Photoswitchable triple hydrogen-bonding motif†‡
Martin
Herder
,
Michael
Pätzel
,
Lutz
Grubert
and
Stefan
Hecht
*
Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, 12489 Berlin, Germany. E-mail: sh@chemie.hu-berlin.de; Fax: +49 30 2093-6940; Tel: +49 30 2093-7365
First published on 27th September 2010
Abstract
Photochromic bis(thiazol-4-yl)maleimides, displaying enhanced binding affinity to complementary melamine receptors in their ring-closed switching state, have been developed and could pave the way to light-responsive supramolecular assemblies.
The “bottom-up” organization of molecules to defined superstructures and resulting materials requires the use of weak and reversible non-covalent interactions between the molecular building blocks as they enable the generation of defect free structures. As structure formation relies on the primary event of molecular recognition, hydrogen-bonds have been extensively exploited as they offer directionality as well as complementarity and can be adjusted in their strength.1,2 Another advantage of using the supramolecular assembly approach relies on its ability to respond to external stimuli giving rise to “smart” materials.3 For this purpose, hydrogen-bonding interactions can be controlled by changes in temperature, solvent, and pH.1,2 The formation of multiple hydrogen bonding interactions can also be tuned by oxidation/reduction, altering the intrinsic ability of one partner to act as a hydrogen-bond acceptor and/or donor.4 In contrast to the above mentioned stimuli, light represents a truly non-invasive stimulus combined with unprecedented spatio-temporal resolution that, in principle, constitutes an exquisite tool to remotely control hydrogen-bonding interactions.2a,c Thus far such photochemical control has been achieved primarily by manipulating the geometry and flexibility of the key building blocks involved in the formation of hydrogen-bonding networks5 and their higher aggregates.6 Another example relies on the photomodulation of secondary interactions and their impact on the formation of a discrete hydrogen-bonding complex.7 Here, we present an alternative approach to photochemically modulate the inherent binding strength of a triple hydrogen-bonding motif by means of changing its electronic nature during the course of a photochromic reaction.8
Inspired by the possibility of influencing the strength of multiple hydrogen-bonding motifs by variation of π-conjugated electron-donating/accepting groups9 and intrigued by Irie's diarylethene photochromes,10 which allow for significant changes in π-conjugation when switching from the open to the closed form, we chose to merge both aspects in our design (Fig. 1).11 The central maleimide core is acting as a triple hydrogen-bonding acceptor–donor–acceptor (ADA) site, which in the open form is largely decoupled from the termini, whereas in the closed form the terminal donor/acceptor groups are in π-conjugation and hence should influence the association with a complementary N,N-dialkylmelamine DAD moiety.
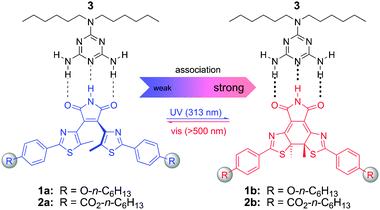 | ||
| Fig. 1 Photoswitchable triple hydrogen-bonding motif: reversible photochemical ring-closure (opening) leads to an enhanced (diminished) binding of the central ADA imide moiety in 1b/2b (1a/2a) to a complementary DAD melamine receptor 3. | ||
In order to readily vary the substitution pattern, a modular synthesis of compounds 1a and 2a was devised, involving Suzuki cross-coupling between the dibromomaleimide core and suitable 5-methyl-2-phenylthiazol-4-ylboronic esters, carrying either electron-donating ether or electron-accepting ester groups in the para-position of the 2-phenyl moiety. Thiazole termini were chosen as they are readily available and offer great performance, in particular with regard to their thermal and photochemical stability.12 To facilitate solubility, n-hexyl chains were attached to both termini in each case (1a, 2a) and furthermore incorporated into the complementary melamine moiety 3. The respective ring-closed isomers 1b and 2b were isolated after preparative irradiations of their ring-open derivatives and subsequent column chromatography.
