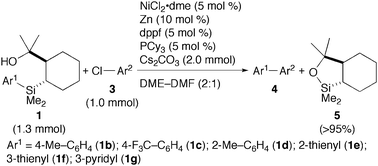
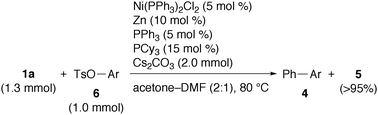
Nickel-catalysed cross-coupling reaction of aryl(trialkyl)silanes with aryl chlorides and tosylates†
Shi
Tang
,
Masahide
Takeda
,
Yoshiaki
Nakao
* and
Tamejiro
Hiyama‡
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto 615-8510, Japan. E-mail: yoshiakinakao@npc05.mbox.media.kyoto-u.ac.jp; Fax: +81 75 383 2445; Tel: +81 75 383 2443
First published on 23rd August 2010
Abstract
Using highly stable, readily accessible, and recyclable 2-(2-hydroxyprop-2-yl)cyclohexyl-substituted arylsilanes activated by a mild carbonate base, nickel-catalysed silicon-based aryl–aryl cross-coupling reaction proceeds for the first time with inexpensive aryl chlorides and tosylates in a highly chemoselective manner.
Biaryls play key roles in many functional organic molecules from pharmaceuticals to optoelectronic materials. Metal-catalysed cross-coupling reactions have innovated synthetic processes to access these structural motifs.1 While a number of protocols have been developed using a range of aryl nucleophiles, increasing demands for chemoselectivity, availability, stability, and non-toxicity have made arylsilanes an attractive alternative to conventionally employed arylmagnesium, -zinc, -tin, and -boron reagents.2 On the other hand, use of inexpensive and abundant metal complexes such as iron and nickel instead of palladium for the cross-coupling reactions has also gained much attention in this field. Nevertheless, such protocols have rarely met with success in the silicon-based cross-coupling reactions except for those with alkyl electrophiles by nickel catalysis.3 With these background in mind, we examined a silicon-based aryl–aryl cross-coupling reaction using a nickel catalyst. We have recently developed novel tetraorganosilicon reagents that participate in a variety of transition metal-catalysed transformations including cross-coupling and carbonyl addition reactions through transmetallation to palladium, copper, and rhodium.4 Formation of pentacoordinate silicates and/or metal alkoxides is likely responsible for the transmetallation under mild conditions with carbonate salts as a mild activator. The new protocol achieves highly chemoselective C–C bond forming reactions using highly robust tetraorganosilicon reagents. Herein, we report that such a reagent design also allows nickel-catalysed aryl–aryl cross-coupling using arylsilanes with inexpensive aryl chlorides5 and tosylates.6 We have found that newly developed 2-(2-hydroxyprop-2-yl)cyclohexyl-substituted arylsilanes (1, Scheme 1) serve as an excellent arylating agent over originally developed 2-(hydroxymethyl)phenyl-substituted ones 2.
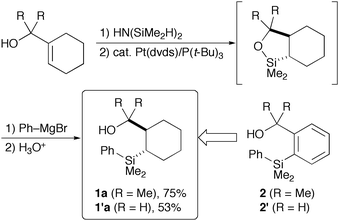 | ||
| Scheme 1 Synthesis of tetraorganosilicon reagents 1a and 1′a. | ||
We have experienced that the original 2-(hydroxymethyl)phenyl-substituted arylsilanes 2 sometimes suffer from cleavage of the substituted phenyl–Si bond depending on reaction conditions.4 We hypothesised that more robust but reactive silicon reagents could be available by making the phenyl group saturated based on the fact that C(sp3)–Si bonds are generally much more stable than C(sp2)–Si bonds. The preparation of 2-(2-hydroxyprop-2-yl)cyclohexyl-(dimethyl)phenylsilane 1a and 2-hydroxymethyl analog 1′a is shown in Scheme 1. O-Silylation of 1-(2-hydroxyalk-2-yl)cyclohexene was performed with HN(SiMe2H)2, and the resulting silyl ethers were subjected to platinum-catalysed intramolecular hydrosilylation7 to give cyclic silyl ethers that underwent ring-opening reactions upon treatment with phenyl Grignard reagent.8 Thus, the new tetraorganosilicon reagents are conveniently prepared in one-pot from readily available cyclohexenylmethanols. The trans geometry of the silicon reagents thus obtained was unambiguously confirmed by X-ray crystallography of 3,5-dinitrobenzoate derived from 1′a (Fig. 1).†
