A new approach to the preparation of poly(p-phenylene terephthalamide) nanofibers
Hongchen Yana,
Jinglong Lia,
Wenting Tiana,
Lianyuan Hea,
Xinlin Tuo*a and
Teng Qiub
aKey Laboratory of Advanced Materials (MOE), Department of Chemical Engineering, Tsinghua University, Beijing 100084, P. R. China
bKey Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: tuoxl@tsinghua.edu.cn
First published on 4th March 2016
Abstract
Poly(p-phenylene terephthalamide) (PPTA) nanofibers were prepared via a polymerization induced self-assembly process with the assistance of methoxy polyethylene glycol (mPEG) for stability and dispersity. In the traditional process of the solution polycondensation of p-phenylenediamine (PPD) and terephthaloyl chloride (TPC), PPTA will aggregate and precipitate with the chain growth due to the liquid crystalline characteristic. The introduction of mPEG can control the aggregation degree of PPTA molecules and stabilize the formed aggregates, which drives the self-assembly of PPTA molecules and results in the formation of nanofibers. The obtained nanofibers specialized by the great length–diameter ratio were characterized in detail. The effects of mPEG addition on the formation and the performance of the fibers were all studied in the work. The nanofibers can disperse in variety of organic solvents and water. Moreover, the nanofibers can be deposited for the formation of thin films with excellent transparency and thermal stability, which has great potential applications such as the separator of lithium ion battery.
Introduction
The study on nanofibers has been highlighted as important to materials science inspired by the rapid developments on carbon nanotubes,1–3 bacterial cellulose nanocrystals,4–6 inorganic or organic–inorganic hybrid fibers at micro- or nanoscale7–9 and polymer nanofibers.10,11 One of the typical polymer nanofibers is the poly(p-phenylene terephthalamide) (PPTA) nanofibers.PPTA is a kind of widely used liquid-crystal polymer. In name of aramid or Kevlar®, PPTA fibers are well known for their high performances, such as high strength, high modulus and excellent heat resistance because of which they have been extensively concerned both in military and civil fields.12–18 However, PPTA can only be dissolved under rigorous conditions like sulfuric acid, limiting the processing and application of PPTA fibers. Development of fine denier PPTA fibers, from PPTA pulp, PPTA fibrid to dispersible PPTA nanofibers, is a direction to solve such problems.19–23 Furthermore, nanofibers of high-performance polymers also provide attractive building-blocks for the fabrication of functional nanocomposites, which will extend PPTA's application to many high-tech areas. For example, PPTA nanofiber/poly(ethylene oxide) (PEO) film was prepared via layer-by-layer method, which was used as a solid ion-conducting medium in batteries.24 PPTA nanofiber-functionalized graphene sheets could act as a novel polymer reinforcement and dramatically increase the mechanical properties, thermal and ultraviolet stabilities of poly(methyl methacrylate) (PMMA) matrix.25 In the building of nanocomposites, dispersible PPTA nanofibers which can form colloidal dispersions in general solvents will provide great convenience.
Although the fabrication of dispersible PPTA nanofibers is of specific advantages, the work is also a challenge for the poor dissolubility of PPTA. To now there have been three main methods to fabricate PPTA nanofibers. The first is based on the electrospinning method. A high electric field is applied to a hanging droplet of PPTA solution contained in a capillary tube in this process. When the applied electric field overcomes surface tension, a charged jet of the solution is ejected. The solvent is removed by coagulation in a non-solvent bath and nanofibers are fabricated. However, the poor solubility of PPTA is still the “bottle-neck” in this approach which has to be conquered by using specialized devices and formulations.26,27 Moreover, the prepared nanofibers can't be dispersed in liquid mediums, limiting their convenience of application. The second method is based on the deprotonation of amide groups on macroscopic PPTA fibers.28,29 Kotov group has proposed the method of chemical cleaving to prepare PPTA nanofibers in dimethylsulphoxide (DMSO) in the presence of KOH.30 The prepared nanofibers dispersed in DMSO are of negatively charged surfaces, thus they can be used as the self-assembly building blocks for the fabrication of ultrathin films. However, the preparation has its own defects such as time-consuming and low reaction concentration. In the third method by a downsizing process, Twaron fibers are disintegrated into nanofibers under mechanical treatment with the help of electrostatic repulsive force.31 In conclusion, all of the three methods are based on the secondary processing of the pre-prepared PPTA fibers. Considering the advantages and potential applications, there is an urgent need to explore novel and effective methods to prepare dispersible PPTA nanofibers.
