Controlled fabrication of polypyrrole capsules and nanotubes in the presence of Rhodamine B†
Yanpeng
Xue
a,
Xiaofeng
Lu
*a,
Yue
Xu
b,
Xiujie
Bian
a,
Lirong
Kong
a and
Ce
Wang
*a
aAlan G. MacDiarmid Institute, Jilin University, Changchun, P. R. China 130012. E-mail: xflu@jlu.edu.cn; Fax: +86-431-85168292; Tel: +86-431-85168292
bTest Science Experiment Center, Jilin University, Changchun, P. R. China 130012
First published on 18th October 2010
Abstract
A novel and simple method for the fabrication of polypyrrole (PPy) capsules and nanotubes via a chemical oxidation polymerization in the presence of Rhodamine B (RB) at room temperature is described.
Nano or microscale hollow polymer structures, especially those with controllable shapes, sizes and compositions,1–9 have attracted a lot of research interest because of their potential applications in absorption, separation, drug delivery, cell and enzyme transplantation, gene therapy, sensing, and environmental research. A typical method for preparing nano or microscale hollow polymer structures mainly involved a multi-step process by the template-based sacrificial-core approach.10 Firstly, the removable templates were synthesized. Then the shell was in situ polymerized or post-covered on the surface of the removable templates. Finally, the templates were removed via chemical etching or other methods. Although it has been shown that hollow polymer structures could be prepared via a self-assembly approach,11 the solution-phase synthesis of such structures with controllable morphologies in one step still remains a great challenge.
During the past few decades, conducting polymers have been extensively studied due to their novel conductive properties and intriguing applications in many fields. Recently, nanostructured conducting polymers have attracted more attention because of their small scale characteristics, which could enhance their performance in sensitive chemical sensors, electronic devices, energy conversion and storage devices etc.11–16 In particular, PPy is one of the most promising conducting polymers due to its high electronic conductivity, excellent environmental stability and potential applications in many kinds of fields. In the past few years, a variety of approaches, including microemulsion polymerization, template method, surfactant-mediated synthesis, and nanofiber seeding etc., have been developed for preparing PPy nanoparticles and solid nanofibers.17–21 While there are several reports describing the preparation of PPy hollow spheres and nanotubes using a template-assisted method, e.g., polystyrene latex spheres, SiO2 spheres, metal, metal halide, metal oxides as templates,22–27 the bulk synthesis of PPy hollow structures directly from pyrrole monomer is still a challenge. Liu and Wan developed a self-assembly method to synthesize PPy microtubes using β-naphthalene sulfonic acid as the dopant by controlling the rate of addition of the oxidant.28 Lu and co-workers reported the fabrication of PPy nanotubes using FeCl3 as an oxidant in the presence of methyl orange by either chemical oxidation polymerization or electrochemical polymerization via a self-assembly process.29,30 However, there are few reports on fabricating other kinds of morphologies of PPy nanostructures besides nanotubes using the self-assembly method.
In this paper, we demonstrate a simple chemical oxidation polymerization approach to create PPy capsules and nanotubes in the presence of Rhodamine B (RB). When certain amount of RB was added to the conventional reagents used to prepare PPy, capsules or nanotubes of PPy could be produced without the need for any specific dopant or as-synthesized template. In our experiment, the complex of the RB–ammonium persulfate (APS) plays an important role in the formation of hollow PPy structures, which act as a self-degrading template for the synthesis of PPy capsules and nanotubes. Furthermore, the template would automatically degrade during the polymerization process due to the reduction of APS, avoiding the need for additional template removal steps. Our procedure affords a facile, scalable and reproducible synthesis of bulk quantities of hollow PPy structures. To the best of our knowledge, this is the first report on the controlled preparation of both PPy capsules and nanotubes in the same reaction by simply adjusting the concentrations of RB.
