Instantaneous dissolution of cellulose in organic electrolyte solutions†‡
Roberto
Rinaldi
*
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, Mülheim an der Ruhr, Germany. E-mail: rinaldi@mpi-muelheim.mpg.de; Fax: +49 0208 306 2995; Tel: +49 0208 306 2368
First published on 25th October 2010
Abstract
Herein a novel class of solvent systems for cellulose is introduced. Surprisingly, organic electrolyte solutions, which contain just a small molar fraction of ionic liquid, dissolve instantaneously large amounts of cellulose. The solvation properties of the solvent systems, required for dissolving the polymer, are discussed here.
One of the major current challenges to the chemical industry is the efficient use of cellulose for the production of performance materials, platform chemicals and biofuels.1,2 Commonly, to process cellulose into desired products requires firstly the dissolution of the natural polymer.2,3 Industrially, this is made by using highly toxic solvents (e.g. CS2).4,5 In 2002, Rogers et al. introduced alkylimidazolium-based ionic liquids (ILs) as green solvents for cellulose.6 This breakthrough opened up new horizons in the chemistry of cellulose.7 Despite efficacy, dissolving cellulose in ILs suffers from several drawbacks,8 such as the slow rate of dissolution,9 the high viscosity of the solutions obtained,10 and not to mention the high costs of ILs. Thus, advances towards improved processes for the dissolution of cellulose are still necessary. Herein, it is shown that cellulose dissolves instantaneously in solvent systems that contain only a minor molar fraction of IL (χIL). Furthermore, the factors responsible for the dissolution of cellulose in the molecular solvents upon addition of IL are discussed in detail.
The agglomeration of the cellulosic fibers is a typical difficulty experienced upon mixing cellulose in ionic liquids. In an attempt to avoid this problem, the following two-step method for the dissolution of cellulose was investigated. First, cellulose (Avicel, 1 g) was dispersed in 1,3-dimethyl-2-imidazolidinone (DMI, 5 g) under magnetic stirring at 100 °C. Next, the solid 1-butyl-3-methylimidazolium chloride (BMIMCl, 5 g) was added into the suspension.
Strikingly, the current method enabled the complete dissolution of cellulose under magnetic stirring at 100 °C after only 3 min. For the sake of comparison, the dissolution of cellulose (5 wt%) in neat BMIMCl takes more than 10 h to be achieved.9 Furthermore, the solvent system BMIMCl/DMI offers clear advantage over the conventional solvents LiCl/DMI11 and LiCl/N,N-dimethylacetamide.12,13 The LiCl-based solvents can dissolve only 2 wt% cellulose under harsh conditions (150 °C for 30 min or, in some cases, for days),11–13 while BMIMCl/DMI dissolves easily 10 wt% cellulose in few minutes.
The behavior of the mixture cellulose/BMIMCl/DMI was studied varying gradually the χIL from 0.07 to 0.40. Fig. 1 shows the appearance of the systems obtained.
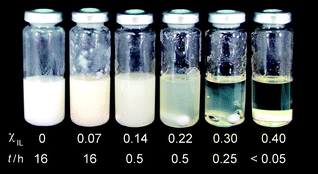 | ||
| Fig. 1 Appearance of the mixtures containing microcrystalline cellulose (Avicel, 10 wt%), DMI and BMIMCl (total volume 10 mL) after stirring at 100 °C for the time indicated in the figure. Note that there is a magnetic bar at the bottom of the vials. | ||
Cellulose is not soluble in DMI. Nevertheless, the polymer becomes heavily swollen when immersed in DMI for 16 h at 100 °C, but no gel is formed. The gelation of Avicel starts upon the addition of BMIMCl into the suspension. First, a white paste is obtained at χIL 0.07. In Fig. 1, the presence of some opaque clumps on the vial wall is still seen even after stirring the suspension for 16 h at 100 °C. From χIL 0.14 on, the agglomerates are no longer visible in the mixture. Additionally, from χIL 0.14 to 0.30, the gels turn gradually more translucent (Fig. 1). At χIL 0.30, a semi-transparent dispersion is obtained immediately after 0.25 h while stirring the mixture at 100 °C. At χIL 0.40, cellulose dissolves completely after only 3 min, yielding a much less viscous solution than that obtained using only the IL. Surprisingly, the solution of cellulose in IL/DMI shows a viscosity low enough to allow vigorous magnetic stirring at 100 °C. Work is in progress to examine the rheological properties of the polymeric solutions obtained.
