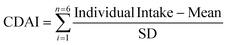Open Access Article
Open Access ArticleAssociation between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011–2018†
Min
Wang
 a,
Zhao-hui
Huang
a,
Yong-hong
Zhu
a,
Ping
He
a and
Qiu-Ling
Fan
*ab
a,
Zhao-hui
Huang
a,
Yong-hong
Zhu
a,
Ping
He
a and
Qiu-Ling
Fan
*ab
aDepartment of Nephrology, First Hospital of China Medical University, Shenyang, Liaoning, China. E-mail: cmufql@163.com
bDepartment of Nephrology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
First published on 29th September 2023
Abstract
Objectives: There is growing evidence that antioxidant-rich diets protect against chronic kidney disease (CKD). However, the relationship between the Composite Dietary Antioxidant Index (CDAI), an important measure of an antioxidant diet, and CKD has received little attention. Therefore, here we investigated the relationship between the CDAI and CKD through a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) 2011–2018 data. Methods: The CDAI was calculated based on the intake of six dietary antioxidants. A survey-based multivariate linear regression analysis was performed to analyze the independent relationship between the CDAI and CKD. Weighted multivariate regression and subgroup analyses were conducted to explore the relationship between the CDAI and CKD. Results: A total of 6874 NHANES participants represented 181.9 million non-institutionalized US residents (mean age, 46.43 ± 0.38 years; 49.87% female; 40.62% non-Hispanic white; 20.24% non-Hispanic black; and 13.94% Mexican American). The weighted linear regression model with full adjustment for confounding variables was −0.0155 (−0.0417, 0.0107) for Q2 (P for trend <0.0001), −0.0052 (−0.0346, 0.0242) for Q3 (P for trend <0.0001), and −0.0305 (−0.0491, −0.0120) for Q4 (P for trend = 0.0094) upon comparison with the lowest quartile of the CDAI. None of the interactions in any subgroup analysis were statistically significant except for individuals with a history of diabetes or the aged population (≥60 years) (P for interaction <0.05). Conclusions: The CDAI was positively associated with a lower prevalence of CKD in adults in the United States. Further large-scale prospective studies are required to analyze the role of the CDAI in CKD.
1. Introduction
Kidney failure is a major health concern worldwide. Chronic kidney disease (CKD) is a chronic disease characterized by proteinuria; normal or reduced estimated glomerular filtration (eGFR) rates; and progressive glomerular, tubular and interstitial damage. CKD, which affects 15–20% of adults globally, is associated with a very high risk of premature death due to cardiovascular disease, the most common cause of death in CKD.1,2 Therefore, clinicians should pay attention to this condition.The Composite Dietary Antioxidant Index (CDAI) is a composite estimate of an individual's overall pro- and antioxidant exposure status.3 The CDAI is an individual antioxidant index based on a combination of dietary antioxidants (manganese, selenium, zinc and vitamins A, C, and E).4 Previous studies reported that a high CDAI is associated with a reduced risk of various types of cancer and diabetes and positively correlated with the plasma levels of S-Klotho, an anti-aging indicator.4–8 A high CDAI is associated with a decreased risk of all-cause and cardiovascular mortality.5 Oxidative stress is correlated with renal damage; however, the relationship between the inflammatory indicator CDAI and CKD remains unclear.9–11
Therefore, this study aimed to explore the association between the CDAI and CKD among the participants of the US National Health and Nutrition Examination Survey (NHANES). We hypothesized that an elevated CDAI would be associated with a lower risk of CKD.