Herein, we proposed a new approach to the preparation of PPTA nanofibers. The work was started from the polycondensation of p-phenylenediamine (PPD) and terephthaloyl chloride (TPC) together with the addition of methoxy polyethylene glycol (mPEG) in N-2-methyl pyrrolidone (NMP) solvent. Employing mPEG as the interfacial tailoring agent as well as the dispersant, the raw product from the polymerization could be dispersed into nanofibers in different organic or inorganic solvents, like methanol, ethanol or water, to form stable dispersions. The long nanofibers in dispersion could be deposited to fabricate thin films with excellent transparency and thermal stability. The resulting nanofibers were detailedly characterized and the mechanism of nanofiber formation was subsequently explored in this work.
Experiment
Materials
PPD (>99%) was purchased from Amino-Chem™ Ltd. (Shanghai). TPC (>99%) was purchased from J&K Scientific Ltd. (Beijing). 4 Å molecular sieve, CaCl2 (>99%), methanol, ethanol and chloroform were provided by Beijing Chemical Works. Methoxy polyethylene glycol (mPEG, Mn = 2000) and polyethylene glycol dimethyl ether (PEG-DME, Mn = 2000) were purchased from Sigma-Aldrich Ltd. (Shanghai). N-2-Methyl pyrrolidone (NMP) was purchased from Modern Oriental Fine Chemistry Ltd. (Beijing) and purified by adding 4 Å molecular sieve before experiments to remove the trace water. Water content after the treatment was less than 100 ppm, measured using a moisture-testing instrument (Mettler Toledo). CaCl2 was heated at 350 °C for 4 hours to eliminate the trace water before the experiments.Preparation of PPTA nanofibers
CaCl2 and mPEG were added and dissolved in NMP at 100 °C in a special designed reactor with N2 sealed and then the reactor was cooled down to 0 °C using ice water bath. PPD was added and dissolved in the NMP solution at 0 °C under stirring. TPC with molar ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.007 (PPD
1.007 (PPD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPC) was added subsequently and the stirring was accelerated to be 2500 rpm to conduct the polycondensation with the reaction temperature kept below 70 °C. The polycondensation was stopped when Weissenberg effect appeared and excess NMP was added under strong shear to crush the formed gel. Then the disperse solvent such as methanol or water was added under the same conditions. With this method, dispersed PPTA nanofibers were formed.
TPC) was added subsequently and the stirring was accelerated to be 2500 rpm to conduct the polycondensation with the reaction temperature kept below 70 °C. The polycondensation was stopped when Weissenberg effect appeared and excess NMP was added under strong shear to crush the formed gel. Then the disperse solvent such as methanol or water was added under the same conditions. With this method, dispersed PPTA nanofibers were formed.
For characterization and preparation of membrane, PPTA nanofibers were washed thoroughly using excess deionized water under strong stirring, followed by filtration to remove the solvents and other residues. The above procedure was repeated three times to ensure the nanofibers were cleaned thoroughly. The obtained PPTA nanofibers were dried at 120 °C for 24 h for characterization. In this way, PPTA nanofiber membranes with different thickness were also prepared by adjusting the amount of nanofiber dispersion.
In the polycondensation process, mPEG was replaced by PEG-DME to confirm the reaction of mPEG with PPTA. Except this replacement, the other conditions kept the same unless otherwise indicated. Moreover, several disperse solvents such as methanol, ethanol, chloroform and water were used to prepare stable and uniform dispersion of PPTA nanofibers.
Characterization
The inherent viscosity method was used to measure the molecular weight of PPTA products. In this experiment, the inherent viscosity of PPTA in sulfuric acid was measured at the concentration of 0.5 g dl−1 by using an Ubbelohde viscometer (Beijing Midwest Group, 1.04–1.07 mm). The relationship between the inherent viscosity and the average molecular weight of PPTA14 is:| Mw = 3902.4η1.556 | (1) |
The microstructure of PPTA nanofibers was observed by using a Scanning Electron Microscope (SEM, Merlin Compact, Zeiss) and transmission electron microscope (TEM, CM120-Biotwin). The topology of PPTA fibers was characterized using a tapping mode by atomic force microscope (AFM, BRUKE Nanoscope V). Thermogravimetric analysis (TGA, TGA2050, TA Instruments) was used to characterize the thermal stability of PPTA nanofibers in N2 atmosphere from 100 °C to 800 °C. A Fourier transform infrared spectrometer (FTIR, Nicolet560, Nicolet) was applied for IR analysis of chemical bonds and structures. The crystal structure of PPTA nanofibers was characterized by X-ray diffraction (XRD, D/maxIIIB, Rigaku) and the crystallinity of PPTA nanofibers was calculated with eqn (2).
