Protein aggregate structure under high pressure†
Andrew J.
Jackson
ab and
Duncan J.
McGillivray
*c
aNIST Center for Neutron Research, Gaithersburg, MD 20899, USA
bDepartment of Chemical Engineering, University of Delaware, Newark, DE 19716, USA
cDepartment of Chemistry, The University of Auckland, Auckland 1142, New Zealand. E-mail: d.mcgillivray@auckland.ac.nz
First published on 12th October 2010
Abstract
We show that casein protein micelles—a complex protein/inorganic phosphate structure—can be subjected to high pressures (up to 350 MPa) while making in situ structural measurements using (ultra-)small angle neutron scattering, to give insight to the protein structure, aggregation and stability under pressure.
In this communication we report on initial detailed structural studies of the behaviour of casein protein aggregates when subjected to high pressure. The casein micelle is a complex consisting of low-structure casein proteins (α-, β- and κ-caseins), existing in a loose and highly hydrated framework which stabilises nanocrystals of insoluble colloidal calcium phosphate (CCP) in solution, and are a major protein constituent of milk.1,2 The detailed structure of the ∼300 nm and roughly spherical micelles, including the existence of any sub-structure, and the disposition of the CCP within the micelle, are questions that have been incompletely addressed by numerous groups.3–7
The current understanding of the casein micelle postulates that the loosely packed structure consists of an outer “hairy” layer enriched in κ-casein, which serves as a steric barrier to prevent micelle flocculation, while the α- and β-caseins bind through phosphoserine residues to the CCP. Controversy exists, however, concerning whether the micelle is formed from the condensation of smaller protein–CCP aggregates that persist in the micelle (“sub-micelles”), or is an un-differentiated protein network.2,3,5,8 Recent synchrotron X-ray scattering results, while giving useful information on the shape of the CCP, were not able to unambiguously resolve whether or not the sub-micelles exist, but the authors suggested an undifferentiated protein matrix.9Neutron scattering work by Holt et al.3,6 also suggest that the protein matrix is undifferentiated, but that the CCP can be stably synthesised as a nanoparticle with a casein protein coating. Earlier neutron scattering work by Hansen et al., however, suggested a sub-micellar structure with a “dense core”.7
Of further recent interest has been understanding of how the casein micelle structure responds to high pressures, and the reversibility of these changes.10,11 Understanding protein conformation changes with high pressure in general can give insight into the native self-organisation that gives rise to protein structure,12 and may give insight into the aggregates such as protein fibrils that can be formed from protein misfolding.13 In the case of the casein micelle, knowledge of how the micelle breaks down may give more information on the internal organisation of the micelle.
Also for casein micelles in particular, there is significant interest in the role of the CCP in stabilising the protein structure. When subjected to high pressures it is well reported, primarily through light scattering measurements, that the casein micelle structure breaks down (with consequent reduction in the solution turbidity).10,11,14 This collapse in the micelle structure has been attributed to the break down of the balance between hydrophobic and electrostatic interaction forces in the aggregate, and is found to be strongly connected to the CCP in the system.11,14 However, there is disagreement as to the ultimate fate of the CCP after pressure release—although this may depend on the extent of micelle disruption.
Detailed structural work performed using (ultra-)small angle neutron scattering ((U)SANS) in multiple isotopic contrasts has allowed us insight into the casein micelle structure over three orders of magnitude in length scales. The casein micelles were then subjected to up to 350 MPa of applied hydrostatic pressure while being continually measured in situ, allowing us to gain the first detailed structural information on the nature of the casein micelle break-down, and the extent of reversibility of the observed changes. This information gives insight into the as-yet unresolved issue of whether the casein micelle is made up of smaller “sub-micelle” fragments and provides a basis from which to further investigate the effects of environmental conditions (for example, pH and temperature) on the protein complex's structural stability.
Measurements of casein micelles were made in the presence of naturally-occurring soluble proteins and other milk components (denoted skim), and also on isolated casein micelles in solution (denoted cas),‡§ suspended in D2O, H2O or a mixed isotope solvent (CM3 - 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume H2O
1 by volume H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O). Samples were measured on the 30 m SANS instrument NG3,15 and a selection was measured on the perfect crystal Bonse-Hart USANS instrument BT5,16 both at the NIST Center for Neutron Research, covering a momentum transfer range (Q/Å−1) of between 1 × 10−4 Å−1 and 0.2 Å−1. The data were reduced and analysed using software from NIST.17 The samples were then subjected to a range of successively higher pressures, up to a maximum of 350 MPa. Reversibility of observed changes in the micelle structure were assessed by returning to low pressure values after each increase in pressure, while stability of the casein micelles at high pressures was determined by continuous monitoring of changes in the measured structure with time.