Different from the conventional template-synthesis method with multiple steps to synthesize hollow structures of conducting polymers, we afforded a simple one-step approach to prepare PPy capsules and nanotubes in aqueous solution via a chemical oxidation polymerization. The polymerization system contained pyrrole monomer, APS and RB. We have observed that the rate of the polymerization and the morphologies of the obtained samples were significantly dependent on the initial concentration of RB. Fig. 1 displays the reaction process for the fabrication of PPy capsules and nanotubes via a chemical oxidation polymerization in the presence of different concentrations of RB. It was found that red precipitate was immediately formed when APS was added to the RB solution, indicating the formation of RB-APS complex. For the reactions in the presence of high concentration of RB (10 mM), the rate of polymerization was fast. The red precipitate has been turned to black dispersions within 5 min, indicating the formation of large quantity of PPy samples. On the other hand, the rate of the polymerization became a little slower when the concentration of RB decreased to 1.25 mM. PPy samples were obtained after 30 min. The morphologies of the synthesized PPy samples in the presence of RB at concentrations of 10 mM and 1.25 mM have been characterized by scannning electron microscopy (SEM) and transmission electron microscopy (TEM) images. Fig. 2A and B show the SEM and TEM images of the obtained PPy sample in the presence of 10 mM RB. It was found that irregular capsules appear to form, some broken capsules in SEM images indicated that the PPy capsules were hollow. The TEM image further proved that the structure of the PPy sample was hollow. And it could be observed that the wall thickness of the PPy capsules was in the range of 30–50 nm from TEM images. On the other hand, when the reaction was performed in the presence of 1.25 mM RB, fiber-like PPy samples were prepared. The TEM image further proved that hollow tubular structures with outer diameters and thickness in the range of 180–280 nm and 20–30 nm, respectively, were formed. It was noted that some irregular nanoparticles and broken nanotubes were also observed in the obtained samples under such conditions, which might be due to the irregular RB-APS complex as the self-degrading template.
![A schematic illustration of the fabrication of PPy capsules and nanotubes via a chemical oxidation polymerization in the presence of different concentrations of RB (top: [RB] = 10 mM, bottom: [RB] = 1.25 mM). The top TEM image showed the sample synthesized from the reaction solution after 5 min. The bottom TEM image shows the sample obtained from the reaction solution after 30 min.](/image/article/2010/PY/c0py00305k/c0py00305k-f1.gif) | ||
| Fig. 1 A schematic illustration of the fabrication of PPy capsules and nanotubes via a chemical oxidation polymerization in the presence of different concentrations of RB (top: [RB] = 10 mM, bottom: [RB] = 1.25 mM). The top TEM image showed the sample synthesized from the reaction solution after 5 min. The bottom TEM image shows the sample obtained from the reaction solution after 30 min. | ||
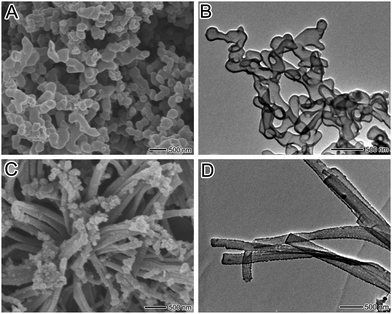 | ||
| Fig. 2 (A, B) SEM and TEM images of as-prepared PPy capsules. (C, D) SEM and TEM images of as-synthesized PPy nanotubes. | ||
The chemical composition of the resulting PPy capsules and nanotubes has been characterized by Fourier-transform infrared (FTIR) spectra and X-ray diffraction (XRD) patterns. Fig. 3A shows the FTIR spectra of PPy capsules and nanotubes, respectively. The two spectra are similar, indicating the resulting PPy capsules and nanotubes have the same polymer backbone. With, for example, PANI capsules, the characteristic bands at 1554 and 1473 cm−1 could be attributed to the antisymmetric and symmetric pyrrole-ring fundamental vibration, respectively. The peaks at 1286 and 1040 cm−1 are assigned to the C–N stretching vibrations and C–H deformation vibrations. The bands near 1199 and 922 cm−1 are related to the doping state of PPy. All of these characteristic bands are consistent with the previous reports, proving the formation of PPy.31Fig. 3B displays the typical XRD patterns of the synthesized PPy capsules and nanotubes. It shows that no sharp peaks are observed in both of the samples, indicating their amorphous nature. The FTIR spectra and XRD patterns fully proved that the concentrations of RB only had a strongly effect on the PPy morphology, and did not influence the chemical and crystalline structures of the resulting PPy.
