Anion conducting poly(dialkyl benzimidazolium) salts†
Owen D.
Thomas
,
Kristen J. W. Y.
Soo
,
Timothy J.
Peckham
,
Mahesh P.
Kulkarni
and
Steven
Holdcroft
*
Department of Chemistry, Simon Fraser University, Burnaby, British Columbia V5A 1S6, Canada. E-mail: holdcroft@sfu.ca
First published on 23rd May 2011
Abstract
Derivatization of poly(benzimidazole) (PBI) with methyl groups generates poly(dimethyl benzimidazolium) (PDMI), an anion exchange material. By using a simple ion exchange process, it is possible to produce PDMI salts with a variety of counter-ions. Anionic conductivity (2.7 ± 0.33 to 8.5 ± 0.5 × 10−3 S cm−1) for the PDMI membranes was found to be surprisingly high even though the membranes generally exhibit very low water uptakes. To the best of our knowledge, this represents the first report on the anionic conductivity of poly(dialkyl benzimidazolium) salts, despite the large body of literature on PBI and on molecular imidazolium salts.
The development of polymeric membranes capable of selective anion transport has garnered renewed interest due to a widening range of applications that includes electrodialysis for water purification and salt production,1–4 battery separators5 and alkaline anion exchange membrane fuel cells (AAEMFCs).6–12 Anion-transporting membranes typically possess quaternary ammonium groups covalently bonded to a polymer backbone,6–12 although there are recent examples based on tethered phosphonium sites.13,14 The majority of reports utilize the reaction of pendant alkyl halides with a tertiary amine to produce quaternized ammonium sites.6–12 An alternative approach employs nitrogen-containing heterocycles, such as imidazoles. The reaction of alkyl halides with imidazole, for example, yields low melting point (T < 373 K), imidazolium salts, commonly referred to as “ionic liquids”. The halides can be easily exchanged with other anions (e.g., PF6−, BF4−, NO3−). The properties of ionic liquids depend upon both the nature of the anion as well as that of the alkyl substituent. The thermal and chemical stability of ionic liquids has led to their use in a number of electrochemical applications.15,16 Composites of ionic liquids imbibed in polymeric matrices have been investigated,15 as have polymers with tethered ionic liquid moieties, derived from vinyl-based monomers, although the polyvinylic nature of the backbone is unlikely to resist high water uptake at high IEC levels. The conductivity of poly(benzimidazole)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid adducts in the dry state has been reported,17,18 but in these systems the acid elutes from the polymer in the presence of water.
acid adducts in the dry state has been reported,17,18 but in these systems the acid elutes from the polymer in the presence of water.
Alkylation of poly(benzimidazole) (PBI) has been previously reported in the literature but the intention was to evaluate the effects of N-substitution upon thermal, mechanical and gas permeability,19–24 rather than ion conductivity, and H3PO4-doped PBI systems have been examined extensively for high temperature proton exchange membrane fuel cells (PEMFCs),25,26 but anionic conductivity is secondary to the high proton conductivity of the phosphoric acid. Surprisingly, there are no reports on the anionic conductivity of dialkylimidazolium salt derivatives of PBI.
In this report, we describe the derivatization of PBI, 1, to form poly(dimethyl benzimidazolium) salts (2-X−−, where X− is an anion). Dimethylated PBI (2-I−−) was synthesized by deprotonation of the acidic hydrogens of 1 using LiH, methylation with CH3I and subsequent methylation of the remaining basic nitrogen sites with excess CH3I (Scheme 1). The degree of methylation was >95% as determined by 1H NMR spectroscopy.19Membranes of 2-I−− were cast from DMSO solution onto glass slides followed by heating at 60 °C and drying under vacuum overnight at 50 °C. Membranes of 2-I−− were treated with aqueous solutions of KX (where X = Br, Cl or HCO3) to exchange iodide for X−. The degree of exchange was determined by treating the membranes with aqueous KNO3 and titrating with AgNO3 using K2CrO4 as an indicator (i.e., Mohr titration).12 In all cases, the degree of exchange was found to be quantitative (see the ESI† for details).