Compounds 1a/b and 2a/b display excellent photochromic behaviour (Fig. 2). Irradiation with UV-light (λirr = 313 nm) of a solution of 1a or 2a in methylene chloride leads to rapid change in colour from yellow to deep-red, reflected in new bands arising at 540 nm and 545 nm, respectively. Isosbestic points at 329 nm in the case of 1a/b and at 256, 336, 352, 379, and 408 nm in the case of 2a/b indicate clean two-component processes. The thus reached photostationary states (PSSs) contain 87% of 1b and 82% of 2b, respectively (Table 1). Subsequent irradiation with visible light (λirr > 500 nm) effects decolorization and the original UV-spectra are completely restored, i.e. the ring-closed isomers are quantitatively converted into their ring-opened forms. Repetitive switching cycles revealed slight decomposition of 1a/b13 while 2a/b showed excellent fatigue resistance (Fig. 2, insets). Furthermore, both ring-closed isomers were found to be thermally stable over extended periods of time (days and weeks). Interestingly, the photochromic performance of 1a/b is strongly dependent on solvent polarity since in acetonitrile prolonged irradiation times are necessary to reach the PSS, which is composed of only 44% of 1b. This finding is further supported by comparing the quantum yields for ring closure, which show a marked decrease when going from methylene chloride to acetonitrile.‡ This effect can be attributed to the generation of a twisted intramolecular charge transfer (TICT) state upon excitation of 1a as suggested previously to explain solvent-dependent photochromism of related donor–acceptor dithienylethenes.14
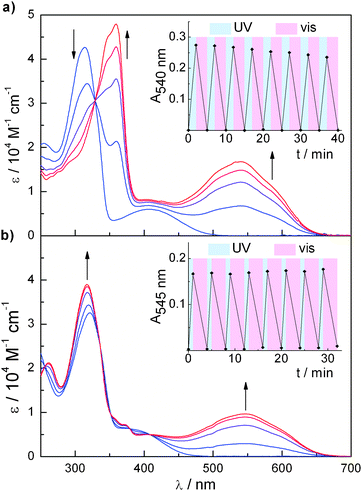 | ||
| Fig. 2 Photochromic behaviour: UV/vis absorption spectra during the course of irradiation (λirr = 313 nm) until reaching the PSS of (a) 1a (time intervals: t = 0, 60, 120, 180, and 500 s) and (b) 2a (time intervals: t = 0, 10, 30, 60, and 120 s) in CH2Cl2 (c = 2 × 10−5 M, 25 °C). Insets show repetitive switching cycles (λirr = 313 nm for ring-closure, λirr > 500 nm for ring-opening) between (a) 1a and 1b as well as (b) 2a and 2b. | ||
| Photochromisma | Associationc | |||||
|---|---|---|---|---|---|---|
| λ max/nm | Φ 313 nm a→b | Φ 546 nm b→a | Conv.b (%) | Δδmax/ppm | K a/M−1 | |
| a In CH2Cl2 at 25 °C. b Composition of the PSS upon irradiation of the open form (1a or 2a) with UV-light (λirr = 313 nm) determined by UPLC. c Derived from NMR-titration data in CDCl3, 25 °C.1 | ||||||
| 1a | 313, 408 | 0.08 | 0.02 | 87 | 7.57 ± 0.02 | 132 ± 11 |
| 1b | 359, 540 | 7.72 ± 0.06 | 231 ± 23 | |||
| 2a | 321, 380 | 0.18 | 0.02 | 82 | 7.75 ± 0.05 | 142 ± 13 |
| 2b | 317, 545 | 7.99 ± 0.03 | 318 ± 30 | |||
To investigate the association behaviour of each photochromic ADA maleimide pair (1a/b and 2a/b), NMR titrations with the complementary DAD receptor (3) were carried out in CDCl3. Changing chemical shifts of the central imide NH-proton were monitored with increasing amount of receptor and show characteristic binding isotherms (Fig. 3). In both cases, the binding curve of ring-closed isomer (1b or 2b) displays a steeper slope that is indicative of a stronger association. Evaluation of the data following established procedures15 allowed us to determine the respective association constants Ka (Table 1), which are in the expected range of related imide–melamine complexes.9d,15c Indeed, the association of each of the ring-closed isomers to the melamine receptor is stronger as compared to the respective ring-opened isomers. The Ka values approximately double upon switching to the ring-closed forms and a slightly stronger enhancement of binding was observed in the case of the ester derivatives 2a/b. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was verified by Job plots showing maxima at molar fractions xreceptor 3 = 0.5.‡ Most importantly, photochemical switching behaviour remains unaffected by the presence of melamine 3, as essentially the same quantum yields are observed even if the majority of the switch is complexed.‡