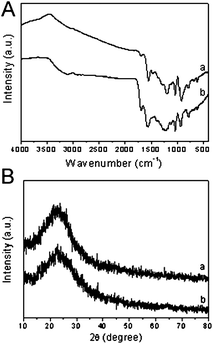 | ||
| Fig. 3 (A) FTIR spectra and (B) XRD patterns of resulting (a) PPy capsules and (b) nanotubes fabricated in the presence of RB at concentration of 10 mM and 1.25 mM, respectively. | ||
During the polymerization process, the concentration of RB plays an important role in the formation of PPy nanostructures. As shown in Fig. 4, we have investigated the influence of the concentration of RB on the morphology of the resulting PPy samples. When the concentration of RB increased to 20 mM, large quantity of solid and hollow PPy spheres were observed. By decreasing the concentration of RB to 10 mM, fine PPy capsules could be synthesized. When the concentration of RB was further decreased to 5.0 mM, not only PPy capsules but also PPy nanotubes were formed. When the concentration of RB decreased to 2.5 mM, large quantities of PPy nanotubes were prepared. Previous studies have shown that organic acids, such as β-naphthalene sulfonic acid, could form a template-like micelle to induce the formation of PPy microtubes.28 It has also been reported that FeCl3 and methyl orange could form a self-degrade fibrillar complex, which guided the formation of PPy nanotubes.29 In our studies, we proposed that the formation of the obtained PPy capsules and nanotubes was related to the complex between APS and RB. In order to prove our assumption, APS was added to the RB solution at a concentration of 10 mM and 1.25 mM, respectively. Immediately, the red transparent solution became red precipitate suspensions for both of the reactions, proving that an RB-APS complex was formed. The RB-APS complex should be attributed to the electrostatic interactions between ammonium cation in RB and persulfate anion in APS, which is similar with the previous report.20 The corresponding TEM images of the resulting RB-APS are depicted in Fig. 5. The morphology of the resulting RB-APS was different for the two reactions. When the concentration of RB was 10 mM, the obtained RB-APS complex exhibited a capsule-like structure, while tube-like structure has been formed when the concentration of RB decreased to 1.25 mM. Such results indicated that the PPy capsules and nanotubes should be formed using RB-APS complex as a self-degrade template. During the polymerization process, pyrrole monomer was polymerized on the surface of RB-APS complex template. With the reaction carrying on, the template would automatically degrade because of the reduction of APS. As a result, PPy hollow structures could be formed.
![TEM image of the as-synthesized PPy samples in the presence of RB at different initial concentrations. (A) [RB] = 20 mM, (B) [RB] = 10 mM, C) [RB] = 5 mM, D) [RB] = 2.5 mM.](/image/article/2010/PY/c0py00305k/c0py00305k-f4.gif) | ||
| Fig. 4 TEM image of the as-synthesized PPy samples in the presence of RB at different initial concentrations. (A) [RB] = 20 mM, (B) [RB] = 10 mM, C) [RB] = 5 mM, D) [RB] = 2.5 mM. | ||
![TEM image of RB-APS complex synthesized in the presence of different concentrations of RB. (A) [RB] = 10 mM; (B) [RB] = 1.25 mM.](/image/article/2010/PY/c0py00305k/c0py00305k-f5.gif) | ||
| Fig. 5 TEM image of RB-APS complex synthesized in the presence of different concentrations of RB. (A) [RB] = 10 mM; (B) [RB] = 1.25 mM. | ||
In summary, we show for the first time that PPy capsules and nanotubes could be fabricated via a chemical oxidation polymerization in the presence of different concentrations of RB. Capsule or tube-like complexes could be formed between RB and APS molecules via electrostatic interactions, providing a self-degraded template for the growth of PPy. This process for fabricating hollow PPy structures does not require removal of the template, allowing easy production on a large scale. It is anticipated that such a facile method could be extended to synthesize other kinds of conducting polymer nanostructures, which have potential applications in the fields of drug delivery, catalyst carrier, supercapacitors, sensors and electronic nanodevices.
Acknowledgements
The financial support from the National 973 project (No. 2007CB936203), and the National Nature Science Foundation of China (Nos. 20904015 and 50973038) are greatly appreciated.Notes and references
- F. Caruso, Adv. Mater., 2001, 13, 11 CrossRef CAS.
- H. Fau, S. Herminghaus, P. Lenz and R. Lipowsky, Science, 1999, 283, 46 CrossRef CAS.
- P. Jiang, F. J. Cizeron and V. L. Colvin, J. Am. Chem. Soc., 1999, 121, 7957 CrossRef CAS.
- D. G. Shuchukin and G. B. Sukhorukov, Adv. Mater., 2004, 16, 671 CrossRef CAS.