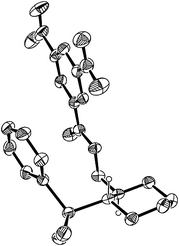 | ||
| Fig. 1 ORTEP drawing for 3,5-dinitrobenzoate of 1′a. | ||
We then examined the nickel-catalysed reaction of the arylsilanes with 4-chloroanisole (3a) (eqn (1)). After screening various parameters, the reaction of 1a (1.3 mmol) with 3a (1.0 mmol) in the presence of NiCl2·dme (5 mol%), Zn [a reducing agent for nickel(II), 10 mol%], dppf (5 mol%), PCy3 (5 mol%), and Cs2CO3 (2.0 mmol) in DME–DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 60 °C for 30 h gave 4-phenylanisole (4aa) in 62% yield after isolation by silica gel chromatography. We also observed quantitative recovery of a cyclic silyl ether 5 by GC. The use of two different phosphorus ligands was critical to achieve a good conversion of 3a: either dppf or PCy3 alone resulted in less than 50% conversion. While rationale for this mixed ligand system is under investigation, similar protocols for nickel-catalysed Suzuki–Miyaura cross-coupling reactions with aryl halides, tosylates, and mesylates have been reported.9 Inexpensive bases such as K2CO3 and K3PO4 were also examined, but gave 4aa in modest yields (∼50%). The cross-coupling reaction using 1′a resulted in a poor yield (<10%) due to competitive oxidation of the hydroxymethyl group.4d The yield obtained with modified original phenylsilane 2 was modest in spite of complete conversion of the silicon reagent, whereas 2′ suffered from the oxidation of the hydroxymethyl group.
1) at 60 °C for 30 h gave 4-phenylanisole (4aa) in 62% yield after isolation by silica gel chromatography. We also observed quantitative recovery of a cyclic silyl ether 5 by GC. The use of two different phosphorus ligands was critical to achieve a good conversion of 3a: either dppf or PCy3 alone resulted in less than 50% conversion. While rationale for this mixed ligand system is under investigation, similar protocols for nickel-catalysed Suzuki–Miyaura cross-coupling reactions with aryl halides, tosylates, and mesylates have been reported.9 Inexpensive bases such as K2CO3 and K3PO4 were also examined, but gave 4aa in modest yields (∼50%). The cross-coupling reaction using 1′a resulted in a poor yield (<10%) due to competitive oxidation of the hydroxymethyl group.4d The yield obtained with modified original phenylsilane 2 was modest in spite of complete conversion of the silicon reagent, whereas 2′ suffered from the oxidation of the hydroxymethyl group.
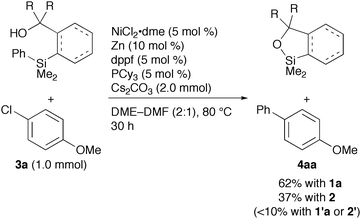 | (1) |
The scope of the present aryl–aryl cross-coupling is shown in Table 1. A range of substrates including aniline, ester, aldehyde, enolisable ketone, and nitrile derivatives participated in the reaction to give respective biaryls in modest to good yields (entries 1–13). The reaction can be performed on a 10 mmol-scale in good yield, allowing recovery of 5 in 93% yield by distillation of a crude mixture (entry 5). The reaction conditions employing the mild carbonate base allows silyl ethers to be tolerated in contrast to the conventional fluoride-activation which results in desilylation (entries 2 and 10). Heteroaryl chlorides also gave phenylated heteroarenes (entries 14–16). Substituted phenylsilanes 1b–1d as well as heteroarylsilanes 1e–1g were prepared similarly from the corresponding aryl Grignard reagents and reacted with aryl chlorides in good yields irrespective of electronic and steric characters of the substituents (entries 17–22).
| Entry | 1 | Cl–Ar2 | Temp/°C | Time/h | Yield of 4a (%) |
|---|---|---|---|---|---|
a Isolated yield.
b Run with acetone–DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a solvent.
c Run on a 10 mmol-scale.
d
5 was also isolated in 93% yield by distillation.
e Run in the absence of PCy3. 1) as a solvent.
c Run on a 10 mmol-scale.
d
5 was also isolated in 93% yield by distillation.
e Run in the absence of PCy3.
|
|||||