Replacing BMIMCl with 1-ethyl-3-methylimidazolium acetate (EMIMAcO) makes the dissolution of cellulose instantaneous. To determine directly the amount of IL required for dissolving cellulose, the IL was dosed into the suspension. The polymer turns soluble in the mixture upon reaching χEMIMAcO 0.18. Likewise, the performance of other molecular solvents in the solvent system was measured. For this purpose, DMI was replaced with other solvents of similar polarity. Table 1 displays the χIL at which the dissolution of the polymer takes place in the solvent systems.
| Entry | Solvent | χ IL a | E T(30)/kcal mol−1 |
|---|---|---|---|
| a χ IL = nIL/(nms + nIL), where χIL is the molar fraction of IL in the electrolyte solution, nms and nIL are the amount in mol of molecular present and of IL in the final mixture, respectively. | |||
| 1 | N,N-Dimethylformamide (DMF) | 0.10 | 43.5 |
| 2 | N,N-Dimethylacetamide (DMA) | 0.27 | 43.0 |
| 3 | Pyrrolidinone | 0.30 | 48.2 |
| 4 | δ-Valerolactam | 0.27 | 44.0 |
| 5 | ε-Caprolactam | 0.42 | 42.1 |
| 6 | N-Methylpyrrolidinone (NMP) | 0.16 | 42.5 |
| 7 | 1,3-Dimethyl-2-imidazolidinone (DMI) | 0.18 | 42.4 |
| 8 | N,N′-Dimethylpropylene urea (DMPU) | 0.33 | 42.1 |
| 9 | N,N,N′,N′-Tetramethylurea | 0.59 | 41.2 |
| 10 | Dimethylsulfoxide (DMSO) | 0.08 | 45.1 |
| 11 | Sulfolane | 0.23 | 45.1 |
| 12 | Acetylacetone | 0.51 | 38.9 |
| 13 | tert-Butanol | 0.48 | 43.7 |
| 14 | tert-Pentanol | 0.49 | 41.2 |
| 15 | EMIMAcO | — | 49.8 |
| 16 | BMIMCl | — | 49.3 |
The systems containing amide-related solvents dissolve the polymer, typically, upon addition of a χIL smaller than 0.30 (Table 1, entries 1 to 4, 6 and 7). In addition, cellulose dissolves immediately also in DMSO and sulfolane at χIL 0.08 and 0.23, respectively (Table 1, entries 10 and 12). The dissolution of cellulose in acetylacetone, tert-butanol and tert-pentanol (Table 1, entries 12–14) only happens when using equimolar amount of the IL. Surprisingly, the solvent system containing N,N,N′,N′-tetramethylurea (Table 1, entry 9), which is a structural analogue of DMI, requires the largest χIL (0.59) to dissolve the polymer.
The polarity of the molecular solvents, ILs and the solvent systems was assessed from the solvatochromic behavior of Reichardt's dye.14 The current ET(30) values of the molecular solvents and ILs (Table 1) are consistent with those published elsewhere.15 The ILs are more polar than the molecular solvents, as indicated by their larger ET(30) values. Fig. 2 shows that IL increases considerably the polarity of the solvent systems. However, in the solvent systems EMIMAcO/1 to 4, 6, 7, 10 and 11, which contain χIL < 0.30, the ET(30) values are still much lower than that found for the neat IL. Yet the dissolution of cellulose is much more efficient in these solvent systems than in EMIMAcO.