2. Materials and methods
2.1 Study population
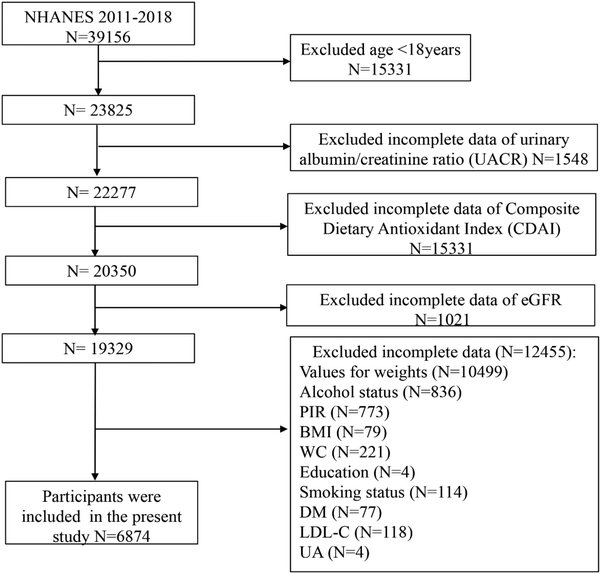
The NHANES is a series of cross-sectional surveys representing the non-institutionalized civilian population of the United States (https://www.cdc.gov/nchs/nhanes/). The NHANES includes demographic, socioeconomic, dietary, and health-related questionnaire data collected through face-to-face interviews, physical and physiological examinations, and extensive laboratory tests. A detailed description of the NHANES was reported previously.12,13 A total of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 participants completed the survey in four NHANES cycles (NHANES 2011–2012, 2013–2014, 2015–2016, and 2017–2018 cycles). Among them, we excluded 15
156 participants completed the survey in four NHANES cycles (NHANES 2011–2012, 2013–2014, 2015–2016, and 2017–2018 cycles). Among them, we excluded 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 participants under 18 years of age, 1548 for whom a urinary albumin/creatinine ratio (UACR) was lacking, 15
331 participants under 18 years of age, 1548 for whom a urinary albumin/creatinine ratio (UACR) was lacking, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 participants for whom a CDAI was lacking, 1021 participants for whom eGFR was lacking, 10
331 participants for whom a CDAI was lacking, 1021 participants for whom eGFR was lacking, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499 participants for whom weight values were unavailable, 836 for whom alcohol status was unavailable, 773 for whom a poverty income ratio (ratio of family income to poverty threshold [PIR]) was unavailable, 79 for whom body mass index (BMI) information was lacking, 221 for whom waist circumference (WC) information was lacking, 4 for whom the education status was lacking, 114 for whom the smoking status was unavailable, 77 without a history of diabetes mellitus (DM), 118 participants for whom low-density lipoprotein cholesterol (LDL-C) information was lacking and 4 participants for whom serum uric acid (UA) information was lacking. Therefore, a total of 6874 participants was included in this study (Fig. 1).
499 participants for whom weight values were unavailable, 836 for whom alcohol status was unavailable, 773 for whom a poverty income ratio (ratio of family income to poverty threshold [PIR]) was unavailable, 79 for whom body mass index (BMI) information was lacking, 221 for whom waist circumference (WC) information was lacking, 4 for whom the education status was lacking, 114 for whom the smoking status was unavailable, 77 without a history of diabetes mellitus (DM), 118 participants for whom low-density lipoprotein cholesterol (LDL-C) information was lacking and 4 participants for whom serum uric acid (UA) information was lacking. Therefore, a total of 6874 participants was included in this study (Fig. 1).
2.2 Data collection
The development of the CDAI was described and verified in a previous report.3 The intake of antioxidants, micronutrients, and total energy was calculated using the US Department of Agriculture's Dietary Research Food and Nutrition Database.14 According to the questionnaire survey, we determined each participant's intake of dietary supplements in the past month, including dose, frequency and number of doses.15 To estimate the CDAI, we standardized each of the same six dietary vitamins and minerals by subtracting the global average and dividing by the global standard deviation. We then calculated the CDAI by adding the standard intake of these vitamins and minerals as follows.
We divided race into non-Hispanic white, non-Hispanic black, Mexican-American, and other groups. Educational background was divided into less than high school (less than 9th grade or 9–11th grade [including 12th grade with no diploma]), high school or equivalent, and more than high school (some college or associate's degree or college graduate or above). The marital status was classified as never married, divorced/widowed/separated, or married/living with a partner. The smoking status was categorized as never/former/now smoker. The diagnostic criteria for alcohol consumption and status were: current heavy drinking (≥4 drinks per day for men, ≥3 drinks per day for women, or binge drinking ≥5 days per month), current moderate drinking (≥3 drinks per day for men, ≥2 drinks per day for women, or binge drinking ≥2 days per month), or current light drinking (not meeting the above criteria), never (had <12 drinks in lifetime), former (had ≥12 dinks in 1 year and did not drink last year, or did not drink last year but drank ≥12 drinks in lifetime).
Hypertension was defined as average systolic blood pressure (SBP) ≥ 140 mmHg and/or average diastolic blood pressure (DBP) ≥ 90 mmHg; or self-reported diagnosis of hypertension and intake of antihypertensive medications.17,18 DM was defined as (1) doctor diagnosed diabetes; (2) glycohemoglobin >6.5%; (3) fasting glucose ≥7.0 mmol L−1; (4) random blood glucose ≥11.1 mmol L−1; (5) two-hour oral glucose tolerance test blood glucose ≥11.1 mmol L−1; and (6) use of diabetes medication or insulin.19 The full measurement technique for these variables is available at https://www.cdc.gov/nchs/nhanes/.