- G. Suhorukov, L. Dahne, J. Hartmann, E. Donath and H. Mohwald, Adv. Mater., 2000, 12, 112 CrossRef CAS.
- Y. Zhang, W. Xu, W. Yao and S. Yu, J. Phys. Chem. C, 2009, 113, 8588 CrossRef CAS.
- X. Yang, T. Dai, M. Wei and Y. Lu, Polymer, 2006, 47, 4596 CrossRef CAS.
- J. Han, G. P. Song and R. Guo, Adv. Mater., 2006, 18, 3140 CrossRef CAS.
- Y. Tan, F. Bai, D. Wang, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2007, 19, 5773 CrossRef CAS.
- Z. Yang, Z. Niu and Y. Lu, Angew. Chem., Int. Ed., 2003, 42, 1943 CrossRef CAS.
- M. Wan, Adv. Mater., 2008, 20, 2926 CrossRef CAS.
- M. Wan, Macromol. Rapid Commun., 2009, 30, 963 CrossRef CAS.
- C. Li, H. Bai and G. Shi, Chem. Soc. Rev., 2009, 38, 2397 RSC.
- D. Li, J. Huang and R. B. Kaner, Acc. Chem. Res., 2009, 42, 135 CrossRef CAS.
- H. D. Tran, D. Li and R. B. Kaner, Adv. Mater., 2009, 21, 1487 CrossRef CAS.
- X. Lu, W. Zhang, C. Wang, T.-C. Wen and Y. Wei, Prog. Polym. Sci., 2010 DOI:10.1016/j.progpolymsci.2010.07.010.
- J. Jang, J. H. Oh and G. D. Stucky, Angew. Chem., Int. Ed., 2002, 41, 4016 CrossRef CAS.
- M. Ikegame, K. Tajima and T. Aida, Angew. Chem., Int. Ed., 2003, 42, 2154 CrossRef CAS.
- W. Zhang, X. Wen and S. Yang, Langmuir, 2003, 19, 4420 CrossRef CAS.
- X. Zhang, J. Zhang, Z. Liu and C. Robinson, Chem. Commun., 2004, 1852 RSC.
- X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 12714 CrossRef CAS.
- Y. Yang, Y. Chu, F. Y. Yang and Y. P. Zhang, Mater. Chem. Phys., 2005, 92, 164 CrossRef CAS.
- X. Liu, H. Wu, F. Ren, G. Qiu and M. Tang, Mater. Chem. Phys., 2008, 109, 5 CrossRef CAS.
- L. Hao, C. Zhu, C. Chen, P. Kang, Y. Hu, W. Fan and Z. Chen, Synth. Met., 2003, 139, 391 CrossRef CAS.
- S. M. Marinakos, J. P. Novak and L. C. Brousseau, J. Am. Chem. Soc., 1999, 121, 8518 CrossRef CAS.
- D. Cheng, H. Xia and H. S. On Chan, Nanotechnology, 2006, 17, 1661 CrossRef CAS.
- X. Lu, H. Mao and W. Zhang, Polym. Compos., 2009, 30, 847 Search PubMed.
- J. Liu and M. Wan, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 997 CrossRef CAS.
- X. Yang, Z. Zhu, T. Dai and Y. Lu, Macromol. Rapid Commun., 2005, 26, 1736 CrossRef CAS.
- X. Yang, T. Dai, Z. Zhu and Y. Lu, Polymer, 2007, 48, 4021 CrossRef CAS.
- T. Dai, X. Yang and Y. Lu, Nanotechnology, 2006, 17, 3028 CrossRef CAS.
Footnote |
| † General procedure for the fabrication of PPy capsules and nanotubes: in a typical synthesis to fabricate PPy capsules, RB (0.144 g) was dissolved in 30 mL of water and vigorously stirred for about 10 min (the initial concentration of RB was 10 mM). Then 120 μL of pyrrole and a solution of APS (0.410 g, 5 mL H2O) were added to the above solution. The resulting solution was vigorously stirred for about one day. The crude product was purified by filtration and washed with water and ethanol until the filtrate became colorless. The solid product was dried in a vacuum oven at 50 °C for 12 h. Other samples were prepared following a similar procedure. The molar ratios between RB, pyrrole and APS were unchangeable. |
| This journal is © The Royal Society of Chemistry 2010 |