|
|||||
| 1 | 1a | Me (3b) | 60 | 36 | 78 (4ab) |
| 2 | 1a | CH2OSiMe2t-Bu (3c) | 60 | 30 | 70 (4ac) |
| 3b | 1a | NH2 (3d) | 60 | 24 | 51 (4ad) |
| 4 | 1a | F (3e) | 75 | 24 | 82 (4ae) |
| 5c | 1a | CO2Et (3f) | 75 | 30 | 82 (4af)d |
| 6e | 1a | CHO (3g) | 75 | 24 | 52 (4ag) |
| 7 | 1a | Ac (3h) | 75 | 24 | 85 (4ah) |
| 8b | 1a | CN (3i) | 75 | 20 | 71 (4ai) |
| 9 | 1a | CF3 (3j) | 75 | 24 | 83 (4aj) |
| 10 | 1a |

|
60 | 30 | 72 (4ak) |
| 11 | 1a |

|
60 | 30 | 73 (4al) |
| 12b | 1h |

|
60 | 30 | 77 (4am) |
| 13e | 1a |

|
60 | 30 | 50 (4an) |
| 14 | 1a |

|
60 | 36 | 67 (4ao) |
| 15b | 1a |

|
60 | 24 | 48 (4ap) |
| 16b | 1a |

|
60 | 24 | 65 (4aq) |
| 17 | 1b | 3f | 75 | 24 | 77 (4bf) |
| 18 | 1c | 3f | 75 | 24 | 84 (4cf) |
| 19 | 1d | 3f | 75 | 24 | 75 (4df) |
| 20 | 1e | 3b | 60 | 36 | 63 (4eb) |
| 21 | 1f | 3b | 60 | 24 | 75 (4fb) |
| 22 | 1g | 3b | 75 | 24 | 56 (4gb) |
Having had succeeded in the biaryl synthesis from aryl chlorides, we turned our attention to that with aryl tosylates. Because of their ready availability from various inexpensive phenol derivatives, the cross-coupling reaction with aryl tosylates and mesylates has gained much importance.10 While the silicon-based cross-coupling with these aryl electrophiles would be of great synthetic potential, only limited examples have been reported using trialkoxy(aryl)silanes and palladium catalysts in the presence of an excess amount of highly nucleophilic and expensive TBAF as a fluoride activator.6 After brief tuning of the reaction conditions, we found that the reaction of 1a (1.3 mmol) with unactivated 4-methoxyphenyl tosylate (6a, 1.0 mmol) proceeded smoothly in the presence of Ni(PPh3)2Cl2 (5 mol%), Zn (10 mol%), extra PPh3 (5 mol%), PCy3 (15 mol%), and Cs2CO3 (2.0 mmol) in acetone–DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C for 24 h to give 4aa in 72% yield (entry 1 of Table 2). A range of functionalised aryl tosylates reacted with 1a to give the corresponding biaryls (entries 2–8). An aryl mesylate also participated in the reaction to give biaryl 4ab in comparable yield (entry 3).
1) at 80 °C for 24 h to give 4aa in 72% yield (entry 1 of Table 2). A range of functionalised aryl tosylates reacted with 1a to give the corresponding biaryls (entries 2–8). An aryl mesylate also participated in the reaction to give biaryl 4ab in comparable yield (entry 3).
| Entry | TsO–Ar | Time/h | Yield of 3a (%) |
|---|---|---|---|
| a Isolated yields. b Run with p-tolyl methanesulfonate. c Run with Ni(PPh2Me)2Cl2 (5 mol%) and PPh2Me (5 mol%) instead of Ni(PPh3)2Cl2 (5 mol%) and PPh3 (5 mol%). | |||