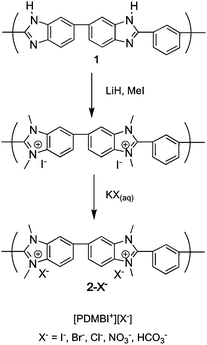 | ||
| Scheme 1 Synthesis of poly(dimethyl benzimidazolium iodide) (2-I−−) from PBI (1) and subsequent anion exchange. | ||
Attempts to prepare 2-OH−−, by treating 2-I−− with alkali metal hydroxide solutions, however, led to decomposition of the polymer. The instability of the hydroxide form was consistent with attempts to produce the small molecule analogue, 3-I−− (see the ESI†), which spontaneously ring-opened to form the linear species 4.
Membrane properties of 2-X−− as a function of X− are shown in Table 1, including values of λ (moles H2O per mole N+), which is related to the measure of water content. Membranes containing Br−, I−, NO3−, and HCO3− possessed relatively low H2O contents (λ = 2, 2, 3, and 5, respectively). Membrane 2-Cl−−, however, reproducibly possessed very large water contents (λ = 167), even when the membranes were dissolved and recast. This is especially surprising given the lower λ values for 2-Br−− and 2-I−−. For alkali halide salts, the free energy of hydration (ΔGhyd) increases as the difference in size between the anion and cation increases,27 which may partially explain why the highest degree of hydration occurs for 2-Cl−−. Moreover, as the ionic character of the imidazolium–halide pair increases, in the order 2-I−− < 2-Br−− < 2-Cl−−, as a result of the increasing hardness of the anion, the hydrophilicity of the salt increases, ultimately leading to exceptionally high λ values for 2-Cl−−.27
| X− | IEC a/meq g−1 | λ c | σ X− d/103 S cm−1 | [X−]e/M | μ′X−f/105 cm2 V−1s−1 | μ X− ∞ g/105 cm2 V−1s−1 |
|---|---|---|---|---|---|---|
| a Ionic exchange capacity, calculated based on 96% quaternization of available nitrogen centres. b Calculated based on 100% exchange of X− for I− (see the ESI†). c Moles H2O per mole N+. d Anionic conductivity of wet membranes. e Analytical anion concentration. f Effective anionic mobility. g Mobility for free anions in H2O at 25 °C at infinite dilution (ref. 30). h Not available (cf. CO32−, μX−∞ = 74.6 × 10−5 cm2 V−1s−1). i Calculated based on uptake of 1.4 mol of I2 per mole of I−. | ||||||
| Cl − | 4.16b | 167 | 7.6 ± 1.1 | 0.32 | 24.62 | 79.1 |
| Br − | 3.48b | 2 | 3.2 ± 0.4 | 4.32 | 0.77 | 80.9 |
| I− | 2.97 | 2 | 3.3 ± 0.4 | 4.31 | 0.79 | 79.6 |
| NO3− | 3.72b | 3 | 4.9 ± 0.4 | 4.96 | 1.02 | 74.0 |
| HCO3− | 3.74b | 5 | 8.5 ± 0.5 | 3.68 | 2.40 | —h |
| I3− | 1.50i | <1 | 2.7 ± 0.3 | 3.29 | 0.85 | — |
The conductivities of 2-X−−membranes were determined in the fully hydrated wet state and are at least 2 orders of magnitude greater than in the dry state (see the ESI†). The data reveal unexpected differences as a function of anion. For example, σX− is similar for X = I− and Br− even though the IEC of the latter is significantly higher. Also, H2O uptakes and IEC are similar for 2-NO33−− and 2-HCO33−− but the conductivity of 2-HCO33−− is significantly higher. Moreover, despite the H2O content for 2-Cl−− being more than 30 times greater than 2-HCO33−−, σX− values are essentially the same. A fuller understanding of the conductivity data can be obtained by examining σX− in the context of eqn (1):
| σX− = F[X−]μ′X− | (1) |
The anions in the membranes 2-X−− exhibit μ′X− that are ∼1 to 2 orders of magnitude lower than the mobility of free anions at infinite dilution in aqueous solutions (μX−∞, listed in Table 1) (except 2-Cl−−).30 This is likely due to the low water content (λ = 2–5) in the membranes. For PEMs, it has been previously demonstrated that the complete separation of the proton and fixed anion does not fully occur unless λ ≥ 6.31 For similar λ values (3–6), the effective proton mobility in Nafion® and sulfonated α–β–β-trifluorostyrene-based BAM® membranes is also ∼1 to 2 orders of magnitude lower than free protons at infinite dilution (3.62 × 10−3 cm2 V−1s−1).28,32 Similarly, the solvent molecules present at low lambda are likely insufficient to fully separate the ion pairs in the case of these anion exchange membranes. It is interesting to note, however, that whereas the infinite mobility values for the anions are within 10% of each other, there is significant variation in the values of μ′X− as a function of X− for 2-X−−. Furthermore, in contrast to Nafion® and BAM® membranes where differences in μ′H+ can be attributed to the different morphological structures, the backbone of 2-X−− is the same in each case. Thus it appears the interaction of X−− with the polymer backbone plays a role in determining the anion mobility warranting further investigation. In the case of 2-Cl−−, the high λ value assures solvent separation of the anions from the fixed cations and thus μ′X− is much closer in value to the mobility of Cl− solutions at infinite dilution.