D2O). Samples were measured on the 30 m SANS instrument NG3,15 and a selection was measured on the perfect crystal Bonse-Hart USANS instrument BT5,16 both at the NIST Center for Neutron Research, covering a momentum transfer range (Q/Å−1) of between 1 × 10−4 Å−1 and 0.2 Å−1. The data were reduced and analysed using software from NIST.17 The samples were then subjected to a range of successively higher pressures, up to a maximum of 350 MPa. Reversibility of observed changes in the micelle structure were assessed by returning to low pressure values after each increase in pressure, while stability of the casein micelles at high pressures was determined by continuous monitoring of changes in the measured structure with time.
Several features can be immediately determined from the scattering data. As can be seen in Fig. 1, a representative set of scattering curves from a skim sample in solvent contrasts at atmospheric pressure, there are two distinct features in the data—a low Q turnover at ∼2 × 10−3 Å−1, representative of the size of the overall casein structure, and a second feature at 3.5 × 10−2 Å−1 which has been attributed to CCP spacing or casein sub-micelles.9 These features are also seen in the cas samples (not shown), suggesting independence from the other protein and sugar components of the skim sample. However, for both skim and cas the feature at 3.5 × 10−2 Å−1 is only weakly visible in the H2O sample, and is therefore not directly linked to the CCP (which has a calculated SLD of 2.5 × 10−6 Å−2), but rather the hydrated protein.
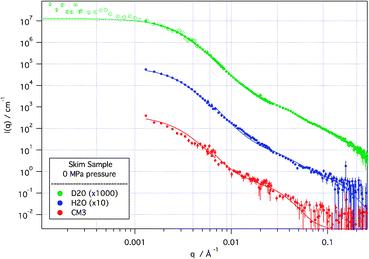 | ||
Fig. 1
SANS and de-smeared USANS (hollow points) scattering curves from skim sample at 0 MPa applied pressure in D2O, H2O and CM3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume D2O 1 by volume D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) solvents. Solid lines are modelled scattering from the model described in the text. D2O and H2O scattering curves are off-set for clarity. Error bars represent ±1 standard deviation. H2O) solvents. Solid lines are modelled scattering from the model described in the text. D2O and H2O scattering curves are off-set for clarity. Error bars represent ±1 standard deviation. | ||
Modelling, with the fits shown in Fig. 1, describes the casein micelle in terms of a polydisperse protein sphere, with internal structure that we assign to a protein-decorated CCP (modelled as a polydisperse core–shell particle) in a protein matrix. The D2O data were fitted first and the other contrasts then fitted, allowing only the solvent and protein scattering length density to vary. In a description similar to that used in the synchrotron X-ray work of Shukla et al.9 the polydispersity accounts, though not explicitly, for the diffuseness of the hairy κ-casein surface layer. The protein-decorated CCP units are fitted as an ∼1 nm radius spherical particle of calcium phosphate, surrounded by a loose spherical protein shell of ∼3 nm radius which is distinct from the background protein matrix. The multiple contrasts show that the protein coating of the CCP is more densely packed than the general protein matrix, consistent with protein bound specifically to the CCP. The volume fraction of these CCP–protein units was found to be ∼50% of the casein micelle.
The protein-decorated CCP units are sufficient to describe the internal structure of the casein micelle as detected by SANS, and can be thought of as a hybrid of the sub-micelle and undifferentiated protein models. They appear to be distinct protein structures of defined size, but they exist in an undifferentiated protein background that is more solvent accessible and can be envisaged as the network that holds the micelle together. Furthermore, each “sub-micelle” appears to contain only one CCP.
This observed structure, however, is strongly and irreversibly modified by the application of high pressure. As can be seen in Fig. 2, when subjected to high pressures the scattering changes smoothly. The changes in structure caused by the applied pressure occur rapidly within the first five minutes from reaching the new pressure, and were stable over a period of hours (data not shown).