1 binding stoichiometry was verified by Job plots showing maxima at molar fractions xreceptor 3 = 0.5.‡ Most importantly, photochemical switching behaviour remains unaffected by the presence of melamine 3, as essentially the same quantum yields are observed even if the majority of the switch is complexed.‡
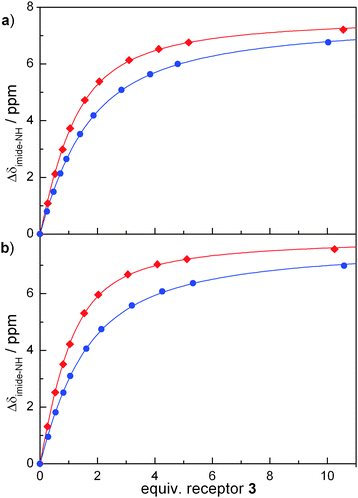 | ||
Fig. 3 Association behaviour: NMR-titration of ring-open isomers (blue circles) and ring-closed isomers (red diamonds) with receptor 3 in CDCl3 at 25 °C for (a) 1a and 1b as well as (b) 2a and 2b (lines show fitted curves for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherms). 1 binding isotherms). | ||
The observed general enhancement in the association of the ring-closed isomers with the receptor can primarily be related to the reduced electron-density of the maleimide core.16 This is nicely reflected in the significantly reduced reduction potential of 2b (Epred = −1.253 V) as compared to its ring-opened analogue 2a (Epred = −1.497 V). In the case of the 1a/b, the reduction potential is also reduced in the ring-closed form yet to a smaller extent (ΔEpred = −74 mV for 1a/b instead of ΔEpred = −244 mV for 2a/b, all values relative to an fc/fc+ standard).‡ Clearly when comparing both ring-closed isomers, the larger Ka value for 2b is due to the presence of the coupled electron-accepting ester groups, which further diminish electron density in the maleimide core. The overall reduction of electron density upon ring-closure is associated with an increased acidity of the central imide N–H group, whose interaction with the melamine's endocyclic N-atom seems to dominate the association event. This finding is in line with the geometry of the five-membered maleimide ring that leads to elongated and hence weaker interactions of both terminal imide carbonyl O-atoms with the two exocyclic N–H groups of the melamine receptor. From our experiments it seems that ring-closure in combination with π-conjugated electron-accepting groups is best suited for achieving enhanced binding constants for the presented photoswitchable imides.
The first encouraging example of a photoswitchable triple hydrogen-bonding motif, based on an electronic modulation of the participating basicity/acidity and therefore binding strength, has been developed. Ongoing efforts in our laboratories are concerned with the design of improved systems, which provide larger differences in association strength (ON/OFF ratios) as well as inherently stronger association. The latter could be accomplished by introducing more hydrogen-bonding sites, for example Meijer's quadruple hydrogen-bonding ureidopyrimidines,2b or simply by working in less polar media.15c In principle, such photoswitchable supramolecular “glue” should allow for the construction of light-responsive soft materials, for example by utilizing multivalent constructs as photochromic monomers or crosslinkers. Furthermore, our approach of exploiting the bridge of diarylethenes as a tunable functionality,16,17 which is electronically modulated by coupling terminal donors/acceptors appears to be a general strategy to photomodulate various functions and work along these lines will be reported in due course.