Recently, Yan and Hickner reported the conductivity of HCO3− in AEMs based on quaternary ammonium-substituted poly(aryl ether sulfone).12 A maximum σHCO3− (2.73 × 10−2 S cm−1) was observed for a membrane possessing an IEC of 2.09 meq g−1. In the present case, the conductivity of 2-HCO33−− was lower but contained a much lower H2O content (λ = 5 vs. 44).
We also examined triiodide (I3−) conductivity of 2-X−− in the solid-state. Samples of 2-I−− were soaked in an I2-saturated methanol (MeOH) solution and air dried for 24 h. The estimated uptake of iodine was 1.4 mol of I2 per mole of I− originally present. Membranes of 2-I33−− possessed even lower H2O content than the parent 2-I−−membranes but a similar (wet) conductivity value (2.7 × 10−3 S cm−1). Dry samples of 2-I33−− exhibited a conductivity 5.0 × 10−4 S cm−1 which is comparable to previously reported values of 5.0 × 10−4 S cm−1 and 8.0 × 10−4 S cm−1 for polyphosphazene33 and poly(ethylene oxide)/poly(vinylidene fluoride)-based34 solid electrolytes respectively that have been used in dye-sensitized solar cells (DSSCs).
In conclusion, a new and adaptable ion exchange membrane material that is resistant to swelling in some anionic forms, and exhibits good anionic conductivity with low H2O contents, has been presented. The morphological properties of these membranes as a function of anion and the development of modified PBI-based AEMs are currently being examined. Moreover, this report should foster research into stable derivatives of 2-OH−− for AAEMFC applications and optimization of 2-I33−− and its derivatives for DSSC applications.
References
- R. Du and J. Zhao, J. Appl. Polym. Sci., 2004, 91, 2721–2728 Search PubMed.
- D. Nwal Amang, S. Alexandrova and P. Schaetzel, Desalination, 2003, 159, 267–271 Search PubMed.
- M. Sadrzadeh, A. Razmi and T. Mohammadi, Sep. Purif. Technol., 2007, 54, 147–156 Search PubMed.
- X. Tongwen and Y. Weihua, J. Membr. Sci., 2001, 190, 159–166 Search PubMed.
- J. Qiu, M. Li, J. Ni, M. Zhai, J. Peng, L. Xu, H. Zhou, J. Li and G. Wei, J. Membr. Sci., 2007, 297, 174–180 CrossRef CAS.
- M. R. Hibbs, C. H. Fujimoto and C. J. Cornelius, Macromolecules, 2009, 42, 8316–8321 CrossRef CAS.
- N. J. Robertson, H. A. Kostalik, T. J. Clark, P. F. Mutolo, H. D. Abruna and G. W. Coates, J. Am. Chem. Soc., 2010, 132, 3400–3404 CrossRef CAS.
- M. Tanaka, M. Koike, K. Miyatake and M. Watanabe, Polym. Chem., 2011, 2, 99–106 RSC.