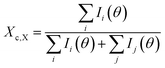 | (2) |
Result and discussion
Preparation of PPTA nanofiber
The evidence of the formation of PPTA nanofibers are shown in Fig. 1. The as-prepared methanol dispersion with PPTA concentration at 3 wt% was translucent and homogenous in macroscopic observation. When radiated by laser, the system showed typical Tyndall effect as Fig. 1(a) shows, which proved the colloidal characteristic of the dispersion. The dispersed PPTA nanofibers were characterized by TEM as Fig. 1(b) shows as the only objects in the observation field. The fibers were uniform in appearance with specific long and entangled morphologies. The topology image obtained in AFM characterization is shown in Fig. 1(c). The TEM and AFM micrographs indicate the diameter of the nanofibers is below 100 nm and the height of the nanofibers is almost the same. | ||
| Fig. 1 Characterization results of PPTA nanofiber dispersion: (a) Tyndall effect of PPTA nanofiber colloid; (b) TEM; (c) AFM. | ||
XRD characterization of the nanofibers is shown in Fig. 2. The peaks at 20.5°, 22.9° and 28.1° are diffraction peaks of (110), (200) and (004) lattice planes in PPTA.32 The XRD patterns of PPTA nanofibers are almost the same as those of the Kevlar fibers and the crystallinity degree of PPTA nanofibers calculated from the result of Gaussian peak fitting shown in Fig. 2(b) is about 73.7%, which is consistent with that of Kevlar fibers. It is known that PPTA molecules are axially oriented in Kevlar fibers and the almost same state of PPTA orientation in nanofibers reveals that the PPTA molecules are highly aggregated and orientated.
 | ||
| Fig. 2 XRD results of PPTA nanofibers: (a) XRD patterns of Kevlar fibers and PPTA nanofibers; (b) result of Gauss peak fitting. | ||
Effects of the addition of mPEG
The effect of the introduction of mPEG in the work could be revealed by Fig. 3. Without mPEG, none PPTA nanofiber is observed. The aligned bundle morphology shown in the SEM characterization (Fig. 3(a)) indicates the strong orientation tendency of the liquid crystalline PPTA. The products were separated out of the system as irregular particles whose dimensions were at microscale. With the addition of mPEG, PPTA molecules tend to form fibrous structure and the increase of mPEG additive amount led to fine and separated PPTA fibers. With the mPEG addition at 5%, fibrous PPTA bundles were formed whose diameter was hundreds of nanometers and the dispersibility was much improved compared with that without mPEG. With the mPEG dosage increased to 10%, monofilament with the diameter of about 50 nm was observed as shown in Fig. 3(c). The morphology evolution with mPEG demonstrated that mPEG should be the key parameter for the formation of nanofibers. Further increasing the mPEG dosage resulted to Fig. 3(d), indicating that increasing the amount of mPEG sequentially led to improved dispersity of PPTA nanofibers. However, the geometric characteristics of the fibers kept almost the same. | ||
| Fig. 3 SEM and TEM micrographs of PPTA nanofibers with different mPEG additive amounts: (a) 0%; (b) 5%; (c) 10%; (d) 15%. | ||
As demonstrated above, mPEG has great contribution to the formation of PPTA nanofibers so that the interaction between PPTA and mPEG was explored. The chemical bonding of mPEG on PPTA via the reaction of hydroxyl in mPEG and acyl chloride in PPTA, as Scheme 1 shows, could be verified by FTIR characterization. The sample was thoroughly washed with water to remove the unreacted mPEG. The typically FTIR spectrum is displayed as the main image in Fig. 4(a). The absorption band at 1647 cm−1 is caused by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations. The deformation coupling vibrations of C–N and N–H cause the absorption bands at 1542 cm−1 and 1252 cm−1, respectively. And the absorption band at 1509 cm−1 is attributed to C
O stretching vibrations. The deformation coupling vibrations of C–N and N–H cause the absorption bands at 1542 cm−1 and 1252 cm−1, respectively. And the absorption band at 1509 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of aromatic ring. These absorption bands are characteristic for PPTA and consistent with the previous literature.33 There is an absorption band at 2872 cm−1 caused by the C–H stretching vibration of aliphatic hydrocarbon, indicating the copolymerization of mPEG with PPTA. The inset in Fig. 4(b) gives the IR spectra of PPTA nanofibers synthesized with different mPEG dosage or PEG-DME. There is none absorption band at 2872 cm−1 when the mPEG dosage is 0% or the PEG-DME is used. On the contrary, the increasing mPEG dosage causes the increasing absorption intensity at 2872 cm−1, suggesting the bonding of mPEG with PPTA.