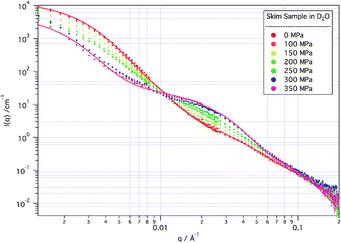 | ||
| Fig. 2 SANS from skim samples in D2O solvent at increasing applied pressure. Modelled fits to the 0 MPa and 350 MPa data are indicated by the solid lines. | ||
Consistent with reported light scattering and turbidity measurements,10,11 little change is apparent at applied pressures up to 100 MPa. Between 100 MPa and 250 MPa there is a reduction in the intensity of the scattering from the micelle as a whole, without significant change in the micelle size (as determined by the observed inflection in the data which remains at around 0.004 Å−1), while there is a gradual growth in structures (associated in modelling with free “sub-micelles”) giving rise to scattering at ∼0.02 Å−1. At the highest pressures measured here (300 and 350 MPa), the growth of these “sub-micellar” structures has increased significantly, and the structure of the micelle as a whole has begun to be affected. Above 250 MPa, in fact, the size of the casein micelle actually increases with increasing pressure, although the volume fraction of micelle in the solution decreases.
The reversibility of the changes in the micelle structure was tested by releasing the pressure on the system after each high pressure measurement, to compare the extent to which the changes in the scattering relaxed back to that from the unperturbed system. Measurements after pressure release were performed multiple times, in an attempt to determine the kinetics of the relaxation process. However, as above it was found that the structural rearrangement occurred on a rapid timescale (less than 5 min) and then remained unchanging with time. Although in each case the scattering did revert somewhat back to the original scattering (see Fig. 3), in no case did the structure fully recover.
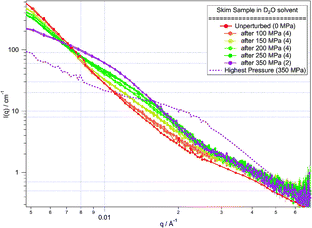 | ||
| Fig. 3 SANS from skim samples in D2O solvent at low pressure after measurement at high pressure. Each low pressure measurement was repeated to determine the sample stability (number of repeats overlaid is shown in brackets). The scattering at the highest applied pressure (350 MPa, dashed line) is shown for comparison. Lines are guides to the eye. | ||
Modelling of the highest pressure sample (Fig. 4), using the same model as was used on the unperturbed system, shows that the fundamental structures in the system do not change. The scattering is still well described by a model of a protein sphere, with internal structure described by a loose protein decoration of CCP. However, a new term is required to account for the growth in free “sub-micellar” components—namely, protein-decorated CCP structures not part of the casein micelle aggregate. These free “sub-micelles” have close to the same size as those that make up the casein micelle aggregate and as their population increases, so there is a commensurate decrease in the population of casein micelles.
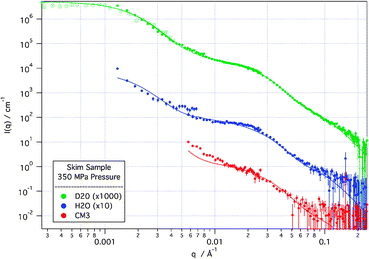 | ||
Fig. 4
SANS (solid points) and de-smeared USANS (hollow points) scattering curves from skim sample at 350 MPa applied pressure in D2O, H2O and CM3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume D2O 1 by volume D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) solvents. Solid lines are modelled scattering from the model described in the text. D2O and H2O scattering curves are off-set for clarity. H2O) solvents. Solid lines are modelled scattering from the model described in the text. D2O and H2O scattering curves are off-set for clarity. | ||
Examining the behaviour of the cas sample in D2O in the unperturbed state and at 350 MPa (not shown) reveals an almost complete disintegration (ca. 98%) of the casein micelles into “sub-micelles”. This suggests that the presence of non-casein proteins in the “skim” samples increases the pressure tolerance of the casein micelle.
It appears significant that the break down of the micelle does not seem to release CCP from a protein coating, free in solution, but rather releases the CCP-containing “sub-micelle” from the larger micelle structure. This reinforces the idea that the “sub-micellar” units are distinct even within the casein micelle, and may make the recovery of the micelle structure on pressure release more feasible. Conversely, the sequestration of calcium from the system, through for example the use of a chelating agent such as EDTA, should lead to the collapse of the “sub-micelle” structure. Further experiments will address these issues.