- J. R. Varcoe and R. C. T. Slade, Fuel Cells, 2005, 5, 187–200 CrossRef CAS.
- J. R. Varcoe, R. C. T. Slade, G. L. Wright and Y. L. Chen, J. Phys. Chem. B, 2006, 110, 21041–21049 CrossRef CAS.
- J. R. Varcoe, R. C. T. Slade, E. L. H. Yee, S. D. Poynton, D. J. Driscoll and D. C. Apperley, Chem. Mater., 2007, 19, 2686–2693 CrossRef CAS.
- J. L. Yan and M. A. Hickner, Macromolecules, 2010, 43, 2349–2356 CrossRef CAS.
- S. Gu, R. Cai, T. Luo, Z. Chen, M. Sun, Y. Liu, G. He and Y. Yan, Angew. Chem., Int. Ed., 2009, 48, 6499–6502 CrossRef CAS.
- S. Gu, R. Cai, T. Luo, K. Jensen, C. Contreras and Y. Yan, ChemSusChem, 2010, 3, 555–558 CrossRef CAS.
- S. Ahmad, Ionics, 2009, 15, 309–321 Search PubMed.
- H. Liu, Y. Liu and J. Li, Phys. Chem. Chem. Phys., 2010, 12, 1685–1697 RSC.
- S. M. Aharoni and M. H. Litt, J. Polym. Sci., Polym. Chem. Ed., 1974, 12, 639–650 Search PubMed.
- S. M. Aharoni and A. J. Signorelli, J. Appl. Polym. Sci., 1979, 23, 2653–2660 Search PubMed.
- M. Hu, E. M. Pearce and T. K. Kwei, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 553–561 Search PubMed.
- S.-K. Kim, T.-H. Kim, J.-W. Jung and J.-C. Lee, Polymer, 2009, 50, 3495–3502 CrossRef CAS.
- S. C. Kumbharkar and U. K. Kharul, Eur. Polym. J., 2009, 45, 3363–3371 Search PubMed.
- S. C. Kumbharkar and U. K. Kharul, J. Membr. Sci., 2010, 357, 134–142 Search PubMed.
- H. Pu and G. Liu, Polym. Int., 2005, 54, 175–179 CrossRef CAS.
- H. Pu, Q. Liu and G. Liu, J. Membr. Sci., 2004, 241, 169–175 CrossRef CAS.
- J. S. Wainright, J. T. Wang, D. Weng, R. F. Savinell and M. Litt, J. Electrochem. Soc., 1995, 142, L121–L123.
- L. Xiao, H. Zhang, E. Scanlon, L. S. Ramanathan, E.-W. Choe, D. Rogers, T. Apple and B. C. Benicewicz, Chem. Mater., 2005, 17, 5328–5333 CrossRef CAS.
- D. W. Oxtoby and N. H. Nachtrieb, Principles of Modern Chemistry, Saunders College Publishing, New York, 1985 Search PubMed.
- T. J. Peckham, J. Schmeisser and S. Holdcroft, J. Phys. Chem. B, 2008, 112, 2848–2858 CrossRef CAS.
- T. J. Peckham, J. Schmeisser, M. Rodgers and S. Holdcroft, J. Mater. Chem., 2007, 17, 3255–3268 RSC.
- P. W. Atkins, Physical Chemistry, W.H. Freeman and Company, New York, 4th edn, 1990 Search PubMed.
- S. J. Paddison, J. New Mater. Electrochem. Syst., 2001, 4, 197 CAS.
- A. A. Adamson, A Textbook of Physical Chemistry, Academic Press, Inc., New York, 1973 Search PubMed.
- S.-H. A. Lee, A.-M. S. Jackson, A. Hess, S.-T. Fei, S. M. Pursel, J. Basham, C. A. Grimes, M. W. Horn, H. R. Allcock and T. E. Mallouk, J. Phys. Chem. C, 2010, 114, 15234–15242 CAS.
- H. W. Han, W. Liu, J. Zhang and X. Z. Zhao, Adv. Funct. Mater., 2005, 15, 1940–1944 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and data on conductivity and hydroxide stability. See DOI: 10.1039/c1py00142f |
| This journal is © The Royal Society of Chemistry 2011 |