2.3 Statistical analyses
Continuous variables are expressed as weighted means ± standard deviation and were compared using weighted linear regression analysis. Categorical variables are described using unweighted frequencies (weighted percentages) and were compared using the chi-squared test. These means and frequencies can be generalized to the US adult population. A multivariate linear regression model was used to study the correlation between the CDAI and CKD. The CDAI is generally converted into categorical variables according to quartiles, and the P values of the trend are calculated. Three models are used in this study. Model 1 was a crude model not adjusted for potential confounding factors. Model 2 was adjusted for age, gender, race and education level. Model 3 was further adjusted for alcohol consumption, smoking status, BMI, WC, PIR, SBP, Glu, UA, TG, eGFR, UACR, hypertension and DM. The association of the CDAI with CKD was further analyzed stratified by gender (male/female), age (<60/≥60 years), smoking status (never/former/now smoker), hypertension (yes/no), and DM (yes/no/preDM). The BMI was categorized as <25.0, 25.0–30.0 and ≥30.0 kg m−2, corresponding to normal weight, overweight and obese, respectively. PIR was categorized as <1.3, 1.3–1.8, and >1.8. These stratification factors were also considered pre-specified potential effect modifiers. An interaction term was also added to test for heterogeneity in the associations between subgroups. All analyses were performed using R software version 4.2.2 (https://www.R-project.org; R Foundation, Austria). Appropriate examination weights were used to represent the complex survey design. Statistical significance was set at P < 0.05.3. Results
3.1 Participant characteristics
A total of 6874 participants (mean age, 46.43 ± 0.38 years; 3428 male, 3446 female) were included. Table 1 shows the weighted baseline characteristics of the study participants. BMI, WC, Glu, Cr, TG, UACR, UA and SBP were lower in the non-CKD versus the CKD group (P < 0.05). Education level and PIR were higher in the non-CKD group. In terms of the smoking status, 29.1% of patients with CKD had a history of smoking or were current smokers. Moderate-drinkers were more often in non-CKD than CKD participants. In contrast, no significant trends were observed for race, ALT, AST, TC, high-density lipoprotein cholesterol (HDL-C) and LDL-C (P > 0.05).| Characteristic | Overall | Non-CKD (N = 5777) | CKD (N = 1097) | P value |
|---|---|---|---|---|
| Data are presented as frequencies (percentages) or mean (SD). CDAI, composite dietary antioxidant index; PIR, poverty income ratio; BMI, the body-mass index is determined as follows: the weight in kilograms (kgs)/height in square meters (m2); WC, circumference waist; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; preDM, prediabetes; Glu, blood glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, serum creatinine; UA, serum uric acid; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration; UACR, urinary albumin/creatinine ratio; and CKD, chronic kidney disease. | ||||
| Age [mean (SD)] | 46.43 (0.38) | 44.58 (0.40) | 59.06 (0.84) | <0.0001 |
| Gender (%) | <0.001 | |||
| Male | 3428 (49.87) | 2897 (88.91) | 531 (11.09) | |
| Female | 3446 (50.13) | 2880 (85.58) | 566 (14.42) | |
| Race/ethnicity (%) | 0.07 | |||
| Non-Hispanic white | 2792 (40.62) | 2306 (87.15) | 486 (12.85) | |
| Non-Hispanic black | 1391 (20.24) | 1145 (84.90) | 246 (15.10) | |
| Mexican American | 958 (13.94) | 814 (88.04) | 144 (11.96) | |
| Other race | 1733 (25.21) | 1512 (88.94) | 221 (11.06) | |
| Education (%) | <0.0001 | |||
| Less than high school | 1290 (19.31) | 1017 (81.32) | 273 (18.68) | |
| High school or equivalent | 1463 (21.9) | 1173 (83.59) | 290 (16.41) | |
| More than high school | 3926 (58.78) | 3402 (89.39) | 524 (10.61) | |
| PIR [mean (SD)] | 2.99 (0.06) | 3.04 (0.06) | 2.68 (0.09) | <0.0001 |
| Alcohol status (%) | <0.0001 | |||
| Never | 986 (14.34) | 810 (83.52) | 176 (16.48) | |
| Former | 873 (12.7) | 657 (77.84) | 216 (22.16) | |
| Mild | 2493 (36.27) | 2097 (87.59) | 396 (12.41) | |
| Moderate | 1131 (16.45) | 993 (90.08) | 138 (9.92) | |
| Heavy | 1391 (20.24) | 1220 (90.55) | 171 (9.45) | |
| Smoking status (%) | <0.0001 | |||
| Never | 3975 (57.83) | 3427 (88.86) | 548 (11.14) | |
| Former | 1595 (23.2) | 1247 (82.69) | 348 (17.31) | |