Generous support by the German Research Foundation (DFGviaSFB 658) and the Fonds der Chemischen Industrie is gratefully acknowledged. Wacker Chemie AG, BASF AG, Bayer Industry Services, and Sasol Germany are thanked for generous donations of chemicals.
Notes and references
- G. A. Jeffrey, An Introduction to Hydrogen Bonding, Oxford University Press, New York, 1997 Search PubMed.
- For multiple hydrogen-bonding motifs: (a) A. J. Wilson, Soft Matter, 2007, 3, 409–425 RSC; (b) R. P. Sijbesma and E. W. Meijer, Chem. Commun., 2003, 5–16 RSC; (c) G. Cooke and V. M. Rotello, Chem. Soc. Rev., 2002, 31, 275–286 RSC; (d) C. Schmuck and W. Wienand, Angew. Chem., 2001, 113, 4493–4499 ( Angew. Chem., Int. Ed. , 2001 , 40 , 4363–4369 ) CrossRef; (e) S. C. Zimmerman and P. S. Corbin, Struct. Bonding, 2000, 96, 63–94 CAS.
- For recent overviews, in particular dealing with photoresponsive materials, see: (a) F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37–54 RSC; (b) M.-M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS.
- (a) E. Breinlinger, A. Niemz and V. M. Rotello, J. Am. Chem. Soc., 1995, 117, 5379–5380 CrossRef CAS; (b) Y. Ge, R. Lilienthal and D. K. Smith, J. Am. Chem. Soc., 1996, 118, 3976–3977 CrossRef CAS; (c) Y. Ge, L. Miller, T. Ouimet and D. K. Smith, J. Org. Chem., 2000, 65, 8831–8838 CrossRef CAS; (d) J. Bu, N. D. Lilienthal, J. E. Woods, C. E. Nohrden, K. T. Hoang, D. Truong and D. K. Smith, J. Am. Chem. Soc., 2005, 127, 6423–6429 CrossRef CAS.
- (a) S. Yagai, T. Karatsu and A. Kitamura, Chem.–Eur. J., 2005, 11, 4054–4063 CrossRef CAS; (b) S. Yagai, T. Nakajima, T. Karatsu, K. Saitow and A. Kitamura, J. Am. Chem. Soc., 2004, 126, 11500–11508 CrossRef CAS; (c) F. Rakotondradany, M. A. Whitehead, A.-M. Lebuis and H. F. Sleiman, Chem.–Eur. J., 2003, 9, 4771–4780 CrossRef CAS.
- (a) M. S. Vollmer, T. D. Clark, C. Steinem and M. R. Ghadiri, Angew. Chem., 1999, 111, 1703–1706 ( Angew. Chem., Int. Ed. , 1999 , 38 , 1598–1601 ) CrossRef; (b) L. N. Lucas, J. van Esch, R. M. Kellog and B. L. Feringa, Chem. Commun., 2001, 759–760 RSC; (c) J. J. D. de Jong, L. N. Lucas, R. M. Kellog, J. van Esch and B. L. Feringa, Science, 2004, 304, 278–281 CrossRef CAS; (d) M. Takeshita, M. Hayashi, S. Kadota, K. H. Mohammed and T. Yamato, Chem. Commun., 2005, 761–763 RSC; (e) M. Takeshita, M. Hayashi and T. Miyazaki, Chem. Lett., 2010, 39, 82–83 CrossRef CAS.
- A. Goodman, E. Breinlinger, M. Ober and V. M. Rotello, J. Am. Chem. Soc., 2001, 123, 6213–6214 CrossRef CAS.