|
|||
| 1 | OMe (6a) | 24 | 72 (4aa) |
| 2 | Me (6b) | 24 | 73 (4ab) |
| 3 | Me (6′b)b | 24 | 71 (4ab) |
| 4c | F (6c) | 20 | 73 (4ae) |
| 5c | CO2Me (6d) | 20 | 83 (4ar) |
| 6c | Ac (6e) | 20 | 73 (4ah) |
| 7c | CN (6f) | 20 | 64 (4ai) |
| 8 |

|
24 | 62 (4ak) |
In summary, we have demonstrated that newly developed arylsilane reagents 1 effect the nickel-catalysed silicon-based aryl–aryl cross-coupling reaction for the first time. Use of highly stable, readily accessible, and recyclable aryl(trialkyl)silanes activated by a mild carbonate base in the presence of a nickel catalyst prepared in situ from inexpensive nickel(II) salts would merit synthetic organic chemists both in academia and industry to perform chemoselective biaryl synthesis.
The authors are grateful to Professor Masaki Shimizu for X-ray crystallographic analysis. This work has been supported financially by a Grant-in-Aid for Priority Areas “Synergy of Elements” from MEXT. S.T. acknowledges the JSPS for a postdoctoral fellowship.
Notes and references
- (a) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 1998 Search PubMed; (b) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed.
- (a) T. Hiyama, Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 1998, pp. 421–453 Search PubMed; (b) T. Hiyama and E. Shirakawa, Top. Curr. Chem., 2002, 219, 61 CAS; (c) S. E. Denmark and R. F. Sweis, in Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd edn, 2004, pp. 163–216 Search PubMed; (d) J. Tsuji, Palladium Reagents and Catalysts, John Wiley & Sons, Chichester, 2004, pp. 338–351 Search PubMed.
- (a) J.-Y. Lee and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 5616 CrossRef CAS; (b) D. A. Powell and G. C. Fu, J. Am. Chem. Soc., 2004, 126, 7788 CrossRef CAS; (c) N. A. Strotman, S. Sommer and G. C. Fu, Angew. Chem., Int. Ed., 2007, 46, 3556 CrossRef CAS; (d) X. Dai, N. A. Strotman and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 3302 CrossRef CAS.
- (a) Y. Nakao, H. Imanaka, A. K. Sahoo, A. Yada and T. Hiyama, J. Am. Chem. Soc., 2005, 127, 6952 CrossRef CAS; (b) Y. Nakao, J. Chen, H. Imanaka, T. Hiyama, Y. Ichikawa, W. L. Duan, R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2007, 129, 9137 CrossRef CAS; (c) J. Chen, M. Tanaka, M. Takeda, A. K. Sahoo, A. Yada, Y. Nakao and T. Hiyama, Bull. Chem. Soc. Jpn., 2010, 83, 554 CrossRef CAS; (d) Y. Nakao, M. Takeda, T. Matsumoto and T. Hiyama, Angew. Chem., Int. Ed., 2010, 49, 4447 CrossRef CAS . (Hydroxymethyl)phenyl-substituted silicon reagents (HOMSi reagents) are available from Advanced Molecular Technologies Pty Ltd (http://www.amtechpl.com).