| Now | 1304 (18.97) | 1103 (88.21) | 201 (11.79) | |
| BMI [mean (SD)] | 29.15 (0.15) | 28.96 (0.15) | 30.43 (0.38) | <0.001 |
| WC[mean (SD)] | 99.59 (0.37) | 98.91 (0.38) | 104.28 (0.89) | <0.0001 |
| SBP [mean (SD)] | 121.41 (0.31) | 119.99 (0.32) | 131.09 (0.69) | <0.0001 |
| DBP [mean (SD)] | 70.15 (0.25) | 70.35 (0.27) | 68.79 (0.51) | 0.01 |
| Hypertension (%) | <0.0001 | |||
| No | 4083 (59.4) | 3757 (93.34) | 326 (6.66) | |
| Yes | 2791 (40.6) | 2020 (76.86) | 771 (23.14) | |
| DM (%) | <0.0001 | |||
| No | 4299 (62.54) | 3893 (92.15) | 406 (7.85) | |
| preDM | 1206 (17.54) | 987 (84.70) | 219 (15.30) | |
| DM | 1369 (19.92) | 897 (68.08) | 472 (31.92) | |
| Glu | 5.91 (0.03) | 5.79 (0.03) | 6.72 (0.09) | <0.0001 |
| Alt | 24.81 (0.24) | 24.90 (0.26) | 24.20 (0.78) | 0.41 |
| Ast | 24.71 (0.24) | 24.51 (0.23) | 26.12 (0.96) | 0.11 |
| Cr | 76.84 (0.36) | 74.39 (0.30) | 93.58 (2.28) | <0.0001 |
| UA | 325.05 (1.48) | 320.11 (1.47) | 358.74 (3.19) | <0.0001 |
| TG | 1.26 (0.02) | 1.24 (0.02) | 1.39 (0.04) | <0.001 |
| TC | 4.89 (0.02) | 4.89 (0.02) | 4.90 (0.04) | 0.78 |
| HDL-C | 1.41 (0.01) | 1.41 (0.01) | 1.43 (0.02) | 0.32 |
| LDL-C | 2.91 (0.02) | 2.92 (0.02) | 2.84 (0.04) | 0.06 |
| eGFR | 96.16 (0.47) | 98.91 (0.45) | 77.37 (1.33) | <0.0001 |
| UACR | 27.96 (2.49) | 7.74 (0.09) | 166.06 (18.76) | <0.0001 |
| CDAI | 0.69 (0.07) | 0.78 (0.08) | 0.06 (0.12) | <0.0001 |
3.2 Association between the CDAI and CKD
Table 2 displays the relationships between the CDAI and diverse variables, such as age, gender, race, education, PIR, alcohol status, smoking status, BMI, WC, SBP, DBP, history of DM and hypertension, and some biochemical indicators. An increase in the CDAI serves as a protective factor against CKD. Table 3 presents the above relationships assessed using multivariate analyses. In this study, three models were constructed to examine the relationship between the CDAI and CKD. Model 1, no covariate adjustment; Model 2 adjustment for age, gender, race and education level; Model 3, Model 2 plus adjustment for the smoking status, alcohol status, BMI, WC, PIR, SBP, Glu, UA, TG, eGFR, UACR, hypertension and DM as covariates. Participants with a higher CDAI had a lower risk of developing CKD. This association was significant both in Model 1 [odds ratio (OR), −0.0048; 95% confidence interval [CI], (−0.0065, −0.0030); P < 0.0001], Model 2 [OR, −0.0037; 95% CI, (−0.0054, −0.0019); P < 0.001] and Model 3 [OR, −0.0017; 95% CI, (−0.0033, −0.0001), P = 0.0399]. For sensitivity analysis, we converted the CDAI from a continuous variable to a quadratic categorical variable for trend testing and obtained consistent findings (Table 3).| Characteristic | B | 95% CI | P value |
|---|---|---|---|
| Data are presented as frequencies (percentages) or mean (SD); OR, odds ratio; 95% CI, 95% confidence interval; CDAI, composite dietary antioxidant index; PIR, poverty income ratio; BMI, body-mass index is determined as follows: the weight in kilograms (kgs)/height in square meters (m2); WC, circumference waist; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; preDM, prediabetes; Glu, blood glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, serum creatinine; UA, serum uric acid; TG, triglycerides; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration; UACR, urinary albumin/creatinine ratio; and CKD, chronic kidney disease. | |||
| Age [mean (SD)] | 0.01 | (0.00, 0.01) | <0.0001 |
| Gender (%) | |||
| Male | Reference | Reference | Reference |
| Female | 0.03 | (0.02, 0.05) | <0.001 |
| Race/ethnicity (%) | |||
| Non-Hispanic white | Reference | Reference | Reference |
| Non-Hispanic black | 0.02 | (0.00, 0.05) | 0.08 |
| Mexican American | 0.01 | (−0.04, 0.02) | 0.51 |
| Other race | 0.02 | (−0.04, 0.01) | 0.12 |
| Education (%) | |||
| Less than high school | Reference | Reference | Reference |
| High school or equivalent | 0.02 | (−0.06, 0.01) | 0.22 |
| More than high school | 0.08 | (−0.11, −0.05) | <0.0001 |
| PIR [mean (SD)] | 0.01 | (−0.02, −0.01) | <0.0001 |
| Alcohol status (%) | |||
| Never | Reference | Reference | Reference |
| Former | 0.06 | (0.01, 0.10) | 0.01 |
| Mild | 0.04 | (−0.08, 0.00) | 0.04 |
| Moderate | 0.07 | (−0.11, −0.03) | 0.002 |
| Heavy | 0.07 | (−0.11, −0.03) | <0.001 |
| Smoking status (%) | |||
| Never | Reference | Reference | Reference |
| Former | 0.06 | (0.04, 0.09) | <0.0001 |
