Supramolecular dendrimer capsules by cooperative binding†‡
Rong
Ju
a,
Matthew
Tessier
b,
Lynda
Olliff
c,
Robert
Woods
b,
Anne
Summers
c and
Yan
Geng
*a
aDepartment of Chemistry, University of Georgia, Athens, GA 30602, USA. E-mail: ygeng@chem.uga.edu
bCCRC, University of Georgia, Athens, GA 30602, USA
cDepartment of Microbiology, University of Georgia, Athens, GA 30602, USA
First published on 5th August 2010
Abstract
We exploit the unique features of dendrimers as molecular nanospheres with many peripheral binding sites to show that maximizing cooperative binding enables a novel modular self-assembly approach to construct supramolecular capsules.
Cooperativity is a general principle that governs multivalent binding between two entities, e.g. multivalent ligand-receptor interactions, as well as subunit binding in self-assembly processes, e.g. collective base pairing in DNA double-helix formation and assembling of protein subunits into viral capsid or cytoskeleton superstructures.1 Cooperative multivalent binding in molecular recognition has been well studied.1 However, limited effort has been made to combine and maximize multivalent and subunit cooperative binding towards constructing functional supramolecular self-assemblies from large numbers of subunits. Here, we explore dendrimers as molecular nanospheres with highly-branched peripheral binding sites to show a new concept that maximizing cooperative binding enables a novel modular self-assembly approach to construct functional supramolecular capsules, Scheme 1.
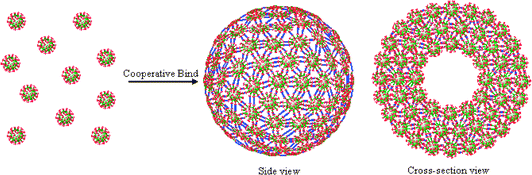 | ||
| Scheme 1 Schematic illustration of the cooperative binding of dendrimers into supramolecular capsules. | ||
Dendrimers have a highly branched architecture emanating from the core, thus can provide many binding sites at the periphery. They are globular in topology, monodispersed in size, and nanometers in dimension (1–10 nm).2Dendrimers and their conjugates are not only of great interest to molecular recognition studies, but have also been vigorously pursued in the fabrication of supramolecular self-assemblies, ranging from dimers, oligomers, megamer clusters, rotaxane complexes, to large assemblies such as vesicles, fibers, gels and liquid crystals.3 Despite tremendous achievements, such dendrimer self-assemblies, however, were largely based on traditional amphiphile hydrophobic effect, guest–host complexation, or dimeric/oligomeric linkages via H-bonding or metal coordination. The unique features of dendrimers as molecular nanospheres with highly-branched binding sites are yet to be fully exploited.
To demonstrate the generality of the cooperative binding concept, here we studied two types of dendrimers that differ significantly in composition, branching motif and rigidity-Fréchet-type dendrimers with 1 → 2 aryl branching motif and ether connectivity, and Newkome-type dendrimers with 1 → 3 C-branching motif and amide connectivity, Fig. 1A and 2A. Each dendrimer was functionalized with carboxyl peripheral groups to allow for potential electrostatic interactions in water. The benzyl ether interior of the Fréchet dendrimers is rather rigid and highly hydrophobic, whereas the Newkome dendrimers have much more conformational freedom around sp3-C branching points, and their amide rich interior is much more hydrophilic. Consistently, our molecular Dynamics (MD) simulations on the Newkome dendrimers demonstrated a large degree of conformational flexibility and revealed water penetration into their amide-rich interior, Fig. 2A(II), ESI. The distinct difference between these two types of dendrimers was also confirmed by solubilization of hydrophobic fluorescent dyes inside the Fréchet dendrimers, but not by the Newkome dendrimers.