C stretching vibrations of aromatic ring. These absorption bands are characteristic for PPTA and consistent with the previous literature.33 There is an absorption band at 2872 cm−1 caused by the C–H stretching vibration of aliphatic hydrocarbon, indicating the copolymerization of mPEG with PPTA. The inset in Fig. 4(b) gives the IR spectra of PPTA nanofibers synthesized with different mPEG dosage or PEG-DME. There is none absorption band at 2872 cm−1 when the mPEG dosage is 0% or the PEG-DME is used. On the contrary, the increasing mPEG dosage causes the increasing absorption intensity at 2872 cm−1, suggesting the bonding of mPEG with PPTA.
 | ||
| Fig. 4 FTIR characterization: (a) FTIR spectra of PPTA nanofibers with main peaks marked; (b) FTIR spectra of PPTA nanofibers with mPEG (from 0% to 15% the mass of PPTA) and PEG-DME. | ||
TGA thermographs of PPTA nanofibers are shown in Fig. 5. It can be seen that the main decomposition of the sample are all up to 550 °C with the weight residual larger than 40%, indicating the good thermal stability of the prepared nanofibers. It could also be observed that with the incorporation of mPEG, the main decomposition curves move to the low temperature with the decomposition temperature descending from 550 °C (0% mPEG) to 330 °C (15% mPEG). At the same time, the residual content also decreases from 45.13% (0% mPEG) to 39.90% (15% mPEG). Additional decomposition at 330–340 °C with the weight loss less than 10% can be observed on the curves of mPEG modified samples but not on the pure PPTA sample. So the decomposition was attributed to that of mPEG. Moreover, the mPEG contents of the samples are lower than the addition amounts, indicating just parts of mPEG molecules participated in the reaction of hydroxyl and acyl chloride. As shown in Table 1, the more mPEG was added, the more mPEG reacted. However, the percentage of the reacted mPEG gradually decreased. mPEG will compete with PPD for the reaction with TPC in the polycondensation, but hydroxyl is less reactive compared with amidogen. Therefore, amidization is the dominant reaction instead of esterification. Further, mPEG reduced the polymerization speed and decreased the molecular weight of PPTA. Without mPEG, the gelation occurred in 5 min during the polymerization of PPTA while the gelation was prolonged to over 8 min with mPEG added. It is worth mentioning that the reaction of mPEG and PPTA changes the molecular structure and the traditional method of the average molecular weight measurement has a little deviation from the reality.
| Additive weight of mPEG (wt%) | mPEG content (wt%) | Mw (×104) |
|---|---|---|
| 0% | — | 2.2 |
| 5% | 2.97% | 1.6 |
| 10% | 4.43% | 1.3 |
| 15% | 5.36% | 1.2 |
According to the characterizations above, we could sketch the possible preparation scheme as polymerization-induced self-assembly.34–38 The initial monomers of PPD and TPC as well as mPEG were soluble in the solvent of NMP while their polymer of PPTA wasn't. Meanwhile, PPTA had strong tendency to form oriented microstructures as a typical liquid crystal polymer (Fig. 6(a)). During polymerization, the orientation of PPTA was tailored by the incorporated PEG segments, which located on the interface to isolate the aligned units via the formation of the intermolecular hydrogen bonds with PPTA. The interfacial location of PEG was favourable in the system as PEG is amphiphilic to both of the polymer and solvent phase. The self-hydrogen bonds of PPTA which resulted in the irregular aggregations were therefore suppressed. In consequence, PPTA would self-assemble into nano-fibrous structure during PPTA polymerization (Fig. 6(b)). At the same time, PPTA chains became insoluble and unstable in NMP along with the chain growth. The strong interaction based on hydrogen bonds and covalent bonds between mPEG and PPTA is believed to be useful for the stability of PPTA. It is noteworthy that it is difficult to characterize the detailed self-assembly process during PPTA nanofiber formation because of the complex phase transition (liquid to gel to solid) and the insolubility of PPTA in common solvents.