| Now | 0.01 | (−0.02, 0.03) | 0.64 |
| BMI [mean (SD)] | 0 | (0.00, 0.01) | <0.001 |
| WC [mean (SD)] | 0 | (0.00, 0.00) | <0.0001 |
| SBP [mean (SD)] | 0 | (0.00, 0.01) | <0.0001 |
| DBP [mean (SD)] | 0 | (0.00, 0.00) | 0.004 |
| Hypertension (%) | |||
| No | Reference | Reference | Reference |
| Yes | 0.16 | (0.14, 0.19) | <0.0001 |
| DM (%) | |||
| No | Reference | Reference | Reference |
| preDM | 0.07 | (0.05, 0.10) | <0.0001 |
| DM | 0.24 | (0.21, 0.27) | <0.0001 |
| Glu | 0.04 | (0.03, 0.05) | <0.0001 |
| ALT | 0 | (0.00, 0.00) | 0.42 |
| AST | 0 | (0.00, 0.00) | 0.13 |
| Cr | 0 | (0.00, 0.00) | <0.0001 |
| UA | 0 | (0.00, 0.00) | <0.0001 |
| TG | 0.03 | (0.02, 0.05) | <0.001 |
| TC | 0 | (−0.01, 0.01) | 0.78 |
| HDL-C | 0.01 | (−0.01, 0.04) | 0.31 |
| LDL-C | −0.01 | (−0.02, 0.00) | 0.06 |
| eGFR | −0.01 | (−0.01, 0.00) | <0.0001 |
| UACR | 0 | (0.00, 0.00) | <0.0001 |
| CDAI | 0 | (−0.01, 0.00) | <0.0001 |
| OR (95% CI), P value | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Model 1: no covariates were adjusted. Model 2: age, gender, race and education were adjusted. Model 3: age, gender, race, education, alcohol status, smoking status; OR, odds ratio; 95% CI, 95% confidence interval; BMI, body-mass index is determined as follows: the weight in kilograms (kgs)/height in square meters (m2); WC, circumference waist; PIR, poverty income ratio; SBP, systolic blood pressure; Glu, blood glucose; UA, serum uric acid; TG, triglycerides; eGFR, estimated glomerular filtration; UACR, urinary albumin/creatinine ratio; and hypertension and DM (diabetes mellitus) were adjusted. | |||
| Continuous | |||
| −0.0048 (−0.0065, −0.0030) | −0.0037 (−0.0054, −0.0019) | −0.0017 (−0.0033, −0.0001) | |
| <0.0001 | <0.001 | 0.0399 | |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 | −0.0257 (−0.0533, 0.0018) | −0.0315 (−0.0599, −0.0031) | −0.0635 (−0.0841, −0.0430) |
| Q3 | −0.0293 (−0.0555, −0.0031) | −0.027 (−0.0562, 0.0022) | −0.0522 (−0.0730, −0.0314) |
| Q4 | −0.0155 (−0.0417, 0.0107) | −0.0052 (−0.0346, 0.0242) | −0.0305 (−0.0491, −0.0120) |
| P for trend | <0.0001 | <0.0001 | 0.0094 |
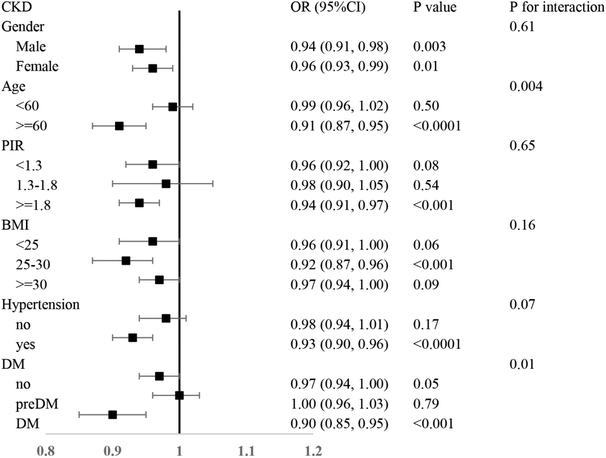
3.3 Subgroup analysis
In the subgroup analysis (Fig. 2), this positive association remained significant in patients older than or equal to 60 years, with a PIR greater than or equal to 1.8 and a BMI between 25 and 30 and in those with a history of hypertension and DM. After stratified gender, age, PIR, BMI, and the history of Hypertension and DM, the interaction test were all observed between the CDAI and CKD (P for interaction <0.05).4. Discussion
In this study, we examined the relationship between the CDAI and the risk of developing CKD. After the adjustment for multiple covariates in the adult population based on the NHANES data, we found a negative association between the CDAI and CKD, suggesting that the CDAI protects against the development of CKD. There was a clear trend toward a lower CKD risk with a higher CDAI.Oxidative stress is an imbalance between the production of antioxidants and pro-oxidants, which subsequently damages the tissues and organs. The accumulation of reactive oxygen species (ROS) can lead to the oxidation of DNA, proteins, carbohydrates, and lipids; apoptosis; and organ dysfunction.20 As an external factor, diet regulates the plasma redox status and protects against ROS and reactive nitrogen species. To maintain a steady biological redox state, antioxidants may scavenge oxidants, thus preventing oxidative stress.21 The exogenous intake of antioxidants prevents inflammation, atherosclerosis, insulin resistance, and oxidative stress in CKD and dialysis patients.22–24
Several clinical studies have examined the association between specific antioxidant micronutrients and CKD; however, the findings have been inconsistent. Some studies have suggested a negative correlation between the intake of vitamins A, C, and E, carotenoids, selenium, zinc and CKD,25–28 whereas others showed no significant relationship.29,30 Previous clinical studies have focused primarily on the effects of individual nutrients on CKD. However, considering the natural combination of nutrients in food, assessing overall dietary antioxidant intake can provide a more comprehensive understanding. The CDAI is a measure of total antioxidant levels in the diet and has been widely used in many studies. Previous studies demonstrated that high CDAI levels reduce the levels of inflammatory factors and lower the risk of various diseases, such as lung cancer, non-alcoholic fatty liver disease, and DM.3,8,31–33 However, existing evidence of the association between higher dietary antioxidant intake and CKD is limited. This study aimed to address this research gap and provide evidence that adequate antioxidant intake may reduce the incidence of CKD.
In accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement, we conducted a subgroup analysis to enhance the utilization of data to reveal the underlying truth. In the subgroup analysis, we observed a significant interaction between dietary antioxidant indicators and the predefined risk factors for CKD. The CDAI showed a strong negative correlation with elderly patients and patients with DM complications. These results are consistent with those of previous studies.6,8 Although the specific reasons for this finding are unclear, it may be because these individuals have higher levels of oxidative stress, and exogenous antioxidant intake appears to be more protective in those with higher innate or acquired ROS-levels.34 Our results suggest that people at high risk of CKD may benefit more from overall dietary antioxidant intake.
There are several limitations to this study. First, due to its retrospective design, it was unable to construct or confirm any causal inferences. Second, despite the adjustment for potential confounding factors, residual confounding factors may still exist, that may affect the relationship between the CDAI and CKD. Third, as the population of this study was American and did not include special populations such as minors, we were unable to analyze special populations or other ethnicities because of the limited sample size. Further studies are required to determine whether the benefits of dietary antioxidants can be extended to other populations.
In summary, this cross-sectional study based on four cycles (2011–2018) of the NHANES detected a negative correlation between the CDAI and CKD in American adults after adjustment for potential confounding factors. This study provides a new way to explore the factors affecting dietary interventions to reduce the incidence of CKD. In the future, more randomized controlled trials or cohort studies are urgently needed to confirm this finding and provide more accurate and effective prevention and treatment options for CKD.
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CDAI | Composite dietary antioxidant index |
| CKD | Chronic kidney disease |
| Cr | Serum creatinine |
| DM | Diabetes |
| DBP | Diastolic blood pressure |
| eGFR | Estimated glomerular filtration |
| Glu | Blood glucose |
| HDL-C | High density lipoprotein cholesterol |
| LDL-C | Low density lipoprotein cholesterol |
| NHANES | National Health and Nutrition Examination Survey |
| OR | Odds ratio |
| PIR | Poverty income ratio |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| TC | Total cholesterol |
| TG | Triglycerides |
| UA | Uric acid |
| UACR | Urinary albumin/creatinine ratio |
| WC | Waist circumference |
| 95% CI | 95% confidence interval |
Author contributions
Min Wang and Qiu-Lin Fan designed the research. Min Wang and Zhao-hui Huang performed the research. Min Wang, Yong-hong Zhu and Ping He analyzed the data. Min Wang wrote the paper. All authors contributed to the article and approved the final manuscript.Data availability
The original contribution presented in this study is included in the article. The dataset was based on the NHANES, which is publicly available and could be found below: https://www.cdc.gov/nchs/nhanes/.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Science and Technology Innovative Leading Talent Fund of Xingliao Talents Program (Liaoning Specially-invited Professors) (XLYC1902080), the Shanghai Pujiang Talent Plan (No. 22PJD061) and the Natural Science Foundation of China (No. 82070754). Thanks to Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, the nhanesR package and webpage, makes it easier for us to explore the NHANES database. We would like to thank Editage (https://www.editage.cn) for English language editing.References
- K. Matsushita, S. H. Ballew, A. Y. Wang, R. Kalyesubula, E. Schaeffner and R. Agarwal, Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease, Nat. Rev. Nephrol., 2022, 18, 696–707 CrossRef PubMed.