- An impressive, conceptually related example of a photoswitchable fulgimide has been described in T. Okuyama, Y. Yokoyama and Y. Yokoyama, Bull. Chem. Soc. Jpn., 2001, 74, 2181–2187 Search PubMed.
- (a) Y. Kyogoku, R. C. Lord and A. Rich, Proc. Natl. Acad. Sci. U. S. A., 1967, 57, 250–256 CAS; (b) C. S. Wilcox, E. Kim, D. Romano, L. H. Kuo, A. L. Burt and D. P. Curran, Tetrahedron, 1995, 51, 621–634 CrossRef CAS; (c) R. Deans, G. Cooke and V. M. Rotello, J. Org. Chem., 1997, 62, 836–839 CrossRef CAS; (d) F. Würthner and S. Yao, J. Org. Chem., 2003, 68, 8943–8949 CrossRef.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS.
- Please note that dithienylethenes have been utilized to photomodulate aggregation via H-bonding, see ref. 6b–e, and via π,π-stacking in combination with hydrophobic forces: (a) T. Hirose, K. Matsuda and M. Irie, J. Org. Chem., 2006, 71, 7499–7508 CrossRef CAS; (b) T. Hirose, M. Irie and K. Matsuda, Adv. Mater., 2008, 20, 2137–2141 CrossRef CAS. Photochromic purine analogues and their base pairing with thymidine have recently been reported in: (c) M. Singer and A. Jäschke, J. Am. Chem. Soc., 2010, 132, 8372–8377 CrossRef CAS.
- (a) K. Uchida, T. Ishikawa, M. Takeshita and M. Irie, Tetrahedron, 1998, 54, 6627–6638 CrossRef CAS; (b) S. Takami, S. Kobatake, T. Kawai and M. Irie, Chem. Lett., 2003, 32, 892–893 CrossRef CAS.
- Upon prolonged UV-irradiation (λirr = 260–380 nm) irreversible bleaching of the absorption band is observed, yet no by-product could be detected.
- (a) M. Irie and K. Sayo, J. Phys. Chem., 1992, 96, 7671–7674 CrossRef; (b) M. Ohsumi, M. Hazama, T. Fukaminato and M. Irie, Chem. Commun., 2008, 3281–3283 RSC.
- (a) K. A. Connors, Binding Constants, Wiley & Sons, New York, 1987 Search PubMed; (b) C. S. Wilcox, in Frontiers of Supramolecular Chemistry and Photochemistry, ed. H. J. Schneider and H. Dürr, VCH, Weinheim, 1991, pp. 123–143 Search PubMed; (c) F. Würthner, C. Thalacker, A. Sautter, W. Schärtl, W. Ibach and O. Hollricher, Chem.–Eur. J., 2000, 6, 3871–3886 CrossRef CAS.
- This effect is somewhat related to photoswitchable changes in Lewis-acidity observed by Branda and coworkers in dioxaborolane bridged dithienylethenes, which interconvert between a (hetero)aromatic open and a cross-conjugated closed isomer: V. Lemieux, M. D. Spantulescu, K. K. Baldridge and N. R. Branda, Angew. Chem., Int. Ed., 2008, 47, 5034–5037 Search PubMed.
- For elegant examples exploiting chemical reactivity differences of the bridge functionality, see: (a) D. Sud, T. J. Wigglesworth and N. R. Branda, Angew. Chem., Int. Ed., 2007, 46, 8017–8019 CrossRef CAS; (b) V. Lemieux, S. Gauthier and N. R. Branda, Angew. Chem., Int. Ed., 2006, 45, 6820–6824 CrossRef CAS; (c) V. Lemieux and N. R. Branda, Org. Lett., 2005, 7, 2969–2972 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Synthetic details and characterization data. See DOI: 10.1039/c0cc02339f |
| This journal is © The Royal Society of Chemistry 2011 |