 | ||
| Fig. 6 Nanostructure of PPTA aggregates: (a) PPTA aggregate without mPEG; (b) PPTA aggregate with 15% mPEG added. | ||
Following with strong shear in organic or inorganic solvent such as methanol, ethanol, chloroform and water, PPTA nanofibers could be isolated as mono-filaments with nanoscale in the radial direction. As the basic fabric units were formed in the polymerization, the geometric parameters of the fibers would not be affected by the disperse medium and the dispersions in different solvents had no obvious difference in appearance (Fig. 7(a)). This observation further confirms that the formation of nanofibers was completed during polymerization process instead of the following dispersion. PPTA thin films could be prepared by the deposition of the nanofibers. Interestingly, the nanofibers can be fabricated into ultrathin membrane with thickness at several micrometer and excellent mechanical properties. Fig. 6(b) shows a dried membrane with thickness of 22 μm, which has smooth surface and excellent transparency. The tensile strength of this membrane is over 50 MPa. Imaged by SEM, tangled network is observed (Fig. 7(c)) which is contributed to the high mechanical properties. Previously it was difficult to produce such a PPTA film whether with film casting of PPTA/sulfuric acid solution or paper making of PPTA pulp. All these results exhibit the availability of PPTA nanofibers for different applications.
 | ||
| Fig. 7 Characterization of PPTA film: (a) PPTA nanofiber dispersion; (b) PPTA film; (c) SEM micrograph of PPTA membrane. | ||
Conclusions
We have introduced a new approach to the preparation of PPTA nanofibers in this work. The preparation was assisted by using mPEG, which was added in NMP before PPD and TPC were dissolved and the polymerization was conducted. With the participation of mPEG, PPTA nanofibers were formed through a polymerization induced self-assembly process. The nanofibers could be dispersed into organic or inorganic solvent like methanol and water for the formation of stable and uniform nanofiber dispersions. The structure, properties and composition of the product were characterized by XRD, IR, TGA, SEM, TEM and AFM, confirming that PPTA nanofibers were successfully prepared and specialized with great length–diameter ratio. SEM and TEM micrographs of the products with different additive amounts of mPEG indicate that the strong interaction between mPEG and PPTA was the key factor in the formation of nanofiber. Incorporation of mPEG onto PPTA via chemical bonding was verified by FTIR and TGA characterization. Moreover, the fibers are of excellent thermal stability with the 50% decomposition temperature higher than 500 °C. Compared with previous methods, such as electrospinning and chemical cleaving, our method present here has its own advantages: convenience, efficiency and brief procedure. The nanofibers in dispersion could be deposited to fabricate thin films with smooth surface and excellent transparency. The PPTA nanofibers would have great potential applications in many fields like the separator of lithium ion battery.Acknowledgements
This work was financially supported by the National Basic Research Program of China (2011CB606102).Notes and references
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- J. R. Potts, D. R. Dreyer, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5–25 CrossRef CAS.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- S. Rose, A. Prevoteau, P. Elziere, D. Hourdet, A. Marcellan and L. Leibler, Nature, 2014, 505, 382–385 CrossRef CAS PubMed.
- S. J. Eichhorn, A. Dufresne, M. Aranguren, N. E. Marcovich, J. R. Capadona, S. J. Rowan, C. Weder, W. Thielemans, M. Roman, S. Renneckar, W. Gindl, S. Veigel, J. Keckes, H. Yano, K. Abe, M. Nogi, A. N. Nakagaito, A. Mangalam, J. Simonsen, A. S. Benight, A. Bismarck, L. A. Berglund and T. Peijs, J. Mater. Sci., 2010, 45, 1–33 CrossRef CAS.
- D. R. Paul and L. M. Robeson, Polymer, 2008, 49, 3187–3204 CrossRef CAS.
- A. A. Mamedov, N. A. Kotov, M. Prato, D. M. Guldi, J. P. Wicksted and A. Hirsch, Nat. Mater., 2002, 1, 190–194 CrossRef CAS PubMed.