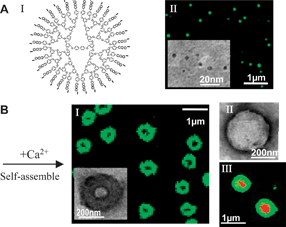 | ||
| Fig. 1 Ca2+ induced self-assembly of Fréchet-COO−dendrimers into supramolecular capsules in water. (A) I. Molecular structure; II. FM and cryo-TEM (inset) imaging on dispersed Fréchet-COO−dendrimers. (B) I. Cross-section projection of the Ca2+ induced capsules imaged by FM and TEM (inset); II. Surface topology of the capsules imaged by negatively-stained TEM; III. Encapsulation of red-fluoresecentl Dox by the capsules. | ||
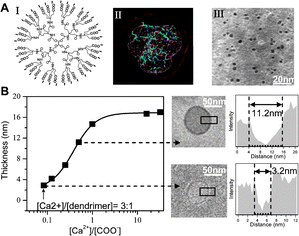 | ||
| Fig. 2 Ca2+ induced self-assembly of Newkome-COO−dendrimers into supramolecular capsules in water. (A) I. Molecular structure; II. MD simulation. Grey/red contour—deviation in conformations. III. Cryo-TEM imaging on dispersed Newkome-COO−dendrimers in water. (B) Scaling and illustration of the capsule thickness with the Ca2+/carboxylate molar ratio. | ||
The carboxyl peripheral groups can completely dissociate into carboxylate anions (–COO−) at neutral pH in aqueous solution, and both types of dendrimers dispersed as individual molecules upon dissolution in water, Fig. 1A(II) and 2A(III). Although the Fréchet dendrimers have highly hydrophobic benzyl ether interiors, their hydrophobic associations into bilayer vesicles are unlikely due to their conformational rigidity. In fact, such Fréchet dendrimers are well known as unimolecular micelles in water, where the hydrophobic benzyl ether interior collapses into a rigid core surrounded by closely packed polar groups at the surface.4 Direct Fluorescent Microscopy (FM) imaging of dye-labeled Fréchet-COO−dendrimers revealed their Brownian motion in water as dispersed individual molecules, Fig. 1A(II). Cryogenic-Transmission Electron Microscopy (Cryo-TEM) with resolution in nanometers showed similar results to those obtained by FM, Fig. 1A (II, inset), where the diameters of the fourth-generation-(G4)-Fréchet-COO−dendrimers (with 32 peripheral groups) and second-generation-(G2)-Fréchet-COO−dendrimers (with 8 peripheral groups) were measured as 5 nm and 3 nm respectively. Cryo-TEM studies on Newkome-COO−dendrimers showed that they are also dispersed molecular nanospheres with average diameters of 4 nm and 2.5 nm for G2 (with 36 peripheral groups) and G1 (with 12 peripheral groups) respectively.
Regardless of core composition and hydrophobicity, branching motif or generations, upon the addition of divalent Ca2+, the dendrimer-COO− nanospheres spontaneously assembled into submicron hollow capsules, Fig. 1B(I) and 2(B). Both FM and TEM imaging of these capsules revealed the projection of hollow cavities and dense membranes. Negative staining in TEM, Fig. 1B(II), further resolved the enclosed, ragged surface topology of these capsules, which appear to be composed of large numbers of tightly bound dendrimer nanospheres. The enclosed capsule structure was further confirmed by their ability to encapsulate guest materials into their cavity, as demonstrated in Fig. 1B(III), using red-fluorescent anticancer drug doxorubicin. Based on the membrane volumes of the capsules and the sizes of the dendrimer nanosphere molecules, we estimate these dendrimer capsules to contain hundreds to more than tens of thousands of dendrimer nanosphere units. These capsules were also observed to be robust and stable against shear and rupture stress.
Although both types of dendrimers formed capsules with Ca2+, they differed distinctively in their response to Ca2+. Fréchet-COO−dendrimers required excess Ca2+ than total carboxylates to assemble, i.e.Ca2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COO− ≥ 4, whereas Newkome-COO−dendrimers, regardless of generations, began to form monolayer capsules at Ca2+
COO− ≥ 4, whereas Newkome-COO−dendrimers, regardless of generations, began to form monolayer capsules at Ca2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dendrimer ∼ 3
dendrimer ∼ 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where the Ca2+ stoichiometry is far below carboxylates, i.e.Ca2+
1, where the Ca2+ stoichiometry is far below carboxylates, i.e.Ca2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COO− = 1
COO− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 for G2, Fig. 2B. Newkome dendrimers also tend to form smaller and thinner capsules than Fréchet-COO−dendrimers. Capsules from G4-Fréchet-COO−dendrimers have average cavity sizes around 100 nm and their membrane thickness can grow up to 100 nm at high Ca2+ concentrations, whereas G2-Newkome dendrimers with comparable number of peripheral groups formed capsules with average cavity sizes around 50 nm and their thickness can grow from monolayer to a maximum of 20 nm with increasing Ca2+ concentrations, Fig. 2B. The size and thickness of the capsules also depended on the generation of the dendrimers. For both types of dendrimers, the smaller lower-generation dendrimers formed smaller cavities with thinner membranes, Fig. S1, ESI.
8 for G2, Fig. 2B. Newkome dendrimers also tend to form smaller and thinner capsules than Fréchet-COO−dendrimers. Capsules from G4-Fréchet-COO−dendrimers have average cavity sizes around 100 nm and their membrane thickness can grow up to 100 nm at high Ca2+ concentrations, whereas G2-Newkome dendrimers with comparable number of peripheral groups formed capsules with average cavity sizes around 50 nm and their thickness can grow from monolayer to a maximum of 20 nm with increasing Ca2+ concentrations, Fig. 2B. The size and thickness of the capsules also depended on the generation of the dendrimers. For both types of dendrimers, the smaller lower-generation dendrimers formed smaller cavities with thinner membranes, Fig. S1, ESI.
Such Ca2+ induced capsule formation is distinctively different from the classical amphiphile hydrophobic-effect driven bilayer vesicles, as this process is independent of hydrophobicity and the membrane thickness can grow from monolayer to far beyond bilayers. Although Ca2+ is commonly used for inducing coil and aggregation of carboxylate based polyelectrolytes,5 the formation of the ordered hollow capsules here is surprising. Hollow capsule formation, such as in viral capsids, generally proceeds by a nucleation and growth pathway, where an oligomeric patch forms first followed by bending and expansion into a shell to minimize the surface energy.6 The dendrimers here share some common features with proteins, as they are nanometers in size and spherical in shape with many binding sites at periphery. Divalent Ca2+ can not only bind with –COO− as counterion, but can also form COO−–Ca2+–COO− salt-bridges linking between dendrimer nanospheres. The highly branched feature of dendrimers would also allow for multiple Ca2+ salt-bridges between dendrimers, as well as three-dimensional binding into multiple layers with sufficient Ca2+, consistent with the observed capsule thickening with the increasing Ca2+.
To better understand the Ca2+/COO− binding that underlies the capsule formation, we used isothermal titration calorimetry (ITC) to study the energetics of the binding process. Ca2+ was added incrementally into the aqueous solution of the more sensitive Newkome-COO−dendrimers. The heat changes of the titration and the integrated heat per mole of added Ca2+versus the molar ratio of Ca2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dendrimer were plotted in Fig. 3. Interestingly, the titration was multiphasic and was best fitted with the two independent binding site model. The most obvious distinction among sites possible in this system is between individual Ca2+–COO− counterion binding and COO−–Ca2+–COO− salt-bridge forming to join two dendrimers. The parameters reported by ITC for two processes were K1 = 3.1 × 103 M−1, ΔH1 = 7.5 kcal/mol, TΔS1 = 12.2 kcal/mol, ΔG1 = −4.7 kcal/mol; and K2 = 4.8 × 104 M−1, ΔH2 = −3.4 kcal/mol, TΔS2 = 1.7 kcal/mol, ΔG = −5.1 kcal/mol. Both binding events are spontaneous processes with release of free energy. The first process is endothermic and entropically driven, consistent with disruption of the ordered water solvation shell upon individual counterion binding.7 Average binding constant K1 is also similar to the previously reported Ca2+–COO− counterion binding constant.8 In contrast, the second Ca2+ binding process is exothermic and enthalpically driven with the average K2 ten times greater than K1. The isotherm is also sigmoidal, indicating a positive cooperative effect. The second process is likely the COO−–Ca2+–COO− salt-bridge binding that assembles dendrimers into capsules, where the formation of the highly ordered dendrimer nanosphere lattices would yield exothermic lattice energy and entropy loss. In addition, as two dendrimers are brought together by the first Ca2+ salt-bridge formation, their proximity would facilitate the subsequent formation of Ca2+ salt bridges between them and maximize the cooperativity. Multiple bonding in turn can enhance the binding strength between dendrimers, and stabilize the self-assembly structure and account for the robustness of these capsules.
Dendrimer were plotted in Fig. 3. Interestingly, the titration was multiphasic and was best fitted with the two independent binding site model. The most obvious distinction among sites possible in this system is between individual Ca2+–COO− counterion binding and COO−–Ca2+–COO− salt-bridge forming to join two dendrimers. The parameters reported by ITC for two processes were K1 = 3.1 × 103 M−1, ΔH1 = 7.5 kcal/mol, TΔS1 = 12.2 kcal/mol, ΔG1 = −4.7 kcal/mol; and K2 = 4.8 × 104 M−1, ΔH2 = −3.4 kcal/mol, TΔS2 = 1.7 kcal/mol, ΔG = −5.1 kcal/mol. Both binding events are spontaneous processes with release of free energy. The first process is endothermic and entropically driven, consistent with disruption of the ordered water solvation shell upon individual counterion binding.7 Average binding constant K1 is also similar to the previously reported Ca2+–COO− counterion binding constant.8 In contrast, the second Ca2+ binding process is exothermic and enthalpically driven with the average K2 ten times greater than K1. The isotherm is also sigmoidal, indicating a positive cooperative effect. The second process is likely the COO−–Ca2+–COO− salt-bridge binding that assembles dendrimers into capsules, where the formation of the highly ordered dendrimer nanosphere lattices would yield exothermic lattice energy and entropy loss. In addition, as two dendrimers are brought together by the first Ca2+ salt-bridge formation, their proximity would facilitate the subsequent formation of Ca2+ salt bridges between them and maximize the cooperativity. Multiple bonding in turn can enhance the binding strength between dendrimers, and stabilize the self-assembly structure and account for the robustness of these capsules.
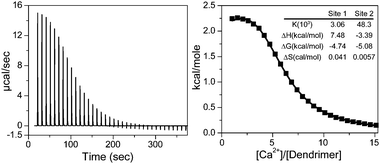 | ||
| Fig. 3 ITC analysis on Ca2+ binding to Newkome-COO−dendrimers. Left panel shows the calorimetric titrations; Right panel displays the integrated heat values as a function of molar ratio. The solid line represents the curve fit to a two independent binding site model. | ||
The Ca2+ induced cooperative binding of dendrimers also explains for the observed differences between Fréchet- and Newkome-COO−dendrimers. As rigid spheres with tightly packed carboxylate groups at the surface, Fréchet dendrimers require addition of more Ca2+ to bind and screen the densely negative surface charges first, before they can be brought to proximity to form Ca2+ salt-bridges.9 In contrast, the carboxylate groups in the highly flexible Newkome dendrimers are more like independent point charges, which can extend to form Ca2+ salt-bridges much more freely. The flexibility of Newkome dendrimers would also enable bending into much smaller shells as observed. Quantitatively, since at least one Ca2+ salt-bridge is needed to link between every two Newkome dendrimers and each dendrimer sphere would be surrounded by six neighbors in close-packing, a minimum ratio of Ca2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dendrimer = 6/2 (3
dendrimer = 6/2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) would be required to induce the capsule formation for Newkome dendrimers, which is consistent with our experimental finding.
1) would be required to induce the capsule formation for Newkome dendrimers, which is consistent with our experimental finding.
Cooperative binding not only allows for flexible supramolecular dendrimer capsule fabrication, but also possesses the advantage of ease in controlling disassembly towards release. Since the capsules are bound by Ca2+ salt-bridges, removal of Ca2+ would cause the capsules to disassemble into dendrimer monomers. Fig. 4 shows that as EDTA, an effective Ca2+ chelator that can withdraw Ca2+ from the capsules, was added to Frèchet-COO−/Ca2+ capsule solutions, the capsules quickly disassembled with time, which was directly observed by FM.