- J. Jankowski, J. Floege, D. Fliser, M. Bohm and N. Marx, Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options, Circulation, 2021, 143, 1157–1172 CrossRef CAS PubMed.
- M. E. Wright, S. T. Mayne, R. Z. Stolzenberg-Solomon, Z. Li, P. Pietinen, P. R. Taylor, J. Virtamo and D. Albanes, Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers, Am. J. Epidemiol., 2004, 160, 68–76 CrossRef PubMed.
- Y. C. Yu, P. Paragomi, R. Wang, A. Jin, R. E. Schoen, L. T. Sheng, A. Pan, W. P. Koh, J. M. Yuan and H. N. Luu, Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the Singapore Chinese Health Study, Int. J. Cancer, 2022, 150, 1599–1608 CrossRef CAS PubMed.
- L. Wang and Z. Yi, Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: A prospective cohort study, Front. Cardiovasc. Med., 2022, 9, 993930 CrossRef PubMed.
- H. He, X. Chen, D. Miao, H. Zhang, Y. Wang, X. He, X. Chen and N. Dai, Composite Dietary Antioxidant Index and Plasma Levels of Soluble Klotho: Insights from NHANES, Oxid. Med. Cell. Longevity, 2023, 2023, 3524611 Search PubMed.
- A. Maugeri, M. Barchitta, R. Magnano San Lio, A. Scalisi and A. Agodi, Antioxidant and inflammatory potential of diet among women at risk of cervical cancer: findings from a cross-sectional study in Italy, Public Health Nutr., 2022, 25, 1577–1585 CrossRef PubMed.
- W. Wang, X. Wang, S. Cao, Y. Duan, C. Xu, D. Gan and W. He, Dietary Antioxidant Indices in Relation to All-Cause and Cause-Specific Mortality Among Adults With Diabetes: A Prospective Cohort Study, Front. Nutr., 2022, 9, 849727 CrossRef PubMed.
- X. Yang, A. Chen, Q. Liang, Q. Dong, M. Fu, X. Liu, S. Wang, Y. Li, Y. Ye, Z. Lan, J. S. Ou, L. Lu and J. Yan, Up-regulation of heme oxygenase-1 by celastrol alleviates oxidative stress and vascular calcification in chronic kidney disease, Free Radicals Biol. Med., 2021, 172, 530–540 CrossRef CAS PubMed.
- M. S. Goligorsky, Oxidative Stress and the Kidney: Riding on the Curve of Hormesis, Antioxid. Redox Signal., 2016, 25, 117–118 CrossRef CAS PubMed.
- R. Samadarsi and D. Dutta, Anti-oxidative effect of mangiferin-chitosan nanoparticles on oxidative stress-induced renal cells, Int. J. Biol. Macromol., 2020, 151, 36–46 CrossRef CAS PubMed.
- S. E. Wu and W. L. Chen, Soluble klotho as an effective biomarker to characterize inflammatory states, Ann. Med., 2022, 54, 1520–1529 CrossRef CAS PubMed.
- H. Xie, N. Li, G. Zhou, Q. Liu, H. Wang, J. Han, L. Shen, P. Yu, J. Chen and X. Chen, Plasma S-Klotho level affects the risk of hyperuricemia in the middle-aged and elderly people, Eur. J. Med. Res., 2022, 27, 262 CrossRef CAS PubMed.
- J. K. Ahuja, A. J. Moshfegh, J. M. Holden and E. Harris, USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice, J. Nutr., 2013, 143, 241S–249S CrossRef CAS PubMed.
- E. D. Kantor, C. D. Rehm, M. Du, E. White and E. L. Giovannucci, Trends in Dietary Supplement Use Among US Adults From 1999–2012, J. Am. Med. Assoc., 2016, 316, 1464–1474 CrossRef PubMed.
- S. G. Adler, B. H. Rovin, J. Barratt, F. Bridoux, K. A. Burdge, T. M. Chan, H. Terence Cook, F. C. Fervenza, K. L. Gibson, R. J. Glassock, D. R. W. Jayne, V. Jha, A. Liew, Z.-H. Liu, J. M. Mejía-Vilet, C. M. Nester, J. Radhakrishnan, E. M. Rave, H. N. Reich, P. Ronco, J.-S. F. Sanders, S. Sethi, Y. Suzuki, S. C. W. Tang, V. Tesar, M. Vivarelli, J. F. M. Wetzels and J. Floege, KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases, Kidney Int., 2021, 100, S1–S276 CrossRef PubMed.
- J. M. Flack and B. Adekola, Blood pressure and the new ACC/AHA hypertension guidelines, Trends Cardiovasc. Med., 2020, 30, 160–164 CrossRef PubMed.
- D. M. Reboussin, N. B. Allen, M. E. Griswold, E. Guallar, Y. Hong, D. T. Lackland, E. P. R. Miller 3rd, T. Polonsky, A. M. Thompson-Paul and S. Vupputuri, Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation, 2018, 138, e595–e616 CrossRef PubMed.
- V. R. A. Boris Draznin, G. Bakris, G. Benson, F. M. Brown, RaS. Freeman, J. Green, E. Huang, D. Isaacs, S. Kahan, J. Leon, S. K. Lyons, A. L. Peters, P. Prahalad, J. E. B. Reusch and D. Young-Hyman, 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022, Diabetes Care, 2022, 45, S17–S38 CrossRef PubMed.
- S. Roumeliotis, A. Roumeliotis, E. Dounousi, T. Eleftheriadis and V. Liakopoulos, Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review, Nutrients, 2019, 11 DOI:10.3390/nu11081911.
- B. Demmig-Adams and W. W. Adams 3rd, Antioxidants in photosynthesis and human nutrition, Science, 2002, 298, 2149–2153 CrossRef CAS PubMed.
- V. Liakopoulos, S. Roumeliotis, A. Bozikas, T. Eleftheriadis and E. Dounousi, Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence?, Oxid. Med. Cell. Longevity, 2019, 2019, 9109473 Search PubMed.
- S. Lupinacci, A. Perri, G. Toteda, D. Vizza, F. Puoci, O. I. Parisi, F. Giordano, D. Lofaro, A. La Russa, M. Bonofiglio and R. Bonofiglio, Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFbeta1 signaling, Cell Biol. Toxicol., 2019, 35, 95–109 CrossRef CAS PubMed.
- H. Xu, Z. Xiong, J. Arnlov, A. R. Qureshi, T. Cederholm, P. Sjogren, B. Lindholm, U. Riserus and J. J. Carrero, Circulating Alpha-Tocopherol and Insulin Sensitivity Among Older Men With Chronic Kidney Disease, J. Renal Nutr., 2016, 26, 177–182 CrossRef CAS PubMed.
- S. F. Rapa, B. R. Di Iorio, P. Campiglia, A. Heidland and S. Marzocco, Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites, Int. J. Mol. Sci., 2019, 21, 263 CrossRef PubMed.
- Q. Zhong, Y. Piao, S. Yin and K. Zhang, Association of serum lycopene concentrations with all-cause and cardiovascular mortality among individuals with chronic kidney disease: A cohort study, Front. Nutr., 2022, 9, 1048884 CrossRef PubMed.
- C. H. Guo, P. C. Chen, G. S. Hsu and C. L. Wang, Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study, Nutrients, 2013, 5, 1456–1470 CrossRef CAS PubMed.
- B. A. Zachara, Selenium and selenium-dependent antioxidants in chronic kidney disease, Adv. Clin. Chem., 2015, 68, 131–151 CAS.
- M. Hodkova, S. Dusilova-Sulkova, M. Kalousova, J. Soukupova, T. Zima, D. Mikova, I. M. Malbohan and J. Bartunkova, Influence of oral vitamin E therapy on micro-inflammation and cardiovascular disease markers in chronic hemodialysis patients, Renal Failure, 2006, 28, 395–399 CrossRef CAS PubMed.
- E. Lonn, S. Yusuf, B. Hoogwerf, J. Pogue, Q. Yi, B. Zinman, J. Bosch, G. Dagenais, J. F. Mann, H. C. Gerstein, H. Study and M.-H. Study, Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy, Diabetes Care, 2002, 25, 1919–1927 CrossRef CAS PubMed.
- A. Salehi-Sahlabadi, A. Mokari, M. Elhamkia, F. Farahmand, M. Jabbari and A. Hekmatdost, Dietary Total Antioxidant Capacity and Risk of Non-Alcoholic Fatty Liver Disease: A Case-Control Study, J. Res. Health Sci., 2020, 20, e00486 CrossRef PubMed.
- D. G. de Oliveira, F. de Faria Ghetti, A. P. B. Moreira, H. H. M. Hermsdorff, J. M. de Oliveira and L. de Castro Ferreira, Association between dietary total antioxidant capacity and hepatocellular ballooning in nonalcoholic steatohepatitis: a cross-sectional study, Eur. J. Nutr., 2019, 58, 2263–2270 CrossRef CAS PubMed.
- H. N. Luu, W. Wen, H. Li, Q. Dai, G. Yang, Q. Cai, Y. B. Xiang, Y. T. Gao, W. Zheng and X. O. Shu, Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels?, Antioxid. Redox Signal., 2015, 22, 951–959 CrossRef CAS PubMed.
- R. I. Salganik, The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population, J. Am. Coll. Nutr., 2001, 20, 464S–472S CrossRef CAS PubMed ; discussion 473S–475S.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo01157g |
| This journal is © The Royal Society of Chemistry 2023 |