- M. H. Al-Saleh and U. Sundararaj, Carbon, 2009, 47, 2–22 CrossRef CAS.
- A. Rahy and D. J. Yang, Mater. Lett., 2008, 62, 4311–4314 CrossRef CAS.
- J. Miao, M. Miyauchi, T. J. Simmons, J. S. Dordick and R. J. Linhardt, J. Nanosci. Nanotechnol., 2010, 10, 5507–5519 CrossRef CAS PubMed.
- Du Pont, US pat., C08J3/02, 3671542, 1972-06-20.
- M. Takayanagi, T. Ogata, M. Morikawa and T. Kai, J. Macromol. Sci., Part B: Phys., 1980, 17(4), 591–615 CrossRef.
- H. Yang, in Kevlar aramid fiber, J. Wiley, Chichester, 1993, pp. 23–46 Search PubMed.
- H. Chae and S. Kumar, J. Appl. Polym. Sci., 2006, 100(1), 791–802 CrossRef CAS.
- Y. Guan, Y. Zheng and J. Cui, Chin. J. Polym. Sci., 2010, 28(2), 257–267 CrossRef CAS.
- D. Tanner, J. Fitzgerald and B. Phillips, Angew. Chem., Int. Ed., 1989, 28(5), 649–654 CrossRef.
- Y. Rao, A. Waddon and R. Farris, Polymer, 2001, 42(13), 5937–5946 CrossRef CAS.
- Du Pont, US pat., D01F6/60, 3869430, 1975-03-04.
- DuPont, Res. Discl., 1975, 8, 13675 Search PubMed.
- DuPont, Res. Discl., 1980, 2, 19037 Search PubMed.
- E. Merriman, Tappi J., 1984, 67(8), 66–68 Search PubMed.
- H. Yoon, Nature, 1987, 326(6113), 580–582 CrossRef CAS.
- S. Tung, S. Ho, M. Yang, R. Zhang and N. Kotov, Nat. Commun., 2015, 6, 6152–6158 CrossRef CAS PubMed.
- J. Fan, Z. Shi, L. Zhang, J. Wang and J. Yin, Nanoscale, 2012, 4(22), 7046–7055 RSC.
- G. Srinivasan and D. Reneker, Polym. Int., 1995, 36(2), 195–201 CrossRef CAS.
- J. Yao, J. Jin, E. Lepore, N. M. Pugno, C. W. M. Bastiaansen and T. Peijs, Macromol. Mater. Eng., 2015, 300, 1238–1245 CrossRef CAS.
- M. Takayanagi and T. Katayose, J. Polym. Sci., Part A: Polym. Chem., 1981, 19, 1133–1145 CrossRef CAS.
- M. Takayanagi, T. Kajiyama and T. Katayose, J. Appl. Polym. Sci., 1982, 27, 3903–3917 CrossRef CAS.
- M. Yang, K. Cao, L. Sui, Y. Qi, J. Zhu, A. Waas, E. Arruda, J. Kieffer, M. Thouless and N. Kotov, ACS Nano, 2011, 5(9), 6945–6954 CrossRef CAS PubMed.
- S. Ifuku, H. Maeta, H. Izawa, M. Morimoto and H. Saimoto, RSC Adv., 2014, 4(76), 40377–40380 RSC.
- Y. Rao, A. J. Waddon and R. J. Farris, Polymer, 2001, 42, 5937–5946 CrossRef CAS.
- G. Litovchenko, T. Sokolova, A. Volokhina, G. Kudryavtsev and S. Papkov, J. Appl. Spectrosc., 1974, 20(3), 345–348 CrossRef.
- S. Sugihara, A. Blanazs, S. Armes, A. Ryan and A. Lewis, J. Am. Chem. Soc., 2011, 133, 15707–15713 CrossRef CAS PubMed.
- A. Blanazs, J. Madsen, G. Battaglia, A. Ryan and S. Armes, J. Am. Chem. Soc., 2011, 133, 16581–16587 CrossRef CAS PubMed.
- W. Wan and C. Pan, Macromolecules, 2010, 43, 2672–2675 CrossRef CAS.
- J. Yuan, S. Soll, M. Drechsler, A. Müller and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 17556–17559 CrossRef CAS PubMed.
- Y. Zhang, L. Wang, Z. Zhang, Y. Zhang and X. Tuo, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2161–2170 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |