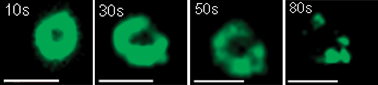 | ||
| Fig. 4 FM imaging on the disassembly of the Frèchet-COO−/Ca2+ capsule by the addition of EDTA. Scale bar: 1 μm. | ||
In summary, we have exploited the unique features of dendrimers as molecular nanospheres with highly-branched binding sites and demonstrated their ability to maximize cooperative binding, which enables a novel modular self-assembly approach to construct supramolecular capsules. Such capsules by cooperative binding are tunable in size and thickness, controllable in disassembly, and can be potentially used for a wide variety of encapsulation applications.
We thank CAUR center of UGA and COMSET center of Clemson University for TEM studies.
Notes and references
- (a) W. P. Jencks, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 4046 CAS; (b) A. D. Hughes and E. V. Anslyn, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6538 CrossRef CAS; (c) M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754 CrossRef.
- (a) G. R. Newkome, C. N. Moorefield and F. Vögtle, Dendritic Macromolecules Concepts, Synthesis, Perspectives, VCH, Wenheim, Germany, 2001 Search PubMed; (b) Dendrimers and Other Dendritic Polymers, ed. J. M. Fréchet and D. A. Tamalia, Wiley, Chichester, UK, 2001 Search PubMed; (c) F. W. Zeng and S. C. Zimmerman, Chem. Rev., 1997, 97, 1681 CrossRef CAS.
- (a) M. D. Giles, S. Liu, R. L. Emanuel, B. C. Gibb and S. M. Grayson, J. Am. Chem. Soc., 2008, 130, 14430 CrossRef CAS; (b) S. C. Zimmerman, F. W. Zeng, D. E. C. Reichert and S. V. Kolotuchin, Science, 1996, 271, 1095 CrossRef CAS; (c) D. A. Tomalia, H. M. Brothers, L. T. Piehler, H. D. Durst and D. R. Swanson, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5081 CrossRef CAS; (d) D. B. Amabilino, P. R. Ashton, V. Balzani, C. L. Brown, A. Credi, J. M. Fréchet, J. W. Leon, F. M. Raymo, N. Spencer, J. F. Stoddart and M. Venturi, J. Am. Chem. Soc., 1996, 118, 12012 CrossRef CAS; (e) P. Wang, C. N. Moorefield, K. U. Jeong, S. H. Hwang, S. Li, S. Z. D. Cheng and G. R. Newkome, Adv. Mater., 2008, 20, 1381 CrossRef CAS; (f) J. C. M. Vanhest, D. A. P. Delnoye, M. W. P. L. Baars, M. H. P. Vangenderen and E. W. Meijer, Science, 1995, 268, 1592 CrossRef CAS; (g) G. Ungar, Y. S. Liu, X. B. Zeng, V. Percec and W. D. Cho, Science, 2003, 299, 1208 CrossRef CAS.
- C. J. Hawker, K. L. Wooley and J. M. Fréchet, J. Chem. Soc., Perkin Trans. 1, 1993, 1287 RSC.
- (a) H. R. Sondjaja, T. A. Hatton and K. C. Tam, Langmuir, 2008, 24, 8501 CrossRef CAS; (b) Y. Li, Y. K. Gong and K. Nakashima, Langmuir, 2002, 18, 6727 CrossRef CAS.
- (a) D. L. Caspar, Biophys. J., 1980, 32, 103 CrossRef CAS; (b) D. Kim, E. Kim, J. Kim, K. M. Park, K. Baek, M. Jung, Y. H. Ko, W. Sung, H. S. Kim, J. H. Suh, C. G. Park, O. S. Na, D. Lee, K. E. Lee, S. S. Han and K. Kim, Angew. Chem., Int. Ed., 2007, 46, 3471 CrossRef.
- B. R. Linton, M. S. Goodman, E. Fan, S. A. Arman and A. D. Hamilton, J. Org. Chem., 2001, 66, 7313 CrossRef CAS.
- N. R. Joseph, J. Biol. Chem., 1946, 164, 529 CAS.
- J. Israelachvili, Intermolecular & Surface Forces, Academic Press, London, 1992 Search PubMed.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Materials and Methods, Fig. S1. See DOI: 10.1039/c0cc01983f |
| This journal is © The Royal Society of Chemistry 2011 |