- For palladium-catalysed silicon-based aryl–aryl cross-coupling with aryl chlorides, see: (a) K.-i. Gouda, E. Hagiwara, Y. Hatanaka and T. Hiyama, J. Org. Chem., 1996, 61, 7232 CrossRef CAS; (b) E. Hagiwara, K.-i. Gouda, Y. Hatanaka and T. Hiyama, Tetrahedron Lett., 1997, 38, 439 CrossRef CAS; (c) H. M. Lee and S. P. Nolan, Org. Lett., 2000, 2, 2053 CrossRef CAS; (d) T. Koide and A. Mori, Synlett, 2003, 1850 CAS; (e) C. Wolf, R. Lerebours and E. H. Tanzini, Synthesis, 2003, 2069 CrossRef CAS; (f) A. K. Sahoo, Y. Nakao and T. Hiyama, Chem. Lett., 2004, 632 CrossRef CAS; (g) A. K. Sahoo, T. Oda, Y. Nakao and T. Hiyama, Adv. Synth. Catal., 2004, 346, 1715 CrossRef CAS; (h) C. Wolf and R. Lerebours, Org. Lett., 2004, 6, 1147 CrossRef CAS; (i) M. Murata, S. Yoshida, S.-i. Nirei, S. Watanabe and Y. Masuda, Synlett, 2006, 118 CrossRef CAS; (j) J.-H. Li, C.-L. Deng and Y.-X. Xie, Synthesis, 2006, 969 CrossRef CAS; (k) E. Alacid and C. Nájera, Adv. Synth. Catal., 2006, 348, 945 CrossRef CAS; (l) M. Endo, T. Sakurai, S. Ojima, T. Katayama, M. Unno, H. Matsumoto, S. Kowase, H. Sano, M. Kosugi and K. Fugami, Synlett, 2007, 749 CAS; (m) L. Ackermann, C. J. Gschrei, A. Althammer and M. Riederer, Chem. Commun., 2006, 1419 RSC; (n) S. E. Denmark, J. D. Baird and C. S. Regens, J. Org. Chem., 2008, 73, 1440 CrossRef CAS; (o) S. E. Denmark, R. C. Smith, W.-T. T. Chang and J. M. Muhuhi, J. Am. Chem. Soc., 2009, 131, 3104 CrossRef CAS; (p) S. E. Denmark and J. D. Baird, Tetrahedron, 2009, 65, 3120 CrossRef CAS; (q) X. Zhang, Q. Xia and W. Chen, Dalton Trans., 2009, 7045 RSC; (r) S. M. Raders, J. V. Kingston and J. G. Verkade, J. Org. Chem., 2010, 75, 1744 CrossRef CAS.
- For palladium-catalysed silicon-based aryl–aryl cross-coupling with aryl tosylates and mesylates, see: (a) L. Zhang and J. Wu, J. Am. Chem. Soc., 2008, 130, 12250 CrossRef CAS; (b) L. Zhang, J. Qing, P. Yang and J. Wu, Org. Lett., 2008, 10, 4971 CrossRef CAS; (c) C. M. So, H. W. Lee, C. P. Lau and F. Y. Kwong, Org. Lett., 2009, 11, 317 CrossRef CAS.
- K. Tamao, Y. Nakagawa, H. Arai, N. Higuchi and Y. Ito, J. Am. Chem. Soc., 1988, 110, 3712 CrossRef CAS.
- See ESI† for details.
- (a) V. Percec, G. M. Golding, J. Smidrkal and O. Weichold, J. Org. Chem., 2004, 69, 3447 CrossRef CAS; (b) V. Kogan, Tetrahedron Lett., 2006, 47, 7515 CrossRef CAS.
- For a review, see: A. Littke, in Modern Arylation Methods, ed. L. Ackermann,Wiley-VCH, Weinheim, 2009, pp. 25–67 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental information, spectral data and crystallographic data (excluding structure factors) for the structure of 3,5-dinitrobenzoate of 1′a. CCDC 779621. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc02173c |
| ‡ Present address: Research & Development Initiative, Chuo University, Bunkyo-ku, Tokyo 112-8551, Japan. |
| This journal is © The Royal Society of Chemistry 2011 |