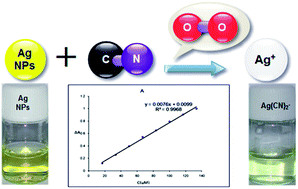
The interaction between aqueous colloidal silver nanoparticles (AgNPs) and cyanide ions was studied using UV-Vis absorption and scanning electron microscopy (SEM) techniques. It was found that AgNPs were oxidized by dissolved oxygen in the presence of cyanide ions, resulting in a considerable decrease in the intensity of the surface plasmon resonance (SPR) absorption band of AgNPs. So, we propose a simple, cost effective, rapid, sensitive and selective colorimetric sensor for the detection of cyanide using AgNPs in aqueous media. There is a linear relationship between the absorbance intensity of AgNPs and the concentration of cyanide ions over the range of 16.7 μmol L−1–133.3 μmol L−1 at 394 nm. The proposed method has been successfully used for the determination of cyanide in water samples.