further, molecular dynamics (MD) simulations of vCP1 were performed. Fig. 4 shows representative nESI mass spectra and ATD data for the +3 charge state of Zn-vCP1, plus structures obtained by MD simulations.10 The experimental collision cross sections of the apo-vCP1 and Zn-vCP1 compare favourably with those obtained from the MD giving confidence in both datasets. Interestingly, the apo form appears to possess some helical character (Fig. 4), although we find these helical regions are transient, particularly when compared to the holo form. When solvent is present in the simulations, however, there is little evidence for any helicity in the apo peptide (Supplementary Information).
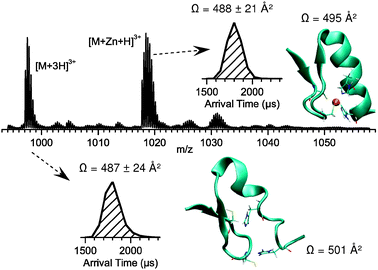 | ||
Fig. 4 nESI mass spectra of the +3 charge state of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn 1 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vCP1 species. Conditions are as for Fig. 3. Insets show the ATDs of apo and holo-vCP1, and representative MD structures, along with collision cross sections obtained experimentally and from the MD simulations. vCP1 species. Conditions are as for Fig. 3. Insets show the ATDs of apo and holo-vCP1, and representative MD structures, along with collision cross sections obtained experimentally and from the MD simulations. | ||
In summary, we have illustrated how solution and gas-phase measurements can be combined to provide information on the binding stoichiometry and specificity of a model metal-binding peptide system. Qualitatively the agreement between the methods is excellent, and some semi-quantitative understanding can be gained also. Moreover, mass spectrometry studies have been extended to give collision cross sections, which give further insight into coordination behaviour as well as the conformational preferences of a zinc-finger peptide. These results corroborate findings of MD studies on the peptide.
We acknowledge the support of the EPSRC, the RSC Analytical Division (who fund YB) and the HCP-Europa2 Scheme. CTA and ALB are funded by the BBSRC. We thank the Mann group (Bristol) for use of their spectrophotometer.
Notes and references
- J. M. Berg, Annu. Rev. Biophys. Biophys. Chem., 1990, 19, 405–421 CrossRef CAS.
- T. Wyttenbach and M. T. Bowers, Annu. Rev. Phys. Chem., 2007, 58, 511–533 CrossRef CAS.
- B. J. McCullough, J. Kalapothakis, H. Eastwood, P. Kemper, D. MacMillan, K. Taylor, J. Dorin and P. E. Barran, Anal. Chem., 2008, 80, 6336–6344 CrossRef CAS.
- S. J. Valentine, A. E. Counterman and D. E. Clemmer, J. Am. Soc. Mass Spectrom., 1999, 10, 1188–1211 CrossRef CAS.
- M. De Cecco, E. S. Seo, D. J. Clarke, B. J. McCullough, K. Taylor, D. Macmillan, J. R. Dorin, D. J. Campopiano and P. E. Barran, J. Phys. Chem. B, 2010, 114, 2312–2318 CrossRef CAS.
- T. Wyttenbach and M. T. Bowers, in Modern Mass Spectrometry, Springer, Berlin/Heidelberg, 2003, pp. 207–232 Search PubMed.
- C. A. Kim and J. M. Berg, Nat. Struct. Biol., 1996, 3, 940–945 CrossRef CAS.
- G. Parraga, S. J. Horvath, A. Eisen, W. E. Taylor, L. Hood, T. Y. Elton and R. E. Klevit, Science, 1988, 241, 1489–1492 CAS.
- Y. Shi, R. D. Beger and J. M. Berg, Biophys. J., 1993, 64, 749–753 CrossRef CAS.
- A. A. Shvartsburg, G. C. Schatz and M. F. Jarrold, J. Chem. Phys., 1998, 108, 2416–2423 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental details for solution phase binding experiments; ion mobility measurements; MD calculations. See DOI: 10.1039/c0cc02445g |
| This journal is © The Royal Society of Chemistry 2011 |