Overall, the application for the first time of in situneutron scattering, in multiple contrast solvents, to casein proteins under high pressure has given insights into both the structure of the casein micelle, and its disruption and breakdown. The methods developed in this work will be extended to further studies of this protein–inorganic complex, and to structural studies of the unfolding of other protein systems.
The authors are grateful for the inspiration for this work provided by Prof. John W. White (Australian National University), useful discussions with Dr Jitendra P. Mata (Bragg Institute, Australian Nuclear Science and Technology Organisation), and experimental assistance by Mr Stewart Pullen (ANSTO). DJM gratefully acknowledges financial support from an Australian Institute of Nuclear Science and Engineering Research Fellowship, and from the NIST Center for Neutron Research Guest Researcher programme. This work utilized facilities supported in part by the National Science Foundation under Agreement No. DMR-0454672.
Notes and references
- D. S. Home, Curr. Opin. Colloid Interface Sci., 2006, 11, 148 CrossRef.
- H. M. Farrell, et al. , Curr. Opin. Colloid Interface Sci., 2006, 11, 135 CrossRef CAS.
- C. Holt, et al. , Colloids Surf., A, 2003, 213, 275 CrossRef CAS.
- D. S. Horne, Curr. Opin. Colloid Interface Sci., 2002, 7, 456 CrossRef CAS; D. J. McMahon and W. R. McManus, J. Dairy Sci., 1998, 81, 2985 CrossRef CAS.
- P. Walstra, Int. Dairy J., 1999, 9, 189 CrossRef CAS.
- C. Holt, et al. , Eur. J. Biochem., 1998, 252, 73 CrossRef CAS.
- S. Hansen, et al. , Eur. Biophys. J., 1996, 24, 143 CAS.
- P. H. Stothart and D. J. Cebula, J. Mol. Biol., 1982, 160, 391 CAS.
- A. Shukla, et al. , Soft Matter, 2009, 5, 2884 RSC.
- T. Huppertz, et al. , Biochim. Biophys. Acta, 2006, 1764, 593 CAS; S. G. Anema, Int. J. Dairy Technol., 2008, 61, 245 CrossRef CAS.
- V. Orlien, et al. , J. Dairy Sci., 2010, 93, 12 CrossRef CAS.
- S. Perrett and J. M. Zhou, Biochim. Biophys. Acta, 2002, 1595, 210 CAS.
- D. Foguel, et al. , Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9831 CrossRef CAS; D. Foguel and J. L. Silva, Biochemistry, 2004, 43, 11361 CrossRef CAS; V. N. Uversky, Cell. Mol. Life Sci., 2003, 60, 1852 CrossRef CAS.
- R. Gebhardt, et al. , Eur. Biophys. J., 2006, 35, 503 CrossRef CAS.
- C. J. Glinka, et al. , J. Appl. Crystallogr., 1998, 31, 430 CrossRef CAS.
- J. G. Barker, et al. , J. Appl. Crystallogr., 2005, 38, 1004 CrossRef CAS.
- S. R. Kline, J. Appl. Crystallogr., 2006, 39, 895 CrossRef CAS.
- R. Jenness and J. Koops, Neth. Milk Dairy J., 1962, 16, 153 CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ The identification of commercial equipment or materials does not constitute endorsement by the NIST, nor does it imply that the equipment or materials identified are necessarily the best for the purpose. |
| § Skim samples were prepared from skim milk powder (Organic Valley Farms, La Farge, Wisconsin) suspended from powder to a concentration of 10% (w/w) using an appropriate isotopic solvent with added sodium azide (0.01% w/w). The final solutions had a measured pH of 6.6. Isolated casein micelles (cas samples) were prepared from the base skim solutions centrifuged at an RCF of 2000 for more than 12 hours, then the casein micelle pellet formed was separated from the supernatant fluid and re-suspended in simulated milk ultra-filtrate, SMUF,18 made up in appropriate contrasts. This process was performed at least twice for each sample. The final casein micelle content in cas samples was made up to be 3% (w/w). Measurements were performed in a high pressure cell designed by Prof. Mark McHugh, from the University of Virginia. |
| This journal is © The Royal Society of Chemistry 2011 |