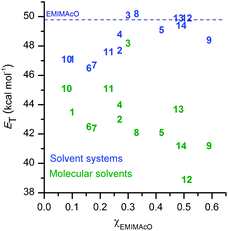 | ||
| Fig. 2 E T(30) values of the molecular solvents (in green) and solvent systems (in blue) ordered according to the χEMIMAcO needed to dissolve cellulose. The ET(30) value of EMIMAcO is indicated by the dash line. | ||
To assess more specifically the terms that compose the solvent polarity, the Kamlet–Taft parameters were determined.16 In this approach, the empirical polarity is defined in terms of the α (hydrogen bond donor/acidity), β (hydrogen bond acceptor/basicity) and π* (polarizability) parameters.17
Fig. 3 displays clearly that the IL has a leveling effect on α, β and π* for most of the solvent systems. However, for the systems containing a χIL < 0.30, the α values are lower than that found for EMIMAcO. This fact shows clearly that a synergism between molecular solvent and IL decreases the hydrogen bond acidity of the solvent system, while the hydrogen bond basicity is kept at the same level of the parent IL.
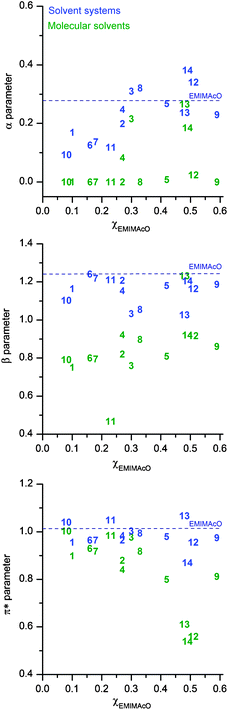 | ||
| Fig. 3 The Kamlet–Taft parameters of the molecular solvents (in green) and solvent systems (in blue) arranged according to the χEMIMAcO needed to dissolve cellulose. The dash line shows the parameters of the IL. | ||
The analysis of the β values of the solvent systems based on pyrrolidinone-like solvents 3 and 6 shows that, although the system EMIMAcO/3 contains much more IL (χIL 0.30) than the EMIMAcO/6 solution (χIL 0.16), the β value found for the mixture EMIMAcO/3 is 1.033, while the blend EMIMAcO/6 shows a β value of 1.241. Likewise, despite the large χIL present in the systems EMIMAcO/4, 5, 11–13, their β values are much lower than the βIL. Taken together, the hydrogen-bond donor solvents appear to interact not only with cellulose, but they also compete with cellulose for the hydrogen bond basicity of the IL. Consequently, a large χIL must be used for leveling the β parameter of the solvent system at a value high enough to allow the dissolution of cellulose. This finding is in line with the current developments of IL for the dissolution of cellulose. For neat ILs, recent studies pointed out that the dissolution of cellulose requires an IL with high hydrogen bond basicity.18
Fig. 4 shows how EMIMAcO modifies α, β and π* parameters of DMI in the entire scale of χIL. Adding IL into DMI causes a sharp rise of both β and π* parameters. From around χIL 0.10 on, it is seen that these values are indeed already identical to those of the IL. Meanwhile, the α parameter experiences otherwise a much more gradual growth towards the value found for EMIMAcO. When cellulose dissolves in the solvent system (χIL 0.18), the medium has nearly identical values of β and π* to those of the parent IL (Fig. 4), while α is about 50% smaller than the αIL.
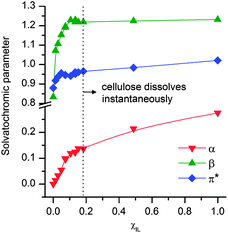 | ||
| Fig. 4 Evolution of the solvatochromic parameters in the DMI–EMIMAcO system. | ||
In summary, the findings of this study have very important implications for future practice. IL-based solvent systems circumvent the problems faced in the conventional dissolution of cellulose in neat ILs. The little time required for the dissolution of cellulose in the IL-based electrolyte solution not only places the solvent systems described here beyond the state-of-art but also brings them closer to the practical utilisation. Furthermore, the current findings add also substantially to our understanding of the dissolution of cellulose. The hydrogen-bond basicity of the solvent system is essential for the dissolution of cellulose. This fact clearly indicates that the polymer behaves preferentially like a hydrogen-bond donor in solution. To reduce the amount of ionic liquid required in the solvent system, both the molecular solvent and the ionic liquid should have high hydrogen-bond basicity. In addition, to avoid the competition between the molecular solvent and cellulose for the hydrogen bond basicity of the IL, the molecular solvents should show, preferentially, no hydrogen-bond acidity.
The author acknowledges the financial support from the Max Planck Society. This work was performed as part of the Cluster of Excellence “Tailor-Made Fuels from Biomass”, which is funded by the Excellence Initiative by the German federal and state governments to promote science and research at German universities.
Notes and references
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef.
- R. Rinaldi and F. Schüth, ChemSusChem, 2009, 2, 1096–1107 CrossRef CAS; R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610–626 RSC.
- R. Rinaldi, N. Meine, J. vom Stein, R. Palkovits and F. Schüth, ChemSusChem, 2010, 3, 266–276 CrossRef CAS; R. Rinaldi, R. Palkovits and F. Schüth, Angew. Chem., Int. Ed., 2008, 47, 8047–8050 CrossRef CAS.
- T. Heinze and A. Koschella, Polimeros, 2005, 15, 84 Search PubMed; B. Lindman, G. Karlström and L. Stigsson, J. Mol. Liq., 2010, 156, 76–81 CrossRef CAS.
- A. F. Turbak, R. B. Hammer, R. E. Davies and H. L. Hergert, Chem. Technol., 1980, 10, 51–57 CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS.
- O. A. El Seoud, A. Koschella, L. C. Fidale, S. Dorn and T. Heinze, Biomacromolecules, 2007, 8, 2629–2647 CrossRef CAS; H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef CAS; A. Pinkert, K. N. Marsh, S. S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS; N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC; M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS; Y. G. Zhang and J. Y. G. Chan, Energy Environ. Sci., 2010, 3, 408–417 RSC; S. D. Zhu, Y. X. Wu, Q. M. Chen, Z. N. Yu, C. W. Wang, S. W. Jin, Y. G. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- S. Zhu, J. Chem. Technol. Biotechnol., 2008, 83, 777–779 CrossRef CAS.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS.
- B. Kosan, C. Michels and F. Meister, Cellulose, 2008, 15, 59–66 CrossRef CAS; B. Kosan, K. Schwikal and F. Meister, Cellulose, 2010, 17, 495–506 CrossRef CAS.
- A. Takaragi, M. Minoda, T. Miyamoto, H. Q. Liu and L. N. Zhang, Cellulose, 1999, 6, 93–102 CrossRef CAS.
- A.-L. Dupont, Polymer, 2003, 44, 4117–4126 CrossRef CAS.
- L. A. Ramos, J. M. Assaf, O. A. El Seoud and E. Frollini, Biomacromolecules, 2005, 6, 2638–2647 CrossRef CAS.
- C. Reichardt, Org. Process Res. Dev., 2007, 11, 105–113 Search PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS; C. Reichardt, Green Chem., 2005, 7, 339–351 RSC.
- M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS.
- Y. Marcus, Chem. Soc. Rev., 1993, 22, 409–416 RSC.
- A. Brandt, J. P. Hallett, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2010, 12, 672–679 RSC; L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC; Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44 RSC; M. Zavrel, D. Bross, M. Funke, J. Buchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Ferdi Schüth on the occasion of his 50th anniversary. |
| ‡ Electronic supplementary information ESI available: Material and Methods. See DOI: 10.1039/c0cc02421j |
| This journal is © The Royal Society of Chemistry 2011 |